
Differential impact of post-neoadjuvant stage on overall survival for surgically treated oesophageal cancer following neoadjuvant chemotherapy or chemoradiation: a retrospective cohort study
Highlight box
Key findings
• Our study reinforced the importance of the post-neoadjuvant “yp” stage as a strong predictor of prognosis in resected oesophageal and gastro-oesophageal junction (GOJ) cancer patients who had undergone neoadjuvant therapy. Patients, who demonstrated a response or down-staging to neoadjuvant treatment, had a statistically significant improvement in overall survival compared to patients who had no change or disease progression.
What is known and what is new?
• Neoadjuvant therapies in the form of chemoradiotherapy or chemotherapy alone prior to surgery have led to significant advances in survival in patients with locally advanced oesophageal and GOJ cancer. Pathological complete response and nodal involvement are independent prognostic factors.
What is the implication, and what should change now?
• Downstaging and deeper pathological response to neoadjuvant therapy was observed to be predictive of improved survival for patients receiving chemotherapy but there was limited evidence of the same relationship for those receiving chemoradiotherapy. This result has the potential to guide judicious use of adjuvant therapies after surgery.
Introduction
Background/rationale
Oesophageal cancer is the eighth most common cancer worldwide affecting 450,000 people each year and is a leading cause of cancer deaths (1). Globally, around half of the oesophageal cancers occur in the middle third of the oesophagus whilst 35% occur in the lower third and gastro-oesophageal junction (GOJ) (2). In Australia, the majority of cases occur in the lower third and GOJ (3). There are two main histological subtypes: adenocarcinoma and squamous cell carcinoma (SCC) (2). SCC makes up most cases worldwide but in western countries there has been a rapid rise in adenocarcinoma (1). In Australia, the 5-year survival is only 22% (4).
Long term prognosis of patients with oesophageal and GOJ cancers is suboptimal and despite multi-modal treatment in resectable oesophageal cancer, post-operative tumour recurrence occurs in approximately half the patients during follow-up after curative surgery (5). Neoadjuvant therapies are now standard of care and aim to improve R0 resection rate, treat early micrometastatic disease, downstage the tumour and improve overall survival (OS) by reducing local and distant recurrences (6).
Many studies have looked at improving survival by adding chemotherapy or chemoradiotherapy prior to surgery. The MAGIC study demonstrated an improved 5-year survival for gastro-oesophageal adenocarcinomas with the addition of peri-operative Epirubicin + Cisplatin + Fluorouracil (EFC) chemotherapy (7). More recently, a German study demonstrated the superiority of FLOT chemotherapy over ECF (8). Both the MAGIC and the German FLOT-4 studies were primarily for patients with gastric cancer but included lower oesophageal and gastro-oesophageal adenocarcinomas with many Australian centres now adopting this approach. Several meta-analyses have demonstrated improved pathological complete response (pCR) rates with the enhanced FLOT based chemotherapy regimen which has translated into better OS outcomes (9).
The outcomes of the CROSS trial (ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study) demonstrated improved survival with a trimodal treatment strategy of neo-adjuvant chemoradiotherapy followed by surgery, compared with surgery alone (median OS 48.6 vs. 24 months) (10). The addition of radiotherapy to neoadjuvant therapy appears to result in a higher pathological response with fewer local recurrences (11) and more R0 resections. The CROSS trial demonstrated a loco-regional recurrence rate of only 14% with neoadjuvant chemoradiotherapy (nCRT) compared with 34% for surgery alone (12). The clinical and pathological lymph node status is an important prognostic parameter and an independent predictor of survival (13). Furthermore, an updated report on the CROSS study demonstrated an ongoing OS benefit for nCRT at 10 years (14).
The pathological response to neoadjuvant treatment is important in predicting treatment outcomes and disease recurrence as reflected in the latest 8th edition of the American Joint Committee on Cancer (AJCC) oesophageal tumor-node-metastasis (TNM) staging guidelines (15,16). In the CROSS trial, the response to nCRT was seen to a greater extent in SCC with a pCR noted in 49% versus 23% in adenocarcinoma (12). Despite the higher rate of pCR with SCC, histological subtype was not a prognostic marker for OS and disease-free survival (11). The Neo-AEGIS study that compared peri-operative chemotherapy to neoadjuvant CRT, failed to show superiority for either option and both remain standard options for patients with resectable oesophageal cancer (17).
Our primary objectives were to gain a greater understanding of the impact value of the post-neoadjuvant “yp” stage as well as the impact of two different neoadjuvant treatment strategies on survival outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-13/rc).
Methods
This retrospective, cohort study design was conducted at a single Australian metropolitan institution (Alfred Health). It involved consecutive patients diagnosed with oesophageal and GOJ cancers (Siewert I, II and III) between the years 2005 to 2019 who received neoadjuvant chemotherapy (nCT) or nCRT prior to surgical resection. Institutional policy was to treat gastro-oesophageal adenocarcinoma with perioperative chemotherapy up until 2015 when a decision was made to adopt nCRT adherent with the CROSS protocol for oesophageal and Siewert I and II GOJ cancers. Some patients still received nCT following the adoption of the CROSS regimen as the standard approach based on discussion within the MDT. Patients were excluded if they did not receive neoadjuvant therapy or had distant disease at diagnosis. Immune checkpoint inhibitors were unavailable at the time these patients were treated.
Data was extracted from the Gastro-oesophageal Surgical Registry and hospital records regarding clinical and pathological stage using the AJCC (v8) manual. The ypTNM stage, i.e., the pathological stage after neoadjuvant therapy, was collected from histological reports. This was categorised from lowest to highest, stages I or II, stage III, and stage IV but adding an additional lowest category of pCR. Downstaging was defined as moving down a stage category between clinical stage and post-neoadjuvant stage, i.e., attaining a partial response (PR) or CR. Progressive disease (PD) was defined as moving up a stage category. Death was ascertained as documented in the medical records.
Differences between patients receiving nCRT and nCT in baseline characteristics and response to neoadjuvant treatment were examined with chi-square tests for categorical variables and rank-sum tests for continuous variables. Associations between clinical and yp stages and OS were assessed with log-rank tests and the survival functions estimated with Kaplan-Meier curves. Analysis time started on the date of surgery with patients censored on the last date of contact, with the latest date being in October 2020. Follow-up was for a maximum of five years after surgery. Further survival analysis was performed by grouping level of response [CR, PR, or no response (NR)/PD] and neoadjuvant treatment type to investigate whether the combination of these influenced survival outcomes. An exploratory examination was also completed for patients with adenocarcinoma, as this was the most frequent histology type and both modes of neoadjuvant treatment were given for this subtype. A P value of <0.05 was considered significant and analysis was done with Stata v16.0MP (College Station, TX, USA). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Alfred Health Human Research Ethics Committee (No. EC00315) and the waiver request of informed consent.
Results
A total of 170 patients were deemed eligible for oesophageal resection with 34 excluded due to receipt of surgery alone. The eligible sample comprised of 136 patients; 65 received nCRT (48%) and 71 nCT (52%) [Table 1, Appendix 1, Figure S1]. The median age was 66 years [interquartile range (IQR): 59 to 73 years] with 107 (79%) male patients. Cancers were most commonly located in the lower oesophagus (n=61, 45%), were adenocarcinomas (n=109, 80%), were clinical stage III (n=95, 70%) and were treated by oesophagectomy (n=110, 81%). nCRT was more frequently prescribed than nCT in 2017–2020 (n=41 vs. n=13) and for squamous cell cancer (n=22 vs. n=3) but no statistically significant differences were noted with respect to age, sex, or clinical stage.
Table 1
| Characteristics | All patients, n=136 | Neoadjuvant chemotherapy, n=71 |
Neoadjuvant chemoradiation, n=65 |
P (chemotherapy vs. chemoradiation) |
|---|---|---|---|---|
| Year of surgery | <0.001 | |||
| 2007–2011 | 22 [16] | 16 [23] | 6 [9.2] | |
| 2012–2016 | 60 [44] | 42 [59] | 18 [28] | |
| 2017–2020 | 54 [40] | 13 [18] | 41 [63] | |
| Age at surgery (years) | ||||
| <55 | 23 [17] | 13 [18] | 10 [15] | >0.9 |
| 55–64 | 38 [28] | 19 [27] | 19 [29] | |
| 65–74 | 52 [38] | 27 [38] | 25 [38] | |
| ≥75 | 23 [17] | 12 [17] | 11 [17] | |
| Median [IQR] | 66 [59–73] | 66 [57–74] | 67 [61–73] | 0.7 |
| Sex | 0.6 | |||
| Female | 29 [21] | 14 [20] | 15 [23] | |
| Male | 107 [79] | 57 [80] | 50 [77] | |
| Site of primary | 0.055* | |||
| Upper oesophagus | 1 [0.7] | 0 | 1 [1.5] | |
| Middle oesophagus | 10 [7.4] | 2 [2.8] | 8 [12] | |
| Lower oesophagus | 61 [45] | 30 [42] | 31 [48] | |
| Siewert 1 | 23 [17] | 12 [17] | 11 [17] | |
| Siewert 2 | 17 [13] | 7 [9.9] | 10 [15] | |
| Siewert 3 | 24 [18] | 20 [28] | 4 [6.2] | |
| Clinical stage | 0.8 | |||
| I/II | 26 [19] | 12 [17] | 14 [22] | |
| III | 95 [70] | 51 [72] | 44 [68] | |
| IV | 15 [11] | 8 [11] | 7 [11] | |
| Neoadjuvant chemotherapy | N/A | |||
| ECF/ECX | 62 [46] | 60 [85] | 2 [3.1] | |
| FLOT | 6 [4.4] | 6 [8.5] | 0 | |
| CROSS regimen | 48 [35] | 0 | 48 [74] | |
| Platinum/5FU | 18 [13] | 4 [5.6] | 14 [22] | |
| Other | 2 [1.5] | 1 [1.4] | 1 [1.5] | |
| Surgery type | 0.002 | |||
| Gastrectomy | 22 [16] | 19 [27] | 3 [4.6] | |
| Oesophagectomy | 110 [81] | 48 [68] | 62 [95] | |
| Both | 2 [1.5] | 2 [2.8] | 0 | |
| Abandoned | 1 [0.7] | 1 [1.4] | 0 | |
| Not recorded | 1 [0.7] | 1 [1.4] | 0 | |
| Histology | <0.001 | |||
| Adenocarcinoma | 109 [80] | 66 [93] | 43 [66] | |
| Squamous | 25 [18] | 3 [4.2] | 22 [34] | |
| Other/not recorded | 2 [1.5] | 2 [2.8] | 0 | |
| Adjuvant therapy | <0.001 | |||
| No | 82 [60] | 24 [34] | 58 [89] | |
| Yes | 54 [40] | 47 [66] | 7 [11] | |
Values are presented in n [%] or median [IQR]. *, oesophageal sites and gastro-oesophageal junction sites combined into two groups for chi-square test. ECF/ECX, epirubicin and cisplatin plus either 5-fluorouracil or capecitabine; FLOT, fluorouracil, leucovorin, oxaliplatin, docetaxel; IQR, interquartile range; 5FU, 5-fluorouracil.
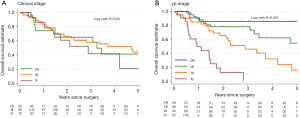
The median length of follow-up in patients still alive was 1.7 years (IQR: 1.0–3.0 years) for nCRT and 3.5 years (IQR: 1.6–5.0 years) for nCT. There were 54 deaths recorded, 16 received nCRT, including 6 with PR and 3 with CR and 38 received nCT (9 PR and 0 CR). Median survival time for nCRT was not reached, for nCT it was 2.8 years (95% CI: 1.5–4.7). In patients with adenocarcinoma, median survival for nCRT was 3.3 years (95% CI: 2.8–NR) and for patients receiving nCT was 2.8 years (95% CI: 1.5–4.7). Clinical stage was not associated with OS in the whole sample (P=0.63) but postneoadjuvant “yp” stage was strongly associated (P<0.001) (Figure 1). Level of response was predictive for OS in the nCT group (P<0.001) but there was no evidence of association in the nCRT group (P=0.51) (Figure 2).
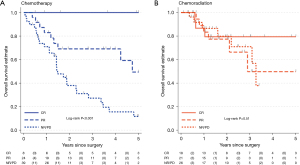
CR was achieved in a statistically significantly higher proportion of patients receiving nCRT than nCT (28% vs. 11%, P=0.015) with numerically more PR + CR, i.e., “downstaged”. Thirty-nine (65%) of patients receiving nCRT were downstaged compared with 32 (45%) in the nCT cohort (Table 2). In the adenocarcinoma only cohort, 24 (56%) of patients receiving nCRT were downstaged compared to 28 (42%) of patients receiving nCT, P=0.17. For patients who were downstaged, no difference was observed in 3-year OS between nCRT 67% (95% CI: 43–82%) versus nCT 76% (95% CI: 56–88%, log-rank P=0.9) (Figure 3). However, for those not downstaged, i.e., with no change in stage category (NR) or an increase in stage (PD) there was a significant difference between neoadjuvant treatment groups favouring nCRT [3-year OS: 71% (95% CI: 41–88%) versus 27% (95% CI: 13–43%), log-rank P=0.024].
Table 2
| Type of neoadjuvant therapy | yp stage, n [row %] | |||
|---|---|---|---|---|
| CR | I/II | III | IV | |
| Chemotherapy | ||||
| I/II | 3 [25]* | 3 [25] | 1 [8.3]** | 5 [42]** |
| III | 4 [7.8]* | 18 [35]* | 18 [35] | 11 [22]** |
| IV | 1 [13]* | 2 [25]* | 4 [50]* | 1 [13] |
| Chemoradiation | ||||
| I/II | 7 [50]* | 6 [43] | 0** | 1 [7.1]** |
| III | 9 [20]* | 16 [36]* | 15 [34] | 4 [9.1]** |
| IV | 2 [29]* | 1 [14]* | 4 [57]* | 0 |
**, indicate progressive disease; * indicate downstaging (partial response and complete response). n, number of patients; (row %), percentage in row that achieved the “yp” stage category. CR, complete response; “yp” stage, post-surgical stage.
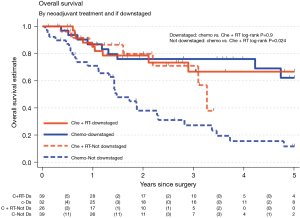
These results were broadly replicated for the 109 patients with an adenocarcinoma histological type. CR was more common for nCRT than nCT groups (19% vs. 9.1%), as was downstaging (56% vs. 42%) (Table S1). Additionally, the 3-year OS for nCRT and nCT patients was similar to the entire sample (downstaged: 67% vs. 80%; not downstaged: 67% vs. 28%) (Figure S2).
Discussion
The 5-year OS in patients with locally advanced oesophageal/GOJ tumours with prior neoadjuvant treatment is between 30% to 47% (18) as these patients experience a high recurrence rate of approximately 50% (19). Neoadjuvant treatment has been shown to improve survival and led to it being the standard of care in the management of locally advanced oesophageal and GOJ cancer, with better outcomes seen in those whose tumours are downstaged (20).
Our study aimed to explore the prognostic value of the pathological stage documented post neoadjuvant therapy i.e., the “yp” stage compared to the clinical stage. The results demonstrated that the initial clinical stage was not a prognostic indicator which is reflective of the current literature (2). However, when one examines the pathological stage post neoadjuvant treatment; there is a markedly significant association of the “yp” stage on OS. This is in line with recent evidence that has shown that disease stage post neoadjuvant therapy is a more accurate prognostic marker than the initial clinical stage (5) and as a result these data have been incorporated into the latest AJCC (v8) staging manual (14).
Another important prognostic marker is the type of response to the neoadjuvant treatment. One of the most important prognostic predictors of survival post neoadjuvant therapy followed by surgical resection is the involvement of lymph nodes (14). Neoadjuvant therapy has been shown to downstage both the depth of tumour invasion and the extent of nodal involvement (20). Patients whose tumours demonstrated a response or downstaging with the neoadjuvant treatment had a statistically significant improvement in OS compared to patients who had no change or disease progression.
Sjoquist et al. conducted a meta-analysis which included 24 clinical trials and 4,188 patients which showed that nCRT showed a survival benefit over nCT where they noted an 8.7% absolute survival benefit at 2 years compared to 5.1% survival benefit with nCT (21). In particular, pathological nodal response rather than primary tumour response to neoadjuvant therapy was one of the key drivers of improved survival (22). In our cohort, numerically more patients achieved a nodal response with nCRT than with nCT (56% compared to 43%) however; there wasn’t a statistically significant difference between the types of neoadjuvant therapy used to achieve the downstaging (Figure 3). Survival seemed to be more impacted whether downstaging was achieved or not.
Similarly, preliminary results from the Neo-AEGIS trial (Neoadjuvant trial in adenocarcinoma of the Oesophagus and Oesophageo-gastric Junction International study) which is a phase III RCT of CROSS versus perioperative chemotherapy (modified MAGIC or FLOT protocol) failed to demonstrate a preferred neoadjuvant approach (23). The Neo-AEGIS trial showed the OS with peri-operative chemotherapy was non-inferior to nCRT (CROSS). It further highlighted that although more patients achieved a pCR with nCRT than perio-operative chemotherapy, OS was similar in both groups (23).
However, our retrospective analysis demonstrated that achieving a pCR with nCT is not equivalent to achieving a pCR with nCRT where other factors may influence the OS seen with nCRT. The pCR rate among the nCT group may be important for survival as it may indicate a better response to micro-metastatic disease, considering the systemic nature of the disease despite being localised on imaging. Cools-Lartigue et al. who compared OS and RFS in oesophageal adenocarcinoma with a pCR following nCT or nCRT in a multi-centre cohort study showed that the rates of pCR were greater following nCRT but this did not translate to improvement in OS or RFS (24). Thus, the biological implication of a pCR differs across neoadjuvant therapy regimens and may not be helpful as a surrogate marker for survival when comparing treatment regimens (24). This observation was also noted in this retrospective cohort study albeit the small size being a limitation.
In this retrospective review of this cohort of patients, there are some inherent biases as to why the patients who received nCRT may have had a survival advantage over the patients who received nCT. Firstly, the cohort of patients who received nCRT was inherently a group with likely better prognostic outcomes. For example, they may have had larger tumours (T) size [which correlate with worse outcomes (25)] as they may not have been suitable for radiotherapy with patients with less bulky tumours receiving nCRT. Furthermore, the latter time period in which nCRT was utilized, included more accurate imaging modalities such as positron emission tomography scans (PET scans) which facilitated more accurate staging as well as minimal invasive surgical techniques. In the subset of patients who received nCT, the majority of the patients received older regimens of chemotherapy of ECF/ECX compared to modern day perioperative FLOT regimens which have been shown to have greater efficacy and treatment response (8). However, despite this, the cohort who would have been impacted most by this, the nCT group demonstrated greater predictive potential on survival based on the post-neoadjuvant stage achieved.
Interestingly, although there were a greater proportion of patients who achieved a pCR with nCRT compared to nCT as shown in Figure 2, 100% of the patients who achieved a pCR with nCT survived. There is a note-worthy trend that those who achieved a pCR with nCT were more likely to survive than those who had a pathological CR following nCRT. This suggests that a pathological CR to chemotherapy alone is very likely to be associated with greater elimination of micro-metastatic disease. Patients who received nCT may have received post-operative chemotherapy which may have had an impact on OS. Although, none of patients receiving nCRT were treated with adjuvant immunotherapy, the Checkmate 577 trial showed that patients who did not achieve a pCR after nCRT had an improved disease-free survival (26) which may have had an impact on OS. These relative differences in post-operative regimens and their impact on OS are yet to be established.
With regards to the patterns of relapse and the fore-mentioned biases associated with the subset of patients who received nCRT, it was not surprising that this subset was associated with less loco-regional and distal recurrences compared to nCT. The pattern of recurrence noted with the use of nCRT wherein there were more distal recurrences (Table S2) suggests that despite improved loco-regional control this approach may not be as good as nCT in preventing systemic relapse (27).
Conclusions
This study adds to our current understanding about the relationship between post neoadjuvant stage and OS in patients with surgically managed oesophageal and GOJ cancer. Downstaging and deeper pathological response to neoadjuvant therapy was observed to be predictive of improved survival for patients receiving chemotherapy but there was limited evidence of the same relationship for those receiving chemoradiotherapy. On the other hand, for non-responding patients, nCRT seems to be associated with better outcomes, perhaps through the impact of better local control. If confirmed in larger studies, these findings have the potential to guide judicious use of adjuvant therapies after surgery.
Acknowledgments
Funding: This work has been supported by the Gastro-oesophageal Surgical Registry at The Alfred.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-13/rc
Data Sharing Statement: Available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-13/dss
Peer Review File: Available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-13/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.org/article/view/10.21037/aoe-23-13/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:582-97. [Crossref] [PubMed]
- Wong MCS, Hamilton W, Whiteman DC, et al. Global Incidence and mortality of oesophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries. Sci Rep 2018;8:4522. [Crossref] [PubMed]
- Cancer Australia. Oesophageal Cancer Statistics in Australia [Internet]. NSW (AU): Cancer Australia; 2012 [updated 2022; cited 2023 Jun 5]. Available online: https://www.canceraustralia.gov.au/affected-cancer/cancer-types/oesophageal-cancer/statistics
- Australasian Gastrointestinal Trials Group (AGITG). Oesophageal cancer. [Internet]. NSW (AU):AGITG; 2017 [updated 2022; cited 2023 Jun 5]. Available online: https://gicancer.org.au/cancer/oesophageal-cancer/#cancer-prognosis
- Butter R, Lagarde SM, van Oijen MGH, et al. Treatment strategies in recurrent esophageal or junctional cancer. Dis Esophagus 2017;30:1-9. [Crossref] [PubMed]
- Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 2014;32:2983-90. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Chidambaram S, Sounderajah V, Maynard N, et al. Evaluation of tumor regression by neoadjuvant chemotherapy regimens for esophageal adenocarcinoma: a systematic review and meta-analysis. Dis Esophagus 2023;36:doac058. [Crossref] [PubMed]
- Wong I, Law S. The CROSS road in neoadjuvant therapy for esophageal cancer: long-term results of CROSS trial. Translational Cancer Research 2016;S415-9. [Crossref]
- Stiles BM, Kamel MK, Harrison SW, et al. Neoadjuvant Therapy for Locally Advanced Esophageal Cancer Should Be Targeted to Tumor Histology. Ann Thorac Surg 2019;107:187-93. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Klevebro F, Nilsson K, Lindblad M, et al. Association between time interval from neoadjuvant chemoradiotherapy to surgery and complete histological tumor response in esophageal and gastroesophageal junction cancer: a national cohort study. Dis Esophagus 2020;33:doz078. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- Soror T, Kho G, Zhao KL, et al. Impact of pathological complete response following neoadjuvant chemoradiotherapy in esophageal cancer. J Thorac Dis 2018;10:4069-76. [Crossref] [PubMed]
- Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014;6:112-20. [Crossref] [PubMed]
- Reynolds J, Preston S, O'Neil B, et al. NEOadjuvant trial in Adenocarcinoma of the oEsophagus and oesophagoGastric junction Study (Neo-AEGIS). BMC Cancer 2017;17:401. [Crossref] [PubMed]
- Donohoe CL, Reynolds JV. Neoadjuvant treatment of locally advanced esophageal and junctional cancer: the evidence-base, current key questions and clinical trials. J Thorac Dis 2017;9:S697-704. [Crossref] [PubMed]
- Kamarajah SK, Navidi M, Wahed S, et al. Significance of Neoadjuvant Downstaging in Carcinoma of Esophagus and Gastroesophageal Junction. Ann Surg Oncol 2020;27:3182-92. [Crossref] [PubMed]
- Zanoni A. Nodal downstaging in esophageal and esophagogastric junction cancer: more important than ever. J Thorac Dis 2017;9:1839-42. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Groth SS, Burt BM, Farjah F, et al. Prognostic value of neoadjuvant treatment response in locally advanced esophageal adenocarcinoma. J Thorac Cardiovasc Surg 2019;157:1682-1693.e1. [Crossref] [PubMed]
- Reynolds JV, Preston SR, O'Neill B, et al. Neo-AEGIS (Neoadjuvant trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): Preliminary results of phase III RCT of CROSS versus perioperative chemotherapy (Modified MAGIC or FLOT protocol). (NCT01726452). J Clin Oncol 2021;39:4004. [Crossref]
- Cools-Lartigue J, Markar S, Mueller C, et al. An International Cohort Study of Prognosis Associated With Pathologically Complete Response Following Neoadjuvant Chemotherapy Versus Chemoradiotherapy of Surgical Treated Esophageal Adenocarcinoma. Ann Surg 2022;276:799-805. [Crossref] [PubMed]
- Mirinezhad SK, Jangjoo AG, Seyednejad F, et al. Impact of tumor length on survival for patients with resected esophageal cancer. Asian Pac J Cancer Prev 2014;15:691-4. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- van Hagen P, Wijnhoven BP, Nafteux P, et al. Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg 2013;100:267-73. [Crossref] [PubMed]
Cite this article as: Gunadasa I, Papa N, Khu YL, Brown W, Burton P, Haydon A, Zalcberg J. Differential impact of post-neoadjuvant stage on overall survival for surgically treated oesophageal cancer following neoadjuvant chemotherapy or chemoradiation: a retrospective cohort study. Ann Esophagus 2024;7:2.


