Intrathoracic anastomotic techniques in robotic assisted minimally invasive esophagectomy: a narrative review
Introduction
Robotic assisted minimally invasive esophagectomy (RAMIE) is being increasingly utilized in the United States and worldwide, with several variations of the technique described by surgeons from many different institutions (1-3). Similar to non-robotic minimally invasive esophagectomy (MIE) or open esophagectomy, technical aspects of anastomosis creation during RAMIE can vary significantly among surgeons (4). Regardless of the technique employed, the principal tenets of creating a tension-free, well-vascularized, and widely patent anastomosis with adequate tumor resection margins remain crucial. Adherence to these key elements will optimally attenuate the risk of leak or stricture, which remain significant sources of morbidity after esophagectomy (5).
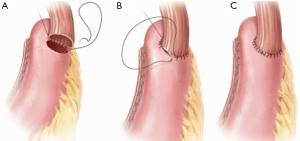
A few factors are considered when describing anastomoses created during esophagectomy. The first is location, whether intrathoracic or cervical. The second is the type of conduit used to re-establish gastrointestinal continuity. This is most commonly the stomach, but jejunum or colon can also be used. The third is the specific technique used to create the anastomosis, which broadly falls into three major categories: circular stapled, handsewn, or linear stapled (Figure 1). The specific anatomic positioning of the esophagus and the conduit, whether end-to-end, end-to-side, or side-to-side, can also be considered a component of the mechanical technique employed to create the anastomosis. This review will focus on intrathoracic gastroesophageal anastomoses created during RAMIE operations using the stomach as a conduit created using either circular-stapled, handsewn, or linear-stapled techniques (Figure 1). The objective of this narrative review is to familiarize surgeons interested in robotic esophageal surgery with these anastomotic techniques. While inclusion of every subtle variation and nuance of these procedures is beyond the scope of this review, the major anastomotic techniques employed during RAMIE are discussed, as well as data regarding outcomes and complications specific to the individual methods. I present this article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-2/rc).
Methods
I performed PubMed searches using the following keywords: robotic esophagectomy, robotic surgery, esophageal cancer, esophageal anastomosis, and robotic assisted minimally invasive esophagectomy. All articles in English published from January 1, 2000 through December 31, 2021 were surveyed to elaborate on this manuscript. Series from experienced centers, meta-analyses, and multicenter studies were prioritized and read in full. I also reviewed my personal practices as a highly experienced robotic thoracic surgeon and a well-established instructor of robotic thoracic surgery (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | January 15, 2022 |
| Databases and other sources searched | PubMed/Medline |
| Search terms used | robotic esophagectomy; robotic surgery; esophageal cancer; esophageal anastomosis; robotic assisted minimally invasive esophagectomy |
| Timeframe | 2000 to 2021 |
| Inclusion and exclusion criteria | Inclusion: English language; Exclusion: Animal studies |
| Selection process | Performed by author I.S.S., MD |
| Any additional considerations, if applicable | Series from experienced centers, meta-analysis and multicenter studies were prioritized; author also retrieved additional publications that he was familiar with as an expert minimally invasive thoracic surgeon |
Narrative
Circular-stapled anastomoses
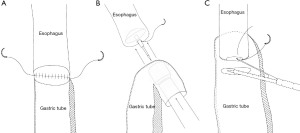
The basic sequence of creating a circular-stapled anastomosis is relatively straightforward and reproducible (Figure 2). The end-to-end anastomotic (EEA) stapler is manufactured by Medtronic, Inc in several sizes ranging most commonly from 25 to 33 mm. The circular-stapled anastomosis requires placement of an anvil within the esophagus, and placement of the circular stapler itself within the conduit. A 4-cm access incision and a skilled bedside assistant are necessary to position and fire the stapler. Once joined, the stapler creates the anastomosis by first approximating the anvil to the housing of the stapler and mechanically affixing the adjoining tissues with full thickness deployment of the staples. The core of central tissue is cut and excised to create the common passage between the gastric conduit and esophagus and is represented by the gastric and esophageal anastomotic rings of tissue remaining on the stem of the anvil. If incomplete rings are observed, the mechanical integrity of the anastomoses must be assumed to be compromised. While the technical execution of this procedure actually creates an end-to-side anastomosis, the anastomosis is functionally an end-to-end anastomosis in most descriptions of the technique with very little or no proximal conduit remaining after completion.
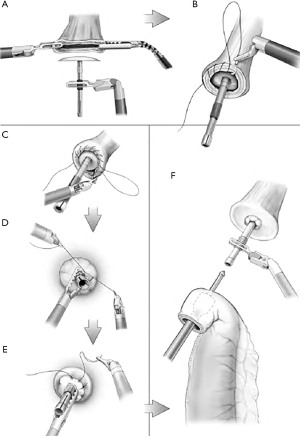
When performing a circular-stapled anastomosis, placement of the anvil is often accomplished with a robotically handsewn purse-string suture. Alternatives include placement of the purse-string suture with an automated device or affixing the OrVil 25 mm EEA stapler (Medtronic, Inc.) to a nasogastric tube that is brought through the oropharynx. This method may obviate the need for a purse-string suture if the esophagus is transected with a stapler. Possible drawbacks of this method include the close apposition of these staple lines, as well as being limited to a smaller 25-mm anvil, with the potential for higher rates of stenosis as compared with larger sizes available for the standard stapler.
Table 2 summarizes several reports on circular-stapled anastomoses that included 10 or more patients. There was relatively varied distribution in the size of anvil used, ranging from 25 to 29 mm. Anastomotic leak rates ranged widely from 0% to 20%, with most reporting anastomotic leak rates of 5–10%. Rates of stenosis/stricture requiring dilation are less frequently reported. Only two of the reports note stenoses requiring dilation. In this author’s own published experience in an initial cohort of 89 patients, 3% required dilation, while Wang and colleagues reported a 7% incidence of dilation and an overall stricture rate of 19% (8,16). One study reported a 17% rate of postoperative dysphagia although details of this were not clearly stated (13).
Table 2
| Authors | Year | Number | Size | Leak rate | Stricture | 30-day mortality | 90-day mortality |
|---|---|---|---|---|---|---|---|
| Sarkaria (8) | 2016 | 89 | 29 mm | 6% | 3% | 0% | 1% |
| Wee (9) | 2016 | 20 | 25 mm, 28 mm | 0% | NR | 0% | 0% |
| Okusanya (10) | 2017 | 23 | 28 mm | 4% | NR | 0% | 0% |
| Meredith (11) | 2018 | 147 | 25 mm OrVil | 3% | NR | 0.7% | 1.4% |
| Zhang (12) | 2018 | 35 | 25 mm | 11% | NR | 0% | NR |
| Zhang (13) | 2019 | 42 | 25mm | 4.8% | 16.7% (post-op dysphagia) | 0% | NR |
| Pötscher (14) | 2019 | 10 | 25 mm OrVil | 20% | NR | NR | NR |
| Tagkalos (15) | 2019 | 50 | 25 mm, 28 mm | 12% | NR | 0% | 4% |
| Wang (16) | 2019 | 31 | 25 mm | 6% | 19% (7% requiring dilation) | 0% | NR |
| de Groot (17) | 2020 | 60 | 29 mm | 17% (5% grade 3) | NR | NR | |
| Pointer (18) | 2020 | 350 | 25 mm OrVil | 15.7% (2% requiring operation) | NR | 2.6% | NR |
| van der Sluis (19) | 2021 | 100 | 25 mm, 28 mm | 8% | 1% | 3% |
NR, not reported; post-op, postoperative.
Linear-stapled anastomosis
The basic sequence of creating the linear-stapled anastomosis is first affixing the conduit and esophagus side to side with significant overlap. The degree of overlap of the esophagus on the gastric conduit will largely determine the extent of the anastomotic orifice created. Each time of the linear stapler is introduced into either the gastric or esophageal lumen through a small fenestration, and single fire is applied to create a common channel between the two. The smaller common defect can then be closed either via stapling or by handsewn techniques at the discretion of the surgeon (Figures 3,4). These may include single- or two-layer closures, multiple single or running sutures, degradable or permanent suture, and use of self-locking barbed sutures. Given the combined stapled and sewn aspect of many of these techniques, the term “hybrid” has been commonly adopted for linear-stapled anastomotic closures. An advantage of this technique is that it is likely the most simple and expeditious to execute. One potential critique of the linear-stapled anastomosis is the potential to sacrifice additional margin to create the necessary side-to-side apposition of the 2 lumens. However, no studies have reported specifically on differences in margin lengths or recurrence rates between anastomotic techniques.
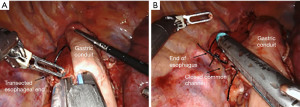
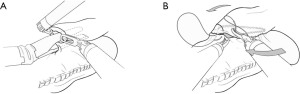
Table 3 summarizes several studies that included 10 or more patients with linear-stapled (hybrid) anastomoses. Anastomotic leak rates after robotic linear stapling were relatively uniform, ranging from 4% to 8%, with rates of stenosis ranging from 6% to 16%. Rates of stenosis requiring dilation were not specifically addressed.
Table 3
| Authors | Year | Number | Technique | Size | Leak rate | Stricture rate | 30-day mortality | 90-day mortality |
|---|---|---|---|---|---|---|---|---|
| Hodari (21) | 2015 | 54 | Stapled, Sewn | 45 mm | 6% | NR | 2% | NR |
| Zhang (13) | 2019 | 35 | – | ~30 mm | 8.6% | 5.7% (post-op dysphagia) | 0% | NR |
| Chouliaras (20) | 2021 | 51 | Stapled, Sewn | 60 mm | 3.9% | 7.6% | 0% | NR |
| Kandagatla (22) | 2021 | 112 | Stapled, Sewn | 45 mm | 8% | 16.1% | 0.9% | 3.6% |
NR, not reported; post-op, postoperative.
Handsewn anastomosis
“Handsewn” anastomoses are those created with suture only and no mechanical device. The robotic platform has greatly aided in the ability to sew intracorporeally as compared with standard laparoscopic or thoracoscopic techniques (Figure 5). Techniques vary in ways that are similar to the different handsewn techniques used for anastomosis during open surgery. Points of variation include the orientation (side to side, end to side, end to end), one- or two-layer closure, and type and number of sutures used. The surgeon must take care to avoid purse-stringing the anastomosis closed if a single running suture is used to complete an entire circular anastomosis. It is advisable to use multiple running sutures fixed at 2–3 points to avoid iatrogenic stenoses. Handsewn anastomoses are generally more time consuming to construct than stapled anastomoses but are entirely within the hands of the console surgeon.
Table 4 summarizes studies reporting outcomes of RAMIE with a handsewn anastomotic technique. The reported leak rate varied significantly from 0% to approximately 30%. Rates of stenosis after handsewn anastomosis with RAMIE have not been reported, however, and are largely unknown.
Table 4
Discussion
In 2020, Plat and colleagues published a systematic review that included 16 studies of robotic esophagectomy. They observed technical variation with increased use of robotic surgery to perform esophagectomy and concluded that all thoracoscopic anastomotic techniques can be adopted using the robotic platform (6). Circular-stapled, linear-stapled (hybrid), and handsewn techniques have all been successfully employed to create the intrathoracic esophagogastric anastomosis necessary for RAMIE. Plat and colleagues observed greater initial adoption of circular-stapled anastomoses as the preferred technique, however, despite the need for an experienced assistant at bedside, and even though linear-stapled and handsewn techniques allow the surgeon complete control over the anastomosis from the console. The authors suggested that the circular-stapled anastomosis is the easiest to reproduce early in the learning curve, and somewhat simplified in comparison with standard MIE given the ability to sew the initial purse string with the robotic platform during RAMIE or the option to use the OrVil technique with introduction of the anvil transorally. Furthermore, the circular-stapled anastomosis may be the technique most familiar to surgeons who routinely perform non-robotic minimally invasive surgery or open esophagectomy.
Similarly, in an international cooperative group consensus statement by Li and colleagues, a 78% consensus was reported recommending mechanical stapling during RAMIE, although the authors acknowledged that the level of evidence supporting the recommendation is weak (1). The cooperative group further stated that after the surgeon accrues enough experience, the robotic platform likely augments performance of a handsewn anastomosis, a difficult task for many when using standard thoracoscopic techniques (1). An analysis of a multicenter, international RAMIE registry identified circular-stapled to be the most common method (52%) for anastomosis, followed by handsewn (30%) and linear-stapled (18%) (2).
I prefer the circular-stapled technique using a 28-mm EEA stapler to minimize the likelihood of stricture formation. I have found that this technique very reproducibly results in an end-to-end anastomosis that maximizes the margins of the resection. A handsewn, end-to-end anastomosis is my 2nd technique of choice. It similarly allows me to maximize the resection margins, and I appreciate that the anastomosis can be created entirely under the direct control of the console operator. I do not use the linear-stapled technique very often. I try to preserve as much stomach and esophagus as possible during RAMIE for resection of esophageal cancer to optimize the margins of the resection. This is more difficult with the linear stapler and might compromise the oncological efficacy of the procedure.
Early studies in patients undergoing open esophagectomy suggested no difference in outcomes with circular-stapled versus handsewn techniques, although potentially higher stricture rates may occur using the circular-stapled technique (26). Zhang and colleagues compared the results of their initial patient cohorts undergoing robotic esophagectomy with linear-stapled versus circular-stapled anastomoses and found the stricture rate was lower in patients with a linear-stapled anastomosis, but the difference was not statistically significant (13).
Although not focused on robotic approaches specifically, a meta-analysis of randomized controlled trials comparing circular-stapled and handsewn anastomoses identified a reduction in operative time when the circular-stapled technique was employed, but no difference in anastomotic leakage or mortality. They did also identify an increased risk of postoperative strictures after circular stapling, however (27). A similar meta-analysis reported like results, increased stricture rates with a circular-stapled anastomosis as compared with handsewn anastomoses and lowest rates of stricture in patients with a linear-stapled anastomosis, but also suggested increased risk of leak in patients with a handsewn anastomosis (28). A meta-analysis looking specifically at handsewn versus linear-stapled side-to-side techniques (both intrathoracic and cervical) corroborated a decreased leak rate overall using a linear-stapled anastomosis, as well as a decreased stricture rate. Importantly, for the purposes of this report, however, there were no differences in leak rates in patients with intrathoracic anastomoses (29). Interestingly, a large multicenter European registry trial comparing five anastomotic techniques used during transthoracic MIE identified higher rates of anastomotic leak with the “double-staple” OrVil technique (23%) versus the linear-stapled (16%) and the circular-stapled “purse-string” techniques (14%) (30). In an analysis of patients who underwent a robot-assisted Ivor Lewis esophagectomy, the highest leak rates were observed with handsewn anastomoses (33%) as compared with circular-stapled (17%) or linear-stapled (15%) (2).
In a detailed analysis of their adoption of a robotic, handsewn anastomotic technique after using a circular-stapled technique, de Groot and colleagues describe several technical refinements, made over time, including switching from an end-to-end anastomosis to an end-to-side anastomosis, switching brands of self-locking suture, and placing tension-release sutures (17). The authors identified a decrease in leak rates (using a moving average over 10 consecutive patients at a time) from 40% to 10% over the 68-patient experience, but acknowledged difficulty in determining what portion of the learning curve had been reached.
All the techniques described in this narrative review are acceptable for completing the intrathoracic anastomosis during RAMIE and, to date, none has been proven superior or been shown to result in fewer complications overall (Table 5). I advise surgeons adopting RAMIE to use the technique they are most comfortable with especially while they are learning. The surgeon should determine which approach he or she wants to use, observe an experienced robotic surgeon using that technique, and practice in a simulation or laboratory setting. Importantly, when they start performing RAMIE, I advise a low threshold early on for conversion to a technique they are experienced with and comfortable completing. The surgeon should convert to video-assisted thoracoscopic surgery (VATS) or even open surgery and should not feel compelled early in the learning curve to complete the anastomosis robotically if they have concerns. Studies of RAMIE adoption have suggested that proctoring and a modular step-up approach, with increasingly more of the esophagectomy procedure being completed using the surgical robot as the surgeon gains experience, may shorten the learning curve and ensure patient safety while the surgeon becomes accustomed to the new platform (31-33).
Table 5
| Anastomotic technique | Pros | Cons |
|---|---|---|
| Circular Stapled | Highly reproducible | Requires experienced user at bedside |
| Different size options (25–31 mm) | Increased stricture rate vs hybrid? | |
| Quick | ||
| OrVil Circular Stapled | Easier to deploy anvil | Limited size options (25 mm only) |
| Higher stricture rate vs standard EEA? | ||
| Requires experienced user at bedside | ||
| Crossed staple lines | ||
| Handsewn | Fully robotic, console-surgeon controlled | Highly operator dependent (variability) |
| Higher stricture rate? (end-to-end) | ||
| Time consuming? | ||
| Linear Stapled | Fully robotic, console-surgeon controlled | Decreased margins? |
| Simplest to execute, relatively quick | ||
| Decreased stricture rates? |
EEA, end-to-end anastomosis.
Conclusions
In conclusion, data regarding the “best” anastomotic technique for robotic assisted esophagectomy is very much in evolution. While linear-stapled anastomotic techniques appear to have the advantage of decreased stricture rates in most studies, similar overall rates of leak and anastomotic failure between the techniques have been suggested but not clearly delineated (Table 5). The circular-stapled method may be the most comfortable to adopt at the outset, while linear-stapled and handsewn techniques may grant the surgeon the ability to perform a complete robotic assisted anastomosis under direct control. Regardless of the technique chosen by any given surgeon, the most important factors likely remain surgical volume, surgeon experience, and technical comfort and facility with the chosen method.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Abbas E. Abbas and Roman V. Petrov) for the series “New Technologies in Esophageal Surgery and Endoscopy” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-2/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-2/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-2/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li B, Yang Y, Toker A, et al. International consensus statement on robot-assisted minimally invasive esophagectomy (RAMIE). J Thorac Dis 2020;12:7387-401. [Crossref] [PubMed]
- Kingma BF, Grimminger PP, van der Sluis PC, et al. Worldwide Techniques and Outcomes in Robot-assisted Minimally Invasive Esophagectomy (RAMIE): Results From the Multicenter International Registry. Ann Surg 2022;276:e386-92. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP. Robotic-assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin 2014;24:211-22. vii. [Crossref] [PubMed]
- Herron R, Abbas G. Techniques of Esophageal Anastomoses for Esophagectomy. Surg Clin North Am 2021;101:511-24. [Crossref] [PubMed]
- Abbas AE, Sarkaria IS. Specific complications and limitations of robotic esophagectomy. Dis Esophagus 2020;33:doaa109. [Crossref] [PubMed]
- Plat VD, Stam WT, Schoonmade LJ, et al. Implementation of robot-assisted Ivor Lewis procedure: Robotic hand-sewn, linear or circular technique? Am J Surg 2020;220:62-8. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Finley DJ, et al. Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development. Eur J Cardiothorac Surg 2013;43:e107-15. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Grosser R, et al. Attaining Proficiency in Robotic-Assisted Minimally Invasive Esophagectomy While Maximizing Safety During Procedure Development. Innovations (Phila) 2016;11:268-73. [Crossref] [PubMed]
- Wee JO, Bravo-Iñiguez CE, Jaklitsch MT. Early Experience of Robot-Assisted Esophagectomy With Circular End-to-End Stapled Anastomosis. Ann Thorac Surg 2016;102:253-9. [Crossref] [PubMed]
- Okusanya OT, Sarkaria IS, Hess NR, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg 2017;6:179-85. [Crossref] [PubMed]
- Meredith K, Huston J, Andacoglu O, et al. Safety and feasibility of robotic-assisted Ivor-Lewis esophagectomy. Dis Esophagus 2018;31: [Crossref] [PubMed]
- Zhang Y, Xiang J, Han Y, et al. Initial experience of robot-assisted Ivor-Lewis esophagectomy: 61 consecutive cases from a single Chinese institution. Dis Esophagus 2018;31: [Crossref] [PubMed]
- Zhang H, Wang Z, Zheng Y, et al. Robotic Side-to-Side and End-to-Side Stapled Esophagogastric Anastomosis of Ivor Lewis Esophagectomy for Cancer. World J Surg 2019;43:3074-82. [Crossref] [PubMed]
- Pötscher A, Bittermann C, Längle F. Robot-assisted esophageal surgery using the da Vinci® Xi system: operative technique and initial experiences. J Robot Surg 2019;13:469-74. [Crossref] [PubMed]
- Tagkalos E, Goense L, Hoppe-Lotichius M, et al. Robot-assisted minimally invasive esophagectomy (RAMIE) compared to conventional minimally invasive esophagectomy (MIE) for esophageal cancer: a propensity-matched analysis. Dis Esophagus 2020;33:doz060. [Crossref] [PubMed]
- Wang WP, Chen LQ, Zhang HL, et al. Modified Intrathoracic Esophagogastrostomy with Minimally Invasive Robot-Assisted Ivor-Lewis Esophagectomy for Cancer. Dig Surg 2019;36:218-25. [Crossref] [PubMed]
- de Groot EM, Möller T, Kingma BF, et al. Technical details of the hand-sewn and circular-stapled anastomosis in robot-assisted minimally invasive esophagectomy. Dis Esophagus 2020;33:doaa055. [Crossref] [PubMed]
- Pointer DT Jr, Saeed S, Naffouje SA, et al. Outcomes of 350 Robotic-assisted Esophagectomies at a High-volume Cancer Center: A Contemporary Propensity-score Matched Analysis. Ann Surg 2022;276:111-8. [Crossref] [PubMed]
- van der Sluis PC, Tagkalos E, Hadzijusufovic E, et al. Robot-Assisted Minimally Invasive Esophagectomy with Intrathoracic Anastomosis (Ivor Lewis): Promising Results in 100 Consecutive Patients (the European Experience). J Gastrointest Surg 2021;25:1-8. [Crossref] [PubMed]
- Chouliaras K, Hochwald S, Kukar M. Robotic-assisted Ivor Lewis esophagectomy, a review of the technique. Updates Surg 2021;73:831-8. [Crossref] [PubMed]
- Hodari A, Park KU, Lace B, et al. Robot-Assisted Minimally Invasive Ivor Lewis Esophagectomy With Real-Time Perfusion Assessment. Ann Thorac Surg 2015;100:947-52. [Crossref] [PubMed]
- Kandagatla P, Ghandour AH, Amro A, et al. Long-term outcomes after robotic-assisted Ivor Lewis esophagectomy. J Robot Surg 2022;16:119-25. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [Crossref] [PubMed]
- Trugeda S, Fernández-Díaz MJ, Rodríguez-Sanjuán JC, et al. Initial results of robot-assisted Ivor-Lewis oesophagectomy with intrathoracic hand-sewn anastomosis in the prone position. Int J Med Robot 2014;10:397-403. [Crossref] [PubMed]
- Egberts JH, Stein H, Aselmann H, et al. Fully robotic da Vinci Ivor-Lewis esophagectomy in four-arm technique-problems and solutions. Dis Esophagus 2017;30:1-9. [Crossref] [PubMed]
- Kim RH, Takabe K. Methods of esophagogastric anastomoses following esophagectomy for cancer: A systematic review. J Surg Oncol 2010;101:527-33. [Crossref] [PubMed]
- Honda M, Kuriyama A, Noma H, et al. Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Ann Surg 2013;257:238-48. [Crossref] [PubMed]
- Liu QX, Min JX, Deng XF, et al. Is hand sewing comparable with stapling for anastomotic leakage after esophagectomy? A meta-analysis. World J Gastroenterol 2014;20:17218-26. [Crossref] [PubMed]
- Deng XF, Liu QX, Zhou D, et al. Hand-sewn vs linearly stapled esophagogastric anastomosis for esophageal cancer: a meta-analysis. World J Gastroenterol 2015;21:4757-64. [Crossref] [PubMed]
- Schröder W, Raptis DA, Schmidt HM, et al. Anastomotic Techniques and Associated Morbidity in Total Minimally Invasive Transthoracic Esophagectomy: Results From the EsoBenchmark Database. Ann Surg 2019;270:820-6. [Crossref] [PubMed]
- Grimminger PP, Tagkalos E, Hadzijusufovic E, et al. Change from Hybrid to Fully Minimally Invasive and Robotic Esophagectomy is Possible without Compromises. Thorac Cardiovasc Surg 2019;67:589-96. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Learning Curve for Robot-Assisted Minimally Invasive Thoracoscopic Esophagectomy: Results From 312 Cases. Ann Thorac Surg 2018;106:264-71. [Crossref] [PubMed]
- Fuchs HF, Müller DT, Leers JM, et al. Modular step-up approach to robot-assisted transthoracic esophagectomy-experience of a German high volume center. Transl Gastroenterol Hepatol 2019;4:62. [Crossref] [PubMed]
Cite this article as: Sarkaria IS. Intrathoracic anastomotic techniques in robotic assisted minimally invasive esophagectomy: a narrative review. Ann Esophagus 2023;6:44.