
Unveiling the complex nexus: dermatomyositis and esophageal adenocarcinoma—a case report
Highlight box
Key findings
• The key findings of this case report include the presentation of a rare case involving a 60-year-old male with dermatomyositis (DM) who developed metastatic esophageal adenocarcinoma. Despite treatment with immunosuppressive agents, the patient’s condition deteriorated, leading to the discovery of advanced esophageal adenocarcinoma. The diagnosis was challenging due to the overlapping symptoms of DM and esophageal malignancy.
What is known and what is new?
• The literature has well-established the association between dermatomyositis and malignancy, with ovarian, colon, breast, lung, gastric, pancreatic, and lymphatic system cancers commonly linked. The diagnostic challenges in cancer screening for DM patients, relying on abnormal laboratory findings and patient-reported symptoms, are recognized in the existing knowledge.
• This manuscript adds to the existing knowledge by presenting a unique case of metastatic esophageal adenocarcinoma in a patient with DM. The rarity of esophageal adenocarcinoma in the context of DM is highlighted, emphasizing the diagnostic challenges posed by the overlapping symptoms of these two conditions. The case underscores the importance of considering esophageal malignancy in DM patients, particularly those with atypical presentations or limited responses to standard treatment.
What is the implication, and what should change now?
• The implications of this case report emphasize the need for heightened vigilance regarding malignancy risk in patients with dermatomyositis, especially when faced with atypical presentations or inadequate responses to standard treatment. The findings also support the notion that DM may be a paraneoplastic event rather than the primary cause of synchronous malignancy. The manuscript highlights the necessity for further research and consensus on screening approaches for malignancy in dermatomyositis patients to guide clinical practice.
Introduction
The association between dermatomyositis (DM) and malignancy is well-documented in the literature, with reported frequencies spanning from 7% to 34% (1,2).
Notably, the most commonly linked malignancies encompass ovarian, colon, breast, lung, gastric, pancreatic, and lymphatic system cancers (2,3). However, reports of esophageal carcinomas in association with DM are relatively scarce (2,3). The process of cancer screening in DM patients presents inherent challenges, often necessitating reliance on abnormal laboratory findings and patient-reported symptoms.
The authors report a rare case of metastatic esophageal adenocarcinoma in a patient with DM. We present this article in accordance with the CARE reporting checklist (available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-23/rc).
Case presentation
A 60-year-old Caucasian male, with a 15-year history of untreated chronic heartburn and a 50 pack-year smoking habit, with no reported additional past medical history or family history, was admitted to the Gastroenterology ward due to a 4-month history of progressive dysphagia to solids and liquids, accompanied by a persistent sensation of food impaction in the lower esophagus.
Seven months before presenting to our emergency room (ER), the patient had sought a dermatology appointment, reporting signs of purple discoloration of the upper eyelids and periorbital skin, violet lichenified lesions on metacarpal-interphalangeal and interphalangeal joints (Gottron papules), and an erythematous pruriginous rash across sun-exposed areas of the back and shoulders. He also complained of symmetric upper and lower limb proximal muscle weakness affecting daily routine activities. Laboratory investigations revealed elevated lactate dehydrogenase at 555 U/L (reference range, 140–280 U/L) and creatine kinase at 754 U/L (reference range, 30–135 U/L). The anti-nuclear antibody titer was 1/320 and the anti-transcription intermediary factor 1 (anti-TIF-1) antibody tested positive. Autoantibody tests were negative for Anti-Mi-2, anti-Jo-1, Sjögren syndrome antigen A (SS-A), Sjögren syndrome antigen B (SS-B), anti-ss-DNA, anti-ds-DNA, anti-Sm, and anti-nuclear ribonucleoprotein (RNP). Electromyography (EMG) displayed a myopathic pattern with positive sharp waves, fibrillations, and increased insertional activity, suggestive of proximal predominant myopathy. The patient declined a muscle biopsy. The diagnosis of DM was established following the Bohan and Peter criteria (1).
Subsequently, he initiated immunosuppressive treatment, namely 20 mg of methotrexate and prednisolone. However, the observed response in ameliorating muscle weakness and skin lesions was moderate, presenting a complex clinical scenario during the patient’s ongoing outpatient dermatological follow-up. Due to the positive anti-TIF-1 antibody test result a comprehensive diagnostic work-up was planned. Computed tomography (CT) scan, colonoscopy, and gastroscopy were prescribed, aimed at a comprehensive investigation and evaluation of potential malignancies, but the patient declined invasive diagnostic tests and failed to attend some scheduled appointments.
In the ensuing months, the patient developed dysphagia, initially intermittent and limited to solid foods. It gradually progressed to dysphagia to solids and liquids, accompanied by regurgitation and chest discomfort. Ultimately, the patient presented to the ER with a sensation of food impaction in the lower esophagus, significant weight loss, and concurrent right ophthalmalgia, associated with a noticeable progressive loss of visual acuity.
On physical examination, the patient exhibited mild erythema affecting the neck and back regions. Vital signs were within normal limits. Upon deep palpation of the epigastric region, abdominal pain was elicited, but no signs of peritoneal irritation were observed. Neurological examination revealed grade 3/5 proximal muscle weakness, particularly in the upper extremities.
Laboratory investigations yielded the following results: Hemoglobin at 12 g/dL (reference range, 13.5–17.5 g/dL), alkaline phosphatase at 439 U/L (reference range, 40–130 U/L), alanine transaminase at 138 U/L (reference range, 10–40 U/L), aspartate transaminase at 107 U/L (reference range, 10–40 U/L), albumin at 32 g/L (reference range, 35–50 g/L), lactate dehydrogenase at 455 U/L (reference range, 140–280 U/L), creatine kinase at 454 U/L (reference, range 30–135 U/L), and C-reactive protein at 21.2 mg/L (reference range, <5 mg/L).
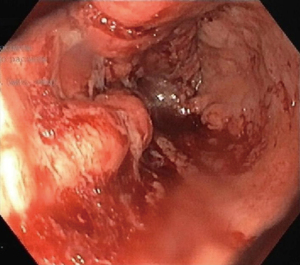
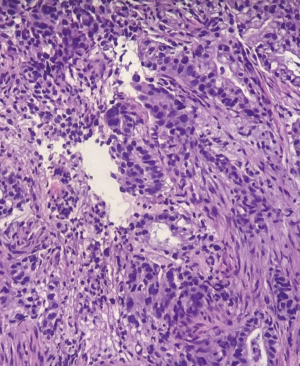
Endoscopic gastroduodenoscopy (EGD) revealed salmon-colored mucosa located 23 cm from the incisors. Beginning at 24 cm and extending onwards, an ulcer-vegetated lesion occupied approximately 2/3 of the luminal circumference. At 34 cm from the incisors, the lesion became circumferential and non-franchisable with the conventional gastroscope (Figure 1). Biopsies were taken from the esophageal lesion, revealing an infiltrative malignant neoplasm characterized by the proliferation of glandular structures displaying pleomorphism and hyperchromasia (Figure 2). This finding was consistent with well-differentiated intestinal subtype esophageal adenocarcinoma.
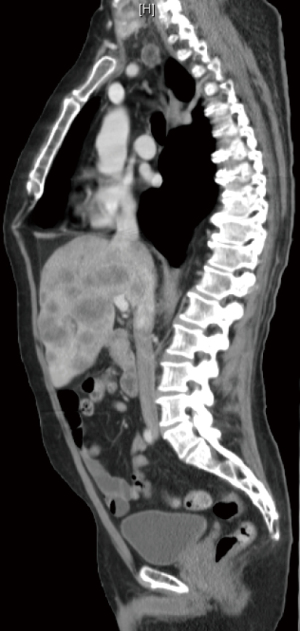
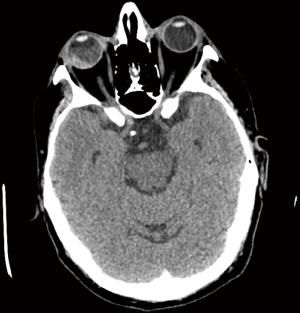
A contrast-enhanced CT scan showed an extensive lesion in the middle and distal segments of the esophagus and at the gastro-esophageal junction, marked by thickened walls and heterogeneous enhancement. Lateral tracheal adenopathies were present. Notably, no hilar adenopathies were observed. In the infradiaphragmatic region, multiple heterogeneous liver nodules infiltrating all segments were found. No abnormalities in the portal vein or bile duct were detected (Figure 3). Furthermore, a right intraocular lesion, approximately 6 mm thick after contrast enhancement, mainly affecting the temporal quadrants, raised suspicion of a secondary deposit (Figure 4).
The final diagnosis was stage IV (T4N+M1b) Esophageal Adenocarcinoma, according to the 8th edition of the American Joint Committee on Cancer staging guidelines Following a multidisciplinary decision meeting, the patient initiated palliative care, including chemotherapy and the placement of a self-expandable esophageal metal stent {measuring 120 mm × [20–26] mm}. The stent placement procedure was carried out without any periprocedural complications.
Subsequently, the patient was admitted to a palliative care unit. Unfortunately, his clinical condition rapidly deteriorated, and he passed away three weeks after the diagnosis was established. All procedures performed in this study were in accordance with the ethical standards of the institutional committee and with the Helsinki Declaration (as revised in 2013). Informed consent was not obtained as the patient passed away during the course of management, and no family members were available for consent.
Discussion
The presented case underscores the intricate relationship between DM and malignancy, notably esophageal adenocarcinoma, a rare association. DM is an idiopathic inflammatory disease that frequently manifests with a symmetrical proximal, extensor inflammatory myopathy and a distinctive cutaneous eruption. In about 10% of cases, patients present with an amyopathic form of the disease, characterized solely by cutaneous manifestations. The precise etiology of DM remains unknown, but it is believed to result from a complex interplay of genetic and environmental factors. Some studies have proposed that viral infections may serve as triggers for the disease in certain instances (1,2).
Classic pathognomonic features of DM encompass the distinctive purple discoloration and edema affecting the upper eyelids and periorbital skin, recognized as the heliotrope rash, along with a violaceous, scaly eruption on the knuckles referred to as the Gottron sign. Additional notable cutaneous hallmarks of DM include a V-sign rash appearing on the neck and chest, as well as a shawl sign extending across the back and shoulders. Importantly, these cutaneous signs typically manifest before the onset of muscular deficits, representing an essential diagnostic characteristic of the condition (1,2).
In cases where DM develops in the context of malignancy, the cutaneous manifestations often precede the emergence of symptoms related to the underlying tumor. DM is linked to an elevated risk of various histological types of cancers, however, the occurrence of DM in association with esophageal adenocarcinomas is relatively infrequent (2,4).
It’s noteworthy that DM exhibits a higher prevalence among females. Nonetheless, it is more frequently observed in males when it is concomitant with malignancy (4).
This case emphasizes the critical importance of considering malignancy risk in DM patients, particularly those displaying atypical presentations or limited response to standard treatment. In fact, the risk of developing cancer appears to be approximately six times higher during the initial year following a DM diagnosis. This risk gradually decreases in subsequent years and is unlikely to be linked to immunosuppressive therapy (1,4,5).
Notably, DM symptoms typically improve with cancer treatment and tend to reoccur when the tumor experiences a relapse. This pattern strongly supports the hypothesis that DM is more likely a paraneoplastic event rather than the primary cause of synchronous malignancy (6-10). Various studies have calculated the frequency of paraneoplastic DM, reporting figures ranging between 15% and 54% of all DM cases (11). Paraneoplastic dermatoses encompass a broad category of non-neoplastic skin alterations dependent on the presence of a neoplasm. One distinguishing characteristic of patients with paraneoplastic DM is a higher incidence of resistance to corticosteroid therapy (12).
In our case, the extended history of untreated heartburn, preceding the onset of dermatological and muscle lesions, coupled with the patient’s resistance to corticosteroid therapy, aligns with the literature and strengthens the hypothesis of DM being a paraneoplastic syndrome secondary to esophageal adenocarcinoma.
Advanced age, the presence of capillary damage on muscle biopsy, and the occurrence of cutaneous necrosis are all recognized as risk factors indicative of potential underlying malignancy. Therefore, these factors necessitate immediate and thorough investigation (13).
Dysphagia, a prevalent and multifactorial symptom in DM, can often be attributed to cricopharyngeal muscle dysfunction, resulting in difficulty initiating swallowing (1,13). In cases where dysphagia persists despite appropriate therapy or when obstructive symptoms are present, it is advisable to pursue further investigation through esophagogastroduodenoscopy.
Immunologic factors have also been implicated in tumor development in this patient population. Notably, the presence of anti-TIF-1γ autoantibodies has been correlated with an elevated risk of malignancy (1,14,15). Indeed, in a recent study, at least 80% of patients with cancer-associated DM had antibodies against either TIF-1γ or nuclear matrix protein NXP-2 (14). Furthermore, a recent meta-analysis comprising six cohort studies demonstrated that anti-TIF-1γ exhibited a sensitivity of 78% and a specificity of 89% for diagnosing cancer association in myositis (16). It is worth noting that cancer predisposition also appears to be associated with Anti-SAE1 patients with DM (17). In our specific case, despite the positive anti-TIF-1 antibody test, the patient chose not to undergo any supplementary examinations and failed to attend the scheduled appointments. This hesitancy to pursue further diagnostic procedures posed a challenge in fully assessing the potential malignancy risk associated with the positive anti-TIF-1 antibody test result, emphasizing the crucial role of patient cooperation in comprehensive healthcare management.
18F-fluorodeoxyglucose-positron emission tomography/CT ([18F] FDG-PET/CT) is a non-invasive imaging modality which exhibits good performance for detecting disease activity, and it proven valuable for cancer screening (18,19). Recent systematic reviews indicate PET/CT performs comparably to conventional work-up methods such as thoraco-abdominal enhanced CT scans, colonoscopy, and gastroscopy (20), demonstrating sensitivity and specificity ranging from 66.7% to 94% and 80% to 97.8%, respectively (21). However, it’s crucial to acknowledge the potential for false-negative results, especially in scenarios involving poorly avid lesions, small-sized tumors (below the camera’s spatial resolution), traditionally PET/CT-negative cancers (e.g., prostate or renal cancer), or non-solid cancers (22). A recent retrospective study highlights limitations, indicating that [18F] FDG-PET/CT may not detect certain occult cancers detected by conventional work-up, emphasizing the need for more prospective and comparative data on its efficacy (18). Addressing concerns about cost and radiation exposure also remains a significant consideration in the ongoing exploration of [18F] FDG-PET/CT’s capabilities (18).
This case report aligns with existing literature, highlighting the significance of immunologic factors, such as anti-TIF-1γ autoantibodies and Anti-SAE1, in correlating with an elevated risk of malignancy in DM patients. This emphasizes the critical need for considering underlying malignancies, especially in cases with atypical presentations or inadequate responses to standard treatment. Clinicians should be vigilant about the potential risk of malignancy in DM patients, prompting comprehensive assessments for timely identification and intervention, in line with care guidelines for DM. Notably, the awareness of the infrequent concurrent occurrence of esophageal cancer with DM is crucial, given the paucity of reports within the English literature documenting the concurrent occurrence of esophageal cancer with DM (3,23-26).
The exceptional clinical scenario outlined in this article underscores the importance for clinicians to conduct comprehensive assessments of patients with DM who develop dysphagia. The diagnosis of metastatic esophageal adenocarcinoma in these patients can be challenging and subject to delays, primarily due to the fact that dysphagia, a common symptom in both conditions, is often disregarded as a potential indicator of an underlying esophageal malignancy.
Conclusions
The risk of having a cancer in patients with DM is higher than in the general population. Thus, DM can also be seen as a paraneoplastic syndrome. A lack of data in approaches to screening for malignancy in DM has led to variability among clinicians. This case highlights the importance of considering an underlying malignancy in patients with DM, particularly in those with atypical presentations or who do not respond to standard treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-23/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-23-23/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-23/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional committee and with the Helsinki Declaration (as revised in 2013). Informed consent was not obtained as the patient passed away during the course of management, and no family members were available for consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lewis M, Fiorentino D. Dermatomyositis. In: Kang S, Amagai M, Bruckner AL, et al., editors. Fitzpatrick's Dermatology, 9e. New York: McGraw Hill; 2019.
- Naik M, Bhat T, Yusuf I, et al. Recurrence of esophageal carcinoma presenting as dermatomyositis. Ann Trop Med Public Health 2013;6:485-6. [Crossref]
- Laidler NK. Dermatomyositis as a paraneoplastic phenomenon in oesophageal cancer. BMJ Case Rep 2018;11:e227387. [Crossref] [PubMed]
- Harrison BA, Heck SI, Hood AF. A fatal case of dermatomyositis with underlying metastatic esophageal adenocarcinoma. Cutis 2008;81:26-8. [PubMed]
- Zhang W, Jiang SP, Huang L. Dermatomyositis and malignancy: a retrospective study of 115 cases. Eur Rev Med Pharmacol Sci 2009;13:77-80. [PubMed]
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 2001;357:96-100. [Crossref] [PubMed]
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med 2001;134:1087-95. [Crossref] [PubMed]
- Basset-Seguin N, Roujeau JC, Gherardi R, et al. Prognostic factors and predictive signs of malignancy in adult dermatomyositis. A study of 32 cases. Arch Dermatol 1990;126:633-7. [Crossref] [PubMed]
- Sunnenberg TD, Kitchens CS. Dermatomyositis associated with malignant melanoma. Parallel occurrence, remission, and relapse of the two processes in a patient. Cancer 1983;51:2157-8. [Crossref] [PubMed]
- Verducci MA, Malkasian GD Jr, Friedman SJ, et al. Gynecologic carcinoma associated with dermatomyositis-polymyositis. Obstet Gynecol 1984;64:695-8. [PubMed]
- Sellami K, Mseddi M, Snoussi M, et al. Malignancy in a retrospective cohort of 17 patients with Dermatomyositis or Polymyositis in southern Tunisia. Rom J Intern Med 2018;56:243-9. [Crossref] [PubMed]
- Liu Y, Xu L, Wu H, et al. Characteristics and predictors of malignancy in dermatomyositis: Analysis of 239 patients from northern China. Oncol Lett 2018;16:5960-8. [Crossref] [PubMed]
- Zouridis S, Tageldin O, Hasa S, et al. S2309 Esophageal Adenocarcinoma Causing Paraneoplastic Dermatomyositis: Preventing Delayed Diagnosis Is Key. Am J Gastroenterol 2022;117:e1554. [Crossref]
- Fiorentino DF, Chung LS, Christopher-Stine L, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum 2013;65:2954-62. [Crossref] [PubMed]
- Sumazaki M, Kaneko K, Ito M, et al. A Case of Dermatomyositis Along with Esophageal Cancer and Screening of Serum Transcriptional Intermediary Factor 1 Gamma Antibodies in Various Cancer Patients. Am J Case Rep 2020;21:e922004. [Crossref] [PubMed]
- Trallero-Araguás E, Rodrigo-Pendás JÁ, Selva-O'Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum 2012;64:523-32. [Crossref] [PubMed]
- Yang H, Peng Q, Yin L, et al. Identification of multiple cancer-associated myositis-specific autoantibodies in idiopathic inflammatory myopathies: a large longitudinal cohort study. Arthritis Res Ther 2017;19:259. [Crossref] [PubMed]
- Yildiz H, D'abadie P, Gheysens O. The Role of Quantitative and Semi-quantitative [18F]FDG-PET/CT Indices for Evaluating Disease Activity and Management of Patients With Dermatomyositis and Polymyositis. Front Med (Lausanne) 2022;9:883727. [Crossref] [PubMed]
- Yildiz H, Lepere C, Zorzi G, et al. [18F]FDG-PET/CT in Idiopathic Inflammatory Myopathies: Retrospective Data from a Belgian Cohort. Diagnostics (Basel) 2023;13:2316. [Crossref] [PubMed]
- Selva-O'Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med 2010;123:558-62. [Crossref] [PubMed]
- Bentick G, Fairley J, Nadesapillai S, et al. Defining the clinical utility of PET or PET-CT in idiopathic inflammatory myopathies: A systematic literature review. Semin Arthritis Rheum 2022;57:152107. [Crossref] [PubMed]
- Maliha PG, Hudson M, Abikhzer G, et al. 18F-FDG PET/CT versus conventional investigations for cancer screening in autoimmune inflammatory myopathy in the era of novel myopathy classifications. Nucl Med Commun 2019;40:377-82. [Crossref] [PubMed]
- Kikuchi K, Seto Y, Matsubara T, et al. Amyopathic dermatomyositis associated with esophageal cancer. Int J Dermatol 2008;47:310-1. [Crossref] [PubMed]
- Iftikhar I, Abdelmannan D, Daw HA. Dermatomyositis and esophageal cancer. South Med J 2006;99:777-9. [Crossref] [PubMed]
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol 2010;146:26-30. [Crossref] [PubMed]
- Karp SJ. Acute dermatomyositis associated with squamous carcinoma of the oesophagus. J R Soc Med 1985;78:770-1. [Crossref] [PubMed]
Cite this article as: Gonçalves A, Simas D, Gomes P, Barbeiro S, Cotrim I, Vasconcelos H. Unveiling the complex nexus: dermatomyositis and esophageal adenocarcinoma—a case report. Ann Esophagus 2024;7:8.


