
Impact of intra-pyloric botulinum toxin injection on delayed gastric emptying following esophagectomy: systematic review and meta-analysis
Highlight box
Key findings
• This meta-analysis suggests botulinum toxin-A (BT-A) use results in no significant impact on rates of delayed gastric emptying, requirement for endoscopic balloon dilatation, anastomotic leaks, respiratory complications or mortality when injected as a pyloric intervention during oesophageal surgery.
What is known and what is new?
• Pyloric interventions are used routinely in many esophagectomy centres;
• Previous meta-analysis of all types of pyloric interventions vs. no intervention have shown no significant benefits to performing them;
• We examined the use of BT-A only as this is a novel technique which may be easier to use in the minimally invasive era.
What is the implication, and what should change now?
• Further well-designed and large randomised controlled trials are required to assess the benefits of pyloric interventions during esophagectomy.
Introduction
Delayed gastric emptying occurs in 15–39% (1) of patients after esophagectomy. It is a result of the transection of the vagal nerve leading to denervation of the pyloric muscle. The condition is defined as the presence of any of two of the following symptoms: early satiety, vomiting, nausea, regurgitation of limited oral intake or delayed contrast passage on contrast imaging post-operatively (2,3). The use of pyloric drainage procedures to mitigate or prevent delayed gastric emptying as part of oesophageal surgery remains controversial. Practice is still variable with some surgeons routinely performing a pyloric intervention and others not (4). Proponents of pyloric intervention argue that it reduces the rate of delayed gastric emptying and associated risks, including aspiration pneumonia and anastomotic leak. Others contend that the risks are low and delayed gastric emptying can be managed if it occurs without the risk incurred by additional operative intervention. It has also been proposed that pyloric intervention may leave patients susceptible to bile reflux (5).
Botulinum toxin-A (BT-A), more commonly called botox, is a bacterial neurotoxin and a potent paralytic agent that inhibits the calcium-dependent release of acetylcholine from cholinergic nerve terminals (6). BT-A has been successfully used in the treatment of achalasia (7), diffuse oesophageal spasm (8) and on the pylorus for the treatment of diabetic gastroparesis (6). For pyloric intervention, it is typically injected endoscopically with 100–200 units (total) injected into 4 quadrants of the pyloric sphincter (5,9-14). The safe nature of BT-A (15) and the relative ease of use compared to other pyloric interventions, especially during laparoscopic surgery make it an attractive option in modern oesophageal surgery. We performed a systematic review and meta-analysis comparing BT-A use to no pyloric intervention in relation to risk of delayed gastric emptying and associated clinical outcomes. We present the following article in accordance with the MOOSE reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-29/rc).
Methods
A systematic literature search of MEDLINE, EMBASE and the Cochrane Library was performed for studies published between January 1990 and July 2021. The search terms were “esophagus” or” esophageal” and “cancer” or “resection” or “esophagectomy” and “botox” or” botulinum”. Additionally, reference lists of all relevant studies were reviewed. We considered cohort studies and randomised clinical trials that compared esophagectomy with BT-A injected into the pylorus either endoscopically or laparoscopically at the time of esophagectomy, to esophagectomy without any pyloric intervention. We excluded studies not including esophagectomy, those in the paediatric population and those which described any reconstructive method apart from gastric conduit were excluded. No non-English studies were found. The records from the initial search were scanned by two authors (A.B. and P.H.P.) to exclude any duplicate and irrelevant studies. Any discrepancies were resolved by discussion and consensus. The outcomes selected were the most clinically relevant including; rates of delayed gastric emptying, post-operative pyloric endoscopic balloon dilatations, anastomotic leaks, respiratory complications and mortality. Results were reported in accordance with the MOOSE (Meta-analysis Of Observational Studies in Epidemiology) guidelines and tabulated in Microsoft Excel (Microsoft, Redmond, Wahington) (16). Study type, type of esophagectomy, method of administration of BT-A, definitions of DGE and definitions of primary outcomes were also extracted. Any missing data resulted in that study being excluded from the individual meta-analysis.
Statistical analysis
Statistical analysis was performed using Review manager (RevMan), version 5.4 (The Cochrane Collaboration, 2020). Pooled outcomes measures were determined using random effects models as described by Mantel-Haenszel. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Heterogeneity among studies was assessed by Galbraith plot. The risk of bias was assessed using the Newcastle-Ottawa Quality Assessment Scale (17). Studies scoring 7 to 9 stars were considered to be of high methodological quality, studies scoring 4 to 6 stars were moderate and 1 to 3 stars were considered to be low quality.
Results
Studies
After evaluation of 103 potentially eligible studies, seven cohort studies met the inclusion criteria (Figure 1), together including a total of 781 patients. No studies were excluded due to published abstracts only. All seven studies provided details of BT-A delivery, given intra-operatively during the esophagectomy (Table 1). Six studies injected BT-A into the pylorus extraluminally (241 patients) and one study injected endoscopically or extraluminally if the tumour was non-traversable (65 patients). The number of units injected ranged from 20 to 200 (median 200) across 2–6 injection sites (median 4 sites).
Table 1
| Author, year, country | Study type | Patient number (n=781) | Type of oesophagectomy | Method of administration of BT-A | NOS score |
|---|---|---|---|---|---|
| Nobel, 2019, USA | Retrospective cohort | 210* | MIO: 2 stage, n=192 (91%); 3 stage, n=18 (9%) | Extraluminal, 200 U, 2 sites | 8 |
| Tham, 2019, UK | Retrospective cohort | 228 | ILO: hybrid, n=113 (50%); open, n=115 (50%) | Endoluminal (or extra if non-traversable), 200 U, 4 sites | 5 |
| Marchese, 2018, UK | Retrospective cohort | 60* | ILO: open, n=60 (100%) | Extraluminal, 200 U, 4 sites & finger fracture | 8 |
| Stewart, 2017, USA | Retrospective cohort | 71 | MIO: 2 stage, n=69 (97%); 3 stage, n=1 (1%); transhiatal, n=1 (1%) | Extraluminal, 20 U, 2 sites | 6 |
| Giugliano, 2017, USA | Retrospective cohort | 49* | MIO: 3 stage, n=23 (47%); 2 stage, n=26 (53%) | Extraluminal, 100 U, 4–6 sites | 7 |
| Fuchs, 2016, USA | Retrospective cohort | 41 | RATE, n=41 (100%) | Extraluminal, 200 U, 4 sites | 7 |
| Cerfolio, 2009, USA | Retrospective cohort | 122* | Open ILO, n=122 (100%) | Extraluminal, 100 U, 4 sties | 7 |
*, studies included patient arms that were excluded as they did not have BT-A or no intervention. MIO, minimally invasive oesophagectomy; ILO, Ivor-Lewis oesophagectomy; NOS, Newcastle-Ottawa Score; BT-A, botulinum toxin-A; RATE, robotic-assisted transhiatal esophagectomy.
Risk of delayed gastric emptying
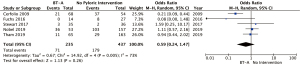
Five studies (672 patients) provided data for rates of delayed gastric emptying (Table 2), each with a different definition of this condition (Table 2) (5,9,10,12,14). Rates of delayed gastric emptying ranged from 6% to 69% in the non-BT-A group vs. 0% to 68% in the BT-A group. Meta-analysis demonstrated a non-significantly reduced rate of DGE in the BT-A use group (pooled OR 0.59, 95% CI: 0.24–1.47; P=0.26) (Figure 2). There was statistically significant heterogeneity between five studies included in this analysis (I2=73%; P=0.005).
Table 2
| Author, year, country | Diagnostic criteria for DGE | Definition of AL and respiratory complications | Timeframe for post-op dilatation data | Outcome data | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Number in each arm, n [%] | Incidence of DGE, n [%] | Dilatation, n [%] | Anastomotic leak, n [%] | Respiratory, n [%] | Mortality, n [%] | LOS (days), SD [range] | ||||
| Nobel, 2019, USA | High NGT output on day 3 >300 mL | Respiratory: pneumonia: clinical, CXR & leukocytosis | EBD categorised within 90 days, 6 & 12 months | BT-A | 53 [25] | 36 [68] | 14 [26] | 8 [15] | 7 [13] | 0 [0] | 8 (IQR, 7–12) |
| AL: not specified | No BT-A | 157 [75] | 103 [66] | 19 [12] | 35 [22] | 31 [20] | 5 [3] | 9 (IQR, 8–11) | |||
| Tham, 2019, UK | NG output >50% of intake or conduit >50% on CXR (1 L of fluids) | Respiratory: N/a | No specifics given | BT-A | 65 [29] | 11 [17] | 8 [12] | * | * | * | 10 [6–70] |
| AL: N/a | No BT-A | 163[71] | 29 [18] | 18 [11] | * | * | * | 9 [7–75] | |||
| Marchese, 2018, UK | No data for DGE | Respiratory: N/a | EBD performed as inpatient & outpatients till follow-up ceased | BT-A | 30 [50] | * | 6 [20] | 1 [3] | * | 1 [3] | 16 [9–71] |
| AL: not specified | No BT-A | 30 [50] | 0 [0] | 0 [0] | * | 0 [0] | 17 [9–42] | ||||
| Stewart, 2017, USA | Not tolerating a PO diet by POD 10—corroborated with contrast swallow or OGD | Respiratory: not specified, numbers given for aspiration pneumonia | EBD within the 1st 12 weeks | BT-A | 35 [49] | 2 [6] | 2 [6] | 8 [23] | 2 [6] | 3 [9] | 11 (IQR, 10–12.5) |
| AL: post-op esophagram | No BT-A | 36 [51] | 3 [8] | 1 [3] | 5 [14] | 2 [6] | 2 [6] | 13 (IQR, 10–18) | |||
| Giugliano, 2017, USA | No data for DGE | Respiratory: N/a | EBD within the 1st 6 months | BT-A | 41 [84] | * | 13 [32] | 7 [17] | * | * | 9 [6–35] |
| AL: not specified | No BT-A | 8 [16] | 1 [12] | 8 [0] | * | * | 9 [6–28] | ||||
| Fuchs, 2016, USA | Clinical diagnosis—nausea and vomiting with swallow and endoscopy to confirm diagnosis | Respiratory: not specified, numbers given for pneumonia | EBD within the 1st 12 months | BT-A | 14 [34] | 0 [0] | 0 [0] | 2 [14] | 0 [0] | 0 [0] | Mean 10.4 [7–21] |
| AL: contrast swallow | No BT-A | 27 [66] | 8 [30] | 8 [30] | 2 [7] | 2 [7] | 1 [4] | Mean 7.4 [6–11] | |||
| Cerfolio, 2009, USA | Contrast swallow on POD 4—gastric emptying taking longer than 10 mins, i.e., majority of contrast not in duodenum | Respiratory: not specified, numbers given for aspiration/pneumonia together | No data | BT-A | 68 [56] | 21 [31] | * | 0 [0] | 9 [13] | 3 [4] | Mean 8.2 |
| AL: swallow completed on day 4 post-operatively | No BT-A | 54 [44] | 37 [69] | * | 1 [2] | 12 [22] | 2 [4] | Mean 7.3 [no range] | |||
*, data not available. BT-A, botulinum toxin-A; DGE, delayed gastric emptying; AL, anastomotic leak; LOS, length of stay; NGT, nasogastric tube; CXR, chest X-ray; EBD, endoscopic balloon dilatation; N/a, non-applicable; PO, per-oral; POD, post-operative day; OGD, oesophagogastroduodenoscopy; IQR, interquartile range; post-op, post-operative.
Requirement for balloon dilatation
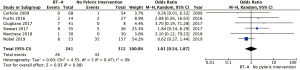
Six studies (659 patients) reported rates of post-operative endoscopic balloon dilatation (9-14). The rates of dilatation ranged from 0% to 30% in the non-BT-A group vs. 0% to 32% in the BT-A group. Two studies compared different time frames for endoscopic balloon dilatation post-operatively (Table 2). One of these studies (n=60) found that both inpatient [BT-A use 4/30 (13%) vs. non-BT-A 0/30 (0%), P=0.032] and outpatient [BT-A use 6/30 (20%) vs. non-BT-A 0/30 (0%), P=0.003] endoscopic balloon dilatation requirement was higher with BT-A use (11). The other study (n=210) found no difference in endoscopic balloon dilatation rates at 90 days [BT-A use 2/53 (4%) vs. non-BT-A 10/157 (6%), P=0.3], but a higher endoscopic balloon dilatation requirements at 6 months [BT-A use 7/48 (14.6%) vs. non-BT-A use 3/141 (2.1%), P=0.009] and a non-significant increase [BT-A use 3/39 (7.7%) vs. non-BT-A use 4/122 (3.3%); P=0.4] at 12 months after surgery (9). In analyses of all timeframes as one variable, BT-A demonstrated a non-significantly increased rate of endoscopic balloon dilatation use in the BT-A group (Figure 3). The pooled OR was 1.75 (95% CI: 0.68–4.48; P=0.24). There was moderate heterogeneity (I2=50%, P=0.07).

Anastomotic leak
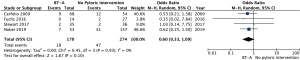
Anastomotic leak rates were reported in 6 studies (comprising 553 patients) (9-14). Anastomotic leaks were defined radiologically in three studies and were undefined in the remaining three. Anastomotic leaks ranged from 0% to 22% in the non-BT-A group vs. 3% to 23% in the BT-A group. Pooled analysis showed that BT-A had no impact on the incidence of anastomotic leaks (pooled OR 1.01, 95% CI: 0.54–1.87; P=0.98) (Figure 4). There was no statistically significant heterogeneity (I2=0%, P=0.47).
Respiratory complications
Four studies reported a total of 65 (15%) respiratory complications, including pneumonia, aspiration pneumonia or aspiration (Table 2) (5,9,12,14). Only one study defined pneumonia with clinical markers (9). Rates in individual studies ranged from 6% to 22% in the non-BT-A group vs. 0% to 13% in the BT-A group. Pooled analysis demonstrated a non-significantly reduced rate of respiratory complications in the BT-A use group (pooled OR 0.60, 95% CI: 0.33–1.09; P=0.10) (Figure 5). There was no statistically significant heterogeneity (I2=0%, P=0.93).
Mortality
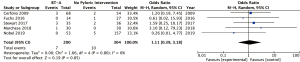
Mortality rates were reported in 5 studies (5,9,11,12,14). It was reported as 30-day mortality in 3 studies. The other two reported 90-day mortality and in-hospital mortality respectively. In total, there were 17 (3%) deaths. Rates in individual studies ranged from 0% to 6% in the non-BT-A group vs. 0% to 9% in the BT-A group. There were no statistically significant differences or trends seen between the two groups on pooled analysis (pooled OR 1.11, 95% CI: 0.39–3.18, P=0.85) (Figure 6). There was no statistically significant heterogeneity (I2=0%).
Quality of studies
Five studies were considered to be of high methodological quality, two of moderate quality and none of low quality. The two studies of moderate quality had risk of bias in either the definition of the exposed or non-exposed groups or adequacy of follow-up (10,12).
Discussion
This is the first systematic review and meta-analysis comparing intra-operative pyloric injection of BT-A with no pyloric intervention to prevent delayed gastric emptying following esophagectomy. It demonstrates no statistically significant benefits to BT-A use in the prevention of delayed gastric emptying or associated outcomes.
Delayed gastric emptying is common after esophagectomy and can increase both rates of pneumonia and length of hospital stay (18). The physiology of delayed gastric emptying after esophagectomy is complex, poorly understood and its aetiology is multifactorial. Disruption of the vagal nerve and hiatal anatomy; the shape and diameter of the conduit (19); negative pressures within the thorax and the conduits route within the mediastinum, all impact on symptoms experienced by patients (3,20,21). With such complexity, it is uncertain if simply mechanically disrupting the pylorus with a pyloric intervention benefits the patients. There are also uncertainties as to the wider management of delayed gastric emptying. In the post-operative period, routine screening for delayed gastric emptying, use of nasogastric tubes and resumption of oral diets are inconsistently utilised (4).
In this review, rates of delayed gastric emptying varied and the need for endoscopic balloon dilatation post-operatively ranged widely between the included studies. Endoscopic balloon dilatation was the most commonly used first-line intervention for delayed gastric emptying across studies. Other options, such as post-operative BT-A injection and pyloroplasty, were rarely used and never in the first instance.
A lack of clarity regarding treatment strategies and outcomes is further complicated by heterogenous diagnostic definitions and management algorithms with reference to pyloric pathology. In this review, the study with the highest reported rate of delayed gastric emptying, for example, had the largest discrepancy between delayed gastric emptying and endoscopic balloon dilatation, suggesting that remaining patients were diagnosed with delayed gastric emptying but deemed not to require treatment. That study defined delayed gastric emptying as nasogastric tube output greater than 300 mL on day 3 (9). The lowest rates of delayed gastric emptying and endoscopic balloon dilatation, conversely, were seen in a different cohort study where a diagnosis of delayed gastric emptying was defined as patients not tolerating an oral diet on post-operative day 10, corroborated with contrast swallow or endoscopy (12).
Further mirroring the lack of consistency in current literature, each of the five studies that defined delayed gastric emptying had a different definition. The lack of consensus on the definition of delayed gastric emptying is a recognised clinical problem. A recent Delphi consensus attempted to address this (2), defining early (i.e., postoperative delayed gastric emptying as >500 mL daily nasogastric tube output between day 5 and 14. This definition requires a nasogastric tube to remain in place for many days, which might not be feasible. In a recent survey, 39% of centres would have removed the tube by day 5 (4) and prolonged use is contrary to enhanced recovery protocols for esophagectomy (22-25). Research is limited by this lack of an appropriate definition and standardised diagnostic criteria.
The use of pyloric interventions is variable with approximately 40% of UK centres performing them routinely. The most frequently used intervention in the UK is surgical pyloroplasty (26%) (4). Since 2010, 16 studies have analysed different approaches to pyloric interventions, and included comparisons of pyloroplasty, pyloromyotomy, BT-A, pre-operative endoscopic balloon dilatation and no intervention. Of these, 13 were cohort studies (9-14,26-32) and three were small randomised clinical trials (33-35). The most recent meta-analysis assessed only pre-operative pyloric endoscopic balloon dilatation and comprised 3 (n=203) cohort studies. It showed that pooled rates of early delayed gastric emptying (16% vs. 39%, P<0.001) and anastomotic leaks (9% vs. 12%, P<0.001) were significantly lower with endoscopic balloon dilatation (36). A meta-analysis from 2015 considered all pyloric drainage interventions (pyloromyotomy, pyloroplasty, BT-A, finger fracture) as one entity. From six comparative studies the meta-analysis found that pyloric drainage showed a non-significant trend toward fewer anastomotic leaks and pulmonary complications and reduced delayed gastric emptying (37). This analysis compared all types of drainage procedures to no intervention rather than individual analysis and it did not analyse rates of subsequent interventions such as post-operative endoscopic balloon dilatation as a measure of treatment efficacy. Previous meta-analyses of pyloric interventions have shown either no impact on rates of delayed gastric emptying and related complications (19) or reduction in delayed gastric emptying, but no effect on other early or late patient outcomes (38). There is a lack of high-quality original studies, particularly large randomised clinical trials, to guide management of the pylorus during esophagectomy.
Although without statistical significance, the present study suggests a trend to reductions in rates of delayed gastric emptying and pneumonia after BT-A, indicating that BT-A may ameliorate some of the early negative effects of delayed gastric emptying. However, there was also a trend towards increased need for endoscopic balloon dilatation in the botox treated group. This incongruousness may be a result of delayed symptoms requiring endoscopic balloon dilatation. Whereas delayed gastric emptying and pneumonia were identified immediately post-operatively, endoscopic balloon dilations were analysed up to 12 months later. Only two studies compared index admission and delayed endoscopic balloon dilatation requirements (9,11). Both found higher rates of delayed endoscopic balloon dilatation with BT-A use when compared to non-use. This was postulated to be due to fibrosis within the muscle once the effects of botulinum stopped (9). It may also simply represent the temporary effect of BT-A. The duration of therapeutic effect with BT-A is thought to be 10±3 weeks, so by 6 months any remaining effects of BT-A would be negligible (39).
BT-A may represent a safe and technically simple method of temporarily reducing early post-operative impact of delayed gastric emptying. Once the pharmacologic effects of BT-A have stopped there may instead be an increase in requirement for endoscopic balloon dilatation. However, after such delay patients would normally have fully recovered from the impact of their operation and would be better able to tolerate the symptoms and sequelae of delayed gastric emptying. Also, some evidence shows that function returns within the gastric conduit over 1–3 years (40-42). A temporary intervention rather than definitive pyloromyotomy or pyloroplasty may be more advantageous in the long-term. Patients may be less susceptible to long-term complications from pyloric intervention such as bile reflux and dumping syndromes (1,3,37).
Among limitations of this systematic review are the retrospective nature of the majority of included studies, the low number of studies, the limited sample sizes, and the heterogeneity amongst outcome definitions. Delayed gastric emptying, anastomotic leakages (whether clinical or radiological and if treated with conservative management on intervened on) and respiratory complications had different definitions in each study and in some they were not defined. Heterogeneity also exists in the different surgical approaches taken. Studies included in the meta-analysis included 2-stage, 3-stage and trans-hiatal operations. For each approach patients will experience different symptoms and rates of complications. Many of these studies are longitudinal, and different techniques to manage the pylorus during esophagectomy may result from evolution in practice or peri-operative protocols over time as well surgeon variation in units. This also reduces the validity of the studies. Four of the studies included other forms of pyloric intervention (5,9,11,13). However, post-operative protocols were similar in each group, allowing for data extraction and a reduction in the risk of bias.
In conclusion, this systematic review and meta-analysis shows no statistically significant benefit to pyloric interventions with BT-A during esophagectomy, but a non-significant trend of reduced rates of delayed gastric emptying and pneumonia as well as an increase in need for endoscopic balloon dilatation. Individual studies, non-significant trends seen on meta-analyses, expert opinions and historical experience mean that pyloric interventions continue to be used routinely. BT-A may represent a safe, simple and temporary way of mitigating the immediate post-operative complications associate with delayed gastric emptying, However, well-designed and large randomised clinical trials comparing a range of surgical approaches to oesophagectomy; and different pyloric interventions are required. This will clarify the role of BT-A and other pyloric interventions during esophagectomy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-29/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-29/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-29/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang R, Zhang L. Management of delayed gastric conduit emptying after esophagectomy. J Thorac Dis 2019;11:302-7. [Crossref] [PubMed]
- Konradsson M, van Berge Henegouwen MI, Bruns C, et al. Diagnostic criteria and symptom grading for delayed gastric conduit emptying after esophagectomy for cancer: international expert consensus based on a modified Delphi process. Dis Esophagus 2020;33:doz074. [Crossref] [PubMed]
- Konradsson M, Nilsson M. Delayed emptying of the gastric conduit after esophagectomy. J Thorac Dis 2019;11:S835-44. [Crossref] [PubMed]
- Bull A, Pucher PH, Maynard N, et al. Nasogastric tube drainage and pyloric intervention after oesophageal resection: UK practice variation and effect on outcomes. Eur J Surg Oncol 2022;48:1033-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Canon CL, et al. Is botulinum toxin injection of the pylorus during Ivor Lewis [corrected] esophagogastrectomy the optimal drainage strategy? J Thorac Cardiovasc Surg 2009;137:565-72. Erratum in: J Thorac Cardiovasc Surg 2009 Jun;137(6):1581. [Crossref] [PubMed]
- Ukleja A, Tandon K, Shah K, et al. Endoscopic botox injections in therapy of refractory gastroparesis. World J Gastrointest Endosc 2015;7:790-8. [Crossref] [PubMed]
- Ramzan Z, Nassri AB. The role of Botulinum toxin injection in the management of achalasia. Curr Opin Gastroenterol 2013;29:468-73. [Crossref] [PubMed]
- Bashashati M, Andrews C, Ghosh S, et al. Botulinum toxin in the treatment of diffuse esophageal spasm. Dis Esophagus 2010;23:554-60. [Crossref] [PubMed]
- Nobel T, Tan KS, Barbetta A, et al. Does pyloric drainage have a role in the era of minimally invasive esophagectomy? Surg Endosc 2019;33:3218-27. [Crossref] [PubMed]
- Tham JC, Nixon M, Ariyarathenam AV, et al. Intraoperative pyloric botulinum toxin injection during Ivor-Lewis gastroesophagectomy to prevent delayed gastric emptying. Dis Esophagus 2019;32:doy112. [Crossref] [PubMed]
- Marchese S, Qureshi YA, Hafiz SP, et al. Intraoperative Pyloric Interventions during Oesophagectomy: a Multicentre Study. J Gastrointest Surg 2018;22:1319-24. [Crossref] [PubMed]
- Stewart CL, Wilson L, Hamm A, et al. Is Chemical Pyloroplasty Necessary for Minimally Invasive Esophagectomy? Ann Surg Oncol 2017;24:1414-8. [Crossref] [PubMed]
- Giugliano DN, Berger AC, Meidl H, et al. Do intraoperative pyloric interventions predict the need for postoperative endoscopic interventions after minimally invasive esophagectomy? Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Fuchs HF, Broderick RC, Harnsberger CR, et al. Intraoperative Endoscopic Botox Injection During Total Esophagectomy Prevents the Need for Pyloromyotomy or Dilatation. J Laparoendosc Adv Surg Tech A 2016;26:433-8. [Crossref] [PubMed]
- Heddle R, Cock C. Role of botulinum toxin injection in treatment of achalasia. Ann Esophagus 2020;3:26. [Crossref]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- Lanuti M, DeDelva P, Morse CR, et al. Management of delayed gastric emptying after esophagectomy with endoscopic balloon dilatation of the pylorus. Ann Thorac Surg 2011;91:1019-24. [Crossref] [PubMed]
- Akkerman RD, Haverkamp L, van Hillegersberg R, et al. Surgical techniques to prevent delayed gastric emptying after esophagectomy with gastric interposition: a systematic review. Ann Thorac Surg 2014;98:1512-9. [Crossref] [PubMed]
- Yang HC, Choi JH, Kim MS, et al. Delayed Gastric Emptying after Esophagectomy: Management and Prevention. Korean J Thorac Cardiovasc Surg 2020;53:226-32. [Crossref] [PubMed]
- Ukegjini K, Vetter D, Fehr R, et al. Functional syndromes and symptom-orientated aftercare after esophagectomy. Langenbecks Arch Surg 2021;406:2249-61. [Crossref] [PubMed]
- Zhang Z, Li H, Yan C, et al. A comparative study on the efficacy of fast-track surgery in the treatment of esophageal cancer patients combined with metabolic syndrome. Oncol Lett 2017;14:4812-6. [Crossref] [PubMed]
- Zhao G, Cao S, Cui J. Fast-track surgery improves postoperative clinical recovery and reduces postoperative insulin resistance after esophagectomy for esophageal cancer. Support Care Cancer 2014;22:351-8. [Crossref] [PubMed]
- Chen L, Sun L, Lang Y, et al. Fast-track surgery improves postoperative clinical recovery and cellular and humoral immunity after esophagectomy for esophageal cancer. BMC Cancer 2016;16:449. [Crossref] [PubMed]
- Huang ZD, Gu HY, Zhu J, et al. The application of enhanced recovery after surgery for upper gastrointestinal surgery: Meta-analysis. BMC Surg 2020;20:3. [Crossref] [PubMed]
- Boshier PR, Adam ME, Doran S, et al. Effects of intraoperative pyloric stretch procedure on outcomes after esophagectomy. Dis Esophagus 2018; [Crossref] [PubMed]
- Hadzijusufovic E, Tagkalos E, Neumann H, et al. Preoperative endoscopic pyloric balloon dilatation decreases the rate of delayed gastric emptying after Ivor-Lewis esophagectomy. Dis Esophagus 2019;32:doy097. [Crossref] [PubMed]
- De Pasqual CA, Weindelmayer J, Gobbi L, et al. Effect of Pyloroplasty on Gastric Conduit Emptying and Patients' Quality of Life After Ivor Lewis Esophagectomy. J Laparoendosc Adv Surg Tech A 2021;31:692-7. [Crossref] [PubMed]
- Deng B, Tan QY, Jiang YG, et al. Prevention of early delayed gastric emptying after high-level esophagogastrostomy by "pyloric digital fracture". World J Surg 2010;34:2837-43. [Crossref] [PubMed]
- Swanson EW, Swanson SJ, Swanson RS. Endoscopic pyloric balloon dilatation obviates the need for pyloroplasty at esophagectomy. Surg Endosc 2012;26:2023-8. [Crossref] [PubMed]
- Antonoff MB, Puri V, Meyers BF, et al. Comparison of pyloric intervention strategies at the time of esophagectomy: is more better? Ann Thorac Surg 2014;97:1950-8. [Crossref] [PubMed]
- Eldaif SM, Lee R, Adams KN, et al. Intrapyloric botulinum injection increases postoperative esophagectomy complications. Ann Thorac Surg 2014;97:1959-65. [Crossref] [PubMed]
- Bagheri R, Fattahi SH, Haghi SZ, et al. Botulinum toxin for prevention of delayed gastric emptying after esophagectomy. Asian Cardiovasc Thorac Ann 2013;21:689-92. [Crossref] [PubMed]
- Mohajeri G, Tabatabaei SA, Hashemi SM, et al. Comparison of pyloromyotomy, pyloric buginage, and intact pylorus on gastric drainage in gastric pull-up surgery after esophagectomy. J Res Med Sci 2016;21:33. [Crossref] [PubMed]
- Mahmodlou R, Badpa N, Nosair E, et al. Usefulness of Pyloromyotomy With Transhiatal Esophagectomy in Improving Gastric Emptying. Gastroenterology Res 2011;4:223-7. [Crossref] [PubMed]
- Abdelrahman M, Ariyarathenam A, Berrisford R, et al. Systematic review and meta-analysis of the influence of prophylactic pyloric balloon dilatation in the prevention of early delayed gastric emptying after oesophagectomy. Dis Esophagus 2022;35:doab062. [Crossref] [PubMed]
- Arya S, Markar SR, Karthikesalingam A, et al. The impact of pyloric drainage on clinical outcome following esophagectomy: a systematic review. Dis Esophagus 2015;28:326-35. [Crossref] [PubMed]
- Urschel JD, Blewett CJ, Young JE, et al. Pyloric drainage (pyloroplasty) or no drainage in gastric reconstruction after esophagectomy: a meta-analysis of randomized controlled trials. Dig Surg 2002;19:160-4. [Crossref] [PubMed]
- Eleopra R, Rinaldo S, Montecucco C, et al. Clinical duration of action of different botulinum toxin types in humans. Toxicon 2020;179:84-91. [Crossref] [PubMed]
- Collard JM, Romagnoli R, Otte JB, et al. The denervated stomach as an esophageal substitute is a contractile organ. Ann Surg 1998;227:33-9. [Crossref] [PubMed]
- Nakabayashi T, Mochiki E, Garcia M, et al. Gastropyloric motor activity and the effects of erythromycin given orally after esophagectomy. Am J Surg 2002;183:317-23. [Crossref] [PubMed]
- Walsh TN, Caldwell MT, Fallon C, et al. Gastric motility following oesophagectomy. Br J Surg 1995;82:91-4. [Crossref] [PubMed]
Cite this article as: Bull A, Pucher PH, Lagergren J, Gossage JA. Impact of intra-pyloric botulinum toxin injection on delayed gastric emptying following esophagectomy: systematic review and meta-analysis. Ann Esophagus 2024;7:1.


