
Impact of radiation related lymphopenia on outcomes in esophageal cancer: a systematic review and meta-analysis of clinical studies
Highlight box
Key findings
• Severe lymphopenia associated with chemoradiation in esophageal cancer is associated with increased risk of disease recurrence and inferior overall survival.
• The dose to circulating immune cell populations and dose to heart is associated with higher odds of lymphocyte depletion.
What is known and what is new?
• The impact of treatment related lymphocyte depletion was relatively unknown.
• Our systematic review provides further evolving evidence that radiation related lymphocyte depletion is associated with increased risk of disease progression.
• The location of the tumor in relation to heart, lung and spleen and the estimated dose to the immune cells all are factors in causing lymphocyte depletion during radiation delivery.
What is the implication, and what should change now?
• Interventions to maintain lymphocyte count during radiotherapy by improving dosimetry or cytokines that increase the lymphocyte count, adoptive T cell transfer may improve esophageal cancer outcomes.
Introduction
Esophageal cancer (EC) is the sixth leading cause for cancer mortality worldwide (1). Most locally advanced ECs are treated with chemo-radiotherapy (CRT) followed by surgery (2). Despite advances in treatments the prognosis of EC remains poor, with an approximate 5-year survival rate of 15–25% for locally advanced EC (3). The inter-individual difference in response and control rates to CRT has been attributed to heterogeneity in tumor biology (4). Studies have shown that circulating lymphocyte depletion is a prognostic factor for inferior overall survival in various solid malignancies. The lymphocytes found in the blood stream are the cells that become resident lymphocytes and hence safeguarding this lymphocyte pool or avenues to increase the circulating lymphocytes pool are potential ways to improve clinical outcomes in EC by increasing immunogenic cell death. Multiple small studies have been conducted in recent years that have tried to assess impact of radiotherapy on lymphocyte counts and further the impact of severe lymphopenia on survival outcomes in EC (5). Esophagus being a serial organ, radiotherapy in different parts of esophagus is expected to result in grossly different doses to heart, lung, spleen, and spine which in turn will have an impact on total dose to immune cells (6). This in turn is expected to cause different rates of lymphopenia based on extent and location of esophageal disease. To better understand the impact of radiation associated lymphopenia in EC, we undertook this systematic review and pooled analysis of clinical studies that have reported radiation related lymphopenia in EC. We present this article in accordance with the PRISMA reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-15/rc).
Methods
Data search
The main three databases PubMed, Cochrane Central, and EMBASE databases were searched with key MeSH terms—radiation; cancer; lymphopenia, Esophagus, and survival. The search methodology with search terms is provided in the Appendix 1. The search duration was from the start of each database till September 6, 2020. We did not use language filter and BPV and RU did the search independently with any conflicts were resolved by mutual discussion. There was no attempt made to reach the authors of the articles for any unpublished data and no automation tools were employed to assist in the systematic review process. The systematic review was performed in accordance with the PRISMA guidelines, and the quality of studies was assessed by Newcastle Ottawa scale.
Eligibility criteria for articles
Any secondary analysis of randomized clinical trials, prospective cohort studies, retrospective cohort studies with radiation used with definitive intent with details on cancer specific outcomes should have been reported. Studies with preclinical data, patients undergoing chemotherapy alone, surgery alone, immunotherapy alone were excluded.
Article review
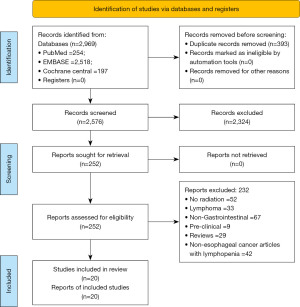
The search was done in accordance with the PRISMA guidelines and the PRISMA flow diagram has been shown in the Figure 1. The articles from the database search were screened and based on the inclusion criteria listed above. The articles found to be relevant to the topic of interest were shortlisted. The full-length paper of the shortlisted articles was assessed for the eligibility criteria. Two reviewers independently extracted the data in a data extraction form.
Statistical analysis
The hazard ratios (HRs), odds ratio (OR), mean difference were presented with forest plots showing the overall survival and progression free survival outcomes between severe lymphopenia and no severe lymphopenia. The variables were reported with 95% confidence interval (CI) and P value <0.05 were considered statistically significant. The Forest plot for HR and mean difference was plotted by Generic inverse variance method; OR by Mantel-Haenszel method. The random-effects model was used for analysis. Study heterogeneity was assessed using the inconsistency Stevel Linindex (I2-statistic) with values of 0–30%, 31–60%, 61–75% and 76–100% indicating low, moderate, substantial, and considerable heterogeneity, respectively. Review Manager Version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) was used for the analysis.
Results
Search results
The systematic search of literature resulted in 2,969 articles of which 252 articles underwent complete review. Twenty studies were included in the final systematic review and 5 studies were included in the meta-analysis. Table 1 summarizes the details of studies reporting on radiation related lymphopenia outcomes in EC.
Table 1
| First author, year | Country/region | No. of patients | Definition of lymphopenia | Incidence of lymphopenia | Cancer specific outcomes | Dosimetric correlations | Comments |
|---|---|---|---|---|---|---|---|
| van Rossum 2020, (7) | USA | 860 | <200 cells/mL | 322 (37%) | PFS, OS | PTV volume (P<0.001); radiation modality (P<0.001); age (P=0.004); BMI (P=0.032); baseline ALC (P<0.001) were independent predictor of grade 4 lymphopenia | Nomogram for lymphopenia-High predicted risk of G4 lymphopenia had a significantly worse PFS and OS compared with patients at low risk |
| Wang 2020, (8) | China | 189 | 0.38×103 cells/μL | 110 (58.2%) | OS, PFS, LRFS | Larger PTV (P=0.01), high lung V5 (P<0.001), high lung V10 (P=0.001), higher heart V5 (P=0.002), V10 (P=0.002), V20 (P=0.029), V30 (P=0.037) correlated with low ALC nadir | Stage III–IVA (P=0.002), low ALC before treatment (P=0.028) associated with low ALC nadir during RT |
| Song 2019, (9) | Taiwan | 105 | NA | NA | OS, TPD, TM | NA | Post-CCRT high platelet count (>300,000/μL) significantly associated with poor OS, TPD, and TM |
| Zhang 2019, (10) | USA | 219 | <200/mm3 | 82 (45%) | OS, RFS | Heart V15 >73%, T-spine V5 >72%, body V10 >18%, aorta V5 >93% were strong predictors of G4 lymphopenia | G-4 lymphopenia is associated with worse median RFS and OS |
| Zhou 2019, (11) | China | 286 | <200 cells per mm3 | 89 (31%) | Treatment response | Greater tumour length (P=0.042), larger PTV (P<0.001). Distal tumour location (P=0.04) were independent predictors for treatment-related lymphopenia | Treatment-related lymphopenia (P=0.043) during CCRT is an independent predictor for poor treatment response |
| Davuluri 2017, (12) | USA | 504 | <200 cells/mL | 134 (27%) | OS, DSS | Proton beam therapy was associated with lesser risk of developing grade 4 lymphopenia on compared to IMRT (P=0.001). Radiation type (proton vs. IMRT) strongly influenced the mean body dose exposure, which was a strong predictor for G4 nadir (P<0.001) | Predictors of G4 nadir included. Distal tumour location, definitive CRT, taxane/5-FU chemotherapy and Photon-based radiation type (vs. proton-based) |
| Fang 2018, (13) | USA | 220 | CTCAE V4 | 86 (39%) | OS, DFS, LRRFS, DMRFS | Treatment with IMRT, compared with PBT (P=0.01), increased age (P=0.03), Larger PTV (P<0.001) are associated with increased risk of grade 4 lymphopenia | PBT reduces the risk of severe, treatment-related lymphopenia, for tumours of the lower oesophagus |
| Routman 2019, (14) | USA | 144 | <200/mm3 | 40% | NA | Body V1-V30Gy (P<0.01), heart V1-V30Gy (P<0.01), liver V1-V35Gy (P<0.01), lung V1-V30Gy (P<0.01), spleen V1-V40Gy (P<0.01) are associated with G4 lymphopenia | Advanced stage (P<0.01), Photon vs. proton (P<0.01), and CTV (P<0.01) were associated with G4L. Low to intermediate dose volumes to body, spleen, liver, lungs, and heart were associated with G4 lymphopenia |
| Shiraishi 2018, (15) | USA | 480 | <200 cells/microliter | 159 (33.1%) | OS, DMFS | Age (P=0.02), larger PTV (P<0.0001). RT modality (P<0.0001) were associated with G4 lymphopenia | PBT was significantly associated with a reduction in grade 4 lymphopenia risk (P<0.0001) |
| Hirano 2018, (16) | Japan | 27 | NA | NA | OS, PFS | NA | PBT enabled a significant reduction in the dose to the lung and heart, compared with 3DCRT or IMRT |
| Xu 2020, (17) | China | 488 | CTCAE V4 | 50% | OS, PFS, DMFS, LRC | Grade 4 lymphopenia resulted in inferior OS, PFS, and DMFS. Higher EDIC (>4.0 Gy) was associated with severe lymphopenia (P<0.001). Increasing EDIC was independently and inversely associated with worse OS, PFS, and DMFS | EDIC can be recommended as a useful tool to predict lymphopenia and inferior clinical outcomes, and it should be below 4 Gy |
| So 2020, (18) | Hong Kong | 92 | 0.5 (109 cells/L) | NA | OS | Low lung dose V1–V25 (P=0.01), EDIC (P<0.01) correlated with lymphocyte nadir | Lymphocyte nadir was a significant independent factor for shorter OS (P<0.001) |
| Chin 2018, (19) | USA | 60 | Grade ≥3 leukopenia | 18 (30%) | NA | Greater decrease in spleen size after radiation therapy and higher spleen V5–V20 were independently associated with a lower risk of severe hematologic toxicity | NA |
| Saito 2018, (20) | Japan | 61 | ALC <0.200×109/I | 48 (79%) | NA | Spleen dose-volume parameters (V5, V10, V20, V30, and mean splenic dose) were significant independent factors negatively influencing the absolute lymphocyte count at nadir. An increase of 1 Gy in mean splenic dose predicted a 2.9% decrease in nadir absolute lymphocyte count | Higher spleen dose-volume parameters were associated with severe lymphopenia during chemoradiotherapy |
PFS, progression free survival; OS, overall survival; PTV, planning target volume; BMI, body mass index; ALC, absolute lymphocyte count; LRFS, locoregional failure free survival; RT, radiotherapy; TPD, time to progressive disease; TM, time to metastases; NA, not applicable; CCRT, chemoradiation; RFS, relapse free survival; DSS, disease specific survival; IMRT, intensity modulated radiation therapy; CRT, chemoradiotherapy; 5-FU, 5-flourouracil; CTCAE V4, common terminology criteria for adverse events; DFS, disease free survival; LRRFS, loco-regional relapse free survival; DMRFS, distant metastases relapse free survival; PBT, proton beam therapy; CTV, clinical target volume; 3DCRT, 3D conformal radiation therapy; DMFS, distant metastases free survival; LRC, loco-regional control; EDIC, effective dose to immune cells.
The Newcastle Ottawa scale assessment was used to assess the quality of the studies included in the systematic review and described in Appendix 2.
Systematic review of included studies
Impact of tumor location on incidence of lymphopenia
Tumor location in the lower third of the esophagus versus mid/upper esophagus was positively associated with grade 4 lymphopenia in a study by Davuluri et al. (P=0.23) (12). In a study by Fang et al., radiotherapy was associated with lymphocyte reduction in patients with tumors in the lower esophagus (P=0.005), but not for those with tumors in the upper or middle esophagus (P=0.32) (13). Similarly, in a study by van Rossum et al., lower third EC patients treated with radiotherapy had a significantly higher incidence of grade 4 lymphopenia (39%) compared with patients of upper/mid third Esophagus (29%; P=0.029) (7). In a study by Zhou et al., patients with lower ECs treated with radiotherapy had a higher OR of developing grade 4 lymphopenia (OR: 2.430; 95% CI: 1.043–5.663; P=0.040) on multi-variate analysis (11).
Impact of proton therapy on incidence of lymphopenia
Proton beam therapy (PBT) was associated with lesser risk of developing grade 4 lymphopenia on multi-variate analysis compared to intensity modulated radiotherapy (IMRT) (P=0.006) in a study by Davuluri et al. (12). Similarly, in a study by Fang et al., treatment with IMRT compared with PBT (OR: 2.13; 95% CI: 1.19–3.81; P=0.01) was associated with increased risk of grade 4 lymphopenia (13). Grade 4 lymphopenia was significantly higher in patients who received photon-based treatment versus those who received proton-based treatment (56% vs. 22%; P=0.01) in another study by Routman et al. On multi-variate analysis, photon-based treatment (OR: 5.13; 95% CI: 2.35–11.18; P=0.001) was associated with severe lymphopenia (14). In a propensity matched analysis of 272 patients (IMRT vs. PBT) by Shiraishi et al., a greater proportion of the IMRT patients (40.4%) developed grade 4 lymphopenia during neo-adjuvant chemo-radiation compared with the PBT patients (17.6%, P=0.0001) (15). In a large retrospective analysis of 860 patients by van Rossum et al. higher percentage of patients receiving IMRT treatment (46%) were associated with grade 4 lymphopenia than patients treated by PBT (22%; P=0.001) (7). The authors went on to create a predictive nomogram for incidence of lymphopenia with radiation modality as one of the factors. As a pointer towards reason of PBT being consistently associated with lower risk of severe lymphopenia, Hirano et al. performed a dosimetric comparison of PBT versus photon beam treatment in ECs and found that PBT enabled a significant reduction in the dose to the lung and heart. This, in turn is expected to reduce dose to circulating immune cells (16).
Impact of dosimetric parameters and incidence of lymphopenia
Impact of target volumes on lymphopenia
Fang et al. performed a propensity matched analysis between IMRT and PBT on 448 patients of EC treated by chemo-radiation. The authors found that larger planning target volume (PTV) volume was associated with a higher risk of grade 4 lymphopenia (P=0.001) on multi-variate analysis (13). Shiraishi et al. reported a 33% incidence of grade 4 lymphopenia amongst 480 patients of EC. Similar to the previous study, larger PTV volume was associated with a higher risk of grade 4 lymphopenia (P=0.009) on multi-variate analysis (15). In a study of 860 patients by van Rossum et al., PTV volume was found to be an independent predictor of grade 4 lymphopenia. The authors went on to create a nomogram for prediction of incidence of lymphopenia with PTV volume as one of the factors (7). Routman et al. reported a 40% incidence of grade 4 lymphopenia amongst 144 patients treated with CRT. Interestingly CTV volume was not associated with increased risk of severe lymphopenia in this study (OR: 1.12; P=0.15) (14).
Impact of integral dose and estimated dose to immune cells (EDIC) on incidence of lymphopenia
Davuluri et al. performed a retrospective analysis of 504 EC patients treated with CRT. Most patients had disease (89%) primarily located in lower esophagus and 27% patients developed grade 4 as per CTCAE v4 grading. In the study the incidence of grade 4 lymphopenia was significantly lower in patients who received a mean body dose (MBD) <10 Gy than in those with an MBD 10 Gy (16.8% vs. 33.7%, P=0.001) (12). Interestingly, in a study by Xu et al. patients with EDIC >4.0 Gy developed more grade 4 lymphopenia than those with EDIC <4.0 Gy (67.3% vs. 40.8%; P=0.001) (17). EDIC was significantly correlated with lymphocyte nadir (Spearman coefficient Z 0.505; P=0.01) in another similar study by So et al. (18).
Impact of doses to spleen on lymphopenia: the splenic conundrum
Chin et al. performed a retrospective analysis of 60 patients of lower EC treated with neo-adjuvant or definitive chemo-radiation. Eighteen patients (30%) patients developed grade 3 or higher leukopenia. No patient developed grade 3 or higher neutropenia and anemia. The authors found that a higher absolute spleen V5–V30 was associated with a greater decrease in spleen volume (P<0.05 each). Interestingly, the authors also found that patients who did not develop grade 3 or higher hematological toxicity had greater percentage decrease in spleen volume at first follow-up (P=0.009) and higher V5–V30 of spleen (P<0.05 each) (19). Contradictory to the above finding, a retrospective study by Saito et al. on 61 patients showed that spleen V5, V10, V20 and V30 significantly affected lymphocyte nadir and an increase of 1 Gy in mean splenic dose predicted a 2.9% decrease in absolute lymphocyte count (ALC) at nadir (20).
Impact of heart dose on incidence of lymphopenia
In a study by Wang et al., high heart V10 (P=0.003) and V20 (P=0.028) were associated with low ALC nadir on multivariate analysis (8). In another study by So et al., mean dose to the heart was highly significant in predicting lymphocyte nadir (Spearman coefficients =−0.502; P=0.01) (18). In a study by Zhang et al., patients with heart V15 >73% were at higher odds of developing severe lymphopenia on multi-variate analysis (OR: 2.39; 95% CI: 1.05–5.46; P=0.05) (10).
Impact of lung dose on incidence of lymphopenia
In a study by So et al., mean dose to the lung was significant in predicting lymphocyte nadir (Spearman coefficient =−0.34; P=0.01) (18). Similarly, Wang et al. found that high lung V5 (r=−0.26; P=0.001) and lung V10 (r=−0.24; P=0.001) strongly correlated with lower ALC nadir (P=0.01) (8).
Impact of doses to bone on incidence of lymphopenia
Chin et al. did not find any association of rib or thoracic spine dose on hematological toxicity (19). Contradictory to the above finding, in another study by Newman et al. multivariable linear regression correlated lymphopenia nadir with vertebral V20, V30 and V40 Gy (P=0.05 each) (21).
Pooled analysis of outcomes of radiation related lymphopenia
Five studies reported on the mortality outcomes in patients with severe lymphopenia. The patients with severe lymphopenia were at increased risk of death with a pooled HR =1.57 (95% CI: 1.35–1.83, I2=0%, P<0.00001) compared to patients with no severe lymphopenia. Two studies reported on risk of progression with severe lymphopenia. The patients with severe lymphopenia were at increased risk of progression with a pooled HR =1.42 (95% CI: 1.19–1.70, I2=0%, P<0.0001). Figure 2A,2B shows the Forest plots for overall survival and progression free survival.

Discussion
Our systematic review provides further evolving evidence that radiation related lymphocyte depletion in EC is associated with inferior overall survival and increased risk of disease progression. The location of the esophageal tumor defines the incidental dose to the heart and other additive factors such as the lung dose, dose to the spleen and the estimated dose to the immune cells all are defining factors in causing lymphocyte depletion during radiation delivery. Currently, CRT (neo-adjuvant or definitive) has become the standard of care treatment for EC. Research into anti-tumor immunity and tumor micro-environment has seen resurgence in recent years with introduction of checkpoint point inhibitors like pembrolizumab and nivolumab (22). EC cells are considered to be highly immunogenic and have been found to induce anti-tumor immunity. Increase in the number of CD8+ tumour infiltrating lymphocytes (TIL) has been found associated with prolonged survival in EC patients, a better pathologic response to neoadjuvant chemotherapy, and a lower rate of lymph node metastasis (6). Increased CD4+ TILs were also associated with significant local regression of EC. The circulating lymphocytes are the cells that eventually become TILs (23). With this context, it becomes imperative to understand the effect of radiotherapy both on the immune cell pool in the human body and on the tumour micro-environment. To answer the first question, we undertook this systematic review and meta-analysis. A larger PTV volume, use of photon beam instead of proton beam in treatment and higher EDIC were consistently associated with higher rates of severe lymphopenia. The dose to circulating immune cells primarily depends on mean heart dose, lung dose, liver dose and integral dose. A larger PTV as well as photon beams result in higher integral dose and presumably also to higher organs at risk viz. heart, lung, bone marrow and bone doses. In addition, we found multiple studies where in higher heart and lung doses correlated with higher incidence of severe lymphopenia (10,18,19). The analyses of dose to spleen on lymphopenia have provided varied results with studies showing both higher and lower risk of lymphopenia with higher splenic doses. Spleen is an organ wherein slowing of circulating blood cells happens. Higher doses to spleen are therefore expected to cause higher rates of lymphopenia. But radiotherapy per se may lead to splenic shrinking which in turn may lead to lesser volume of blood cells in the spleen at any given time point (17,24). This dual effect of radiotherapy, i.e., direct killing of lymphocytes within the spleen and induction of fibrosis of spleen probably explains the variation in findings between the studies. Finally, we performed a pooled analysis to find the impact of grade 4 lymphopenia on survival. The results were in line with the expectation that grade 4 lymphopenia was found to have a higher hazard of death as well as progressive disease.
Our study does come with a few limitations. All the included studies are retrospective, and this brings in various forms of bias. No study performed evaluation of tumour micro-environment to check for TILs and dendritic cells and therefore various correlations have been derived from unselected population. But the present study to our knowledge is the first meta-analysis performed to pool all available data on this topic. This may be considered the strength of the study.
Conclusions
Severe lymphopenia with CRT is associated with worse overall and progression free survival. Larger PTV, higher EDIC, heart and lung doses result in higher risk of severe lymphopenia. The relation of lymphopenia with splenic doses seems more complicated and merits further evaluation. Interventions to maintain lymphocyte count during radiotherapy by improving dosimetry or interventions like adoptive T cell transfer may improve EC outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-15/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-15/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-15/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Duarte MBO, Pereira EB, Lopes LR, et al. Chemoradiotherapy With or Without Surgery for Esophageal Squamous Cancer According to Hospital Volume. JCO Glob Oncol 2020;6:828-36. [Crossref] [PubMed]
- Short MW, Burgers KG, Fry VT. Esophageal Cancer. Am Fam Physician 2017;95:22-8. [PubMed]
- Lin L, Lin DC. Biological Significance of Tumor Heterogeneity in Esophageal Squamous Cell Carcinoma. Cancers (Basel) 2019;11:1156. [Crossref] [PubMed]
- Venkatesulu BP, Mallick S, Lin SH, et al. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018;123:42-51. [Crossref] [PubMed]
- Wang X, Wang P, Zhao Z, et al. A review of radiation-induced lymphopenia in patients with esophageal cancer: an immunological perspective for radiotherapy. Ther Adv Med Oncol 2020;12:1758835920926822. [Crossref] [PubMed]
- van Rossum PSN, Deng W, Routman DM, et al. Prediction of Severe Lymphopenia During Chemoradiation Therapy for Esophageal Cancer: Development and Validation of a Pretreatment Nomogram. Pract Radiat Oncol 2020;10:e16-26. [Crossref] [PubMed]
- Wang X, Zhao Z, Wang P, et al. Low Lymphocyte Count Is Associated With Radiotherapy Parameters and Affects the Outcomes of Esophageal Squamous Cell Carcinoma Patients. Front Oncol 2020;10:997. [Crossref] [PubMed]
- Song Q, Wu JZ, Wang S. Perioperative change in lymphocyte count and prognosis in esophageal squamous cell carcinoma. J Thorac Dis 2019;11:2332-9. [Crossref] [PubMed]
- Zhang E, Deng M, Egleston B, et al. Dose to Heart, Spine, Aorta, and Body Predict for Severe Lymphopenia and Poor Survival in Patients Undergoing Chemoradiation for Esophageal Cancer. Int J Radiat Oncol Biol Phys 2019;105:E206-7. [Crossref]
- Zhou XL, Zhu WG, Zhu ZJ, et al. Lymphopenia in Esophageal Squamous Cell Carcinoma: Relationship to Malnutrition, Various Disease Parameters, and Response to Concurrent Chemoradiotherapy. Oncologist 2019;24:e677-e686. [Crossref] [PubMed]
- Davuluri R, Jiang W, Fang P, et al. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2017;99:128-35. [Crossref] [PubMed]
- Fang P, Jiang W, Davuluri R, et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol 2018;128:584-90. [Crossref] [PubMed]
- Routman DM, Garant A, Lester SC, et al. A Comparison of Grade 4 Lymphopenia With Proton Versus Photon Radiation Therapy for Esophageal Cancer. Adv Radiat Oncol 2019;4:63-9. [Crossref] [PubMed]
- Shiraishi Y, Fang P, Xu C, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol 2018;128:154-60. [Crossref] [PubMed]
- Hirano Y, Onozawa M, Hojo H, et al. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol 2018;13:23. [Crossref] [PubMed]
- Xu C, Jin JY, Zhang M, et al. The impact of the effective dose to immune cells on lymphopenia and survival of esophageal cancer after chemoradiotherapy. Radiother Oncol 2020;146:180-6. [Crossref] [PubMed]
- So TH, Chan SK, Chan WL, et al. Lymphopenia and Radiation Dose to Circulating Lymphocytes With Neoadjuvant Chemoradiation in Esophageal Squamous Cell Carcinoma. Adv Radiat Oncol 2020;5:880-8. [Crossref] [PubMed]
- Chin AL, Aggarwal S, Pradhan P, et al. The role of bone marrow and spleen irradiation in the development of acute hematologic toxicity during chemoradiation for esophageal cancer. Adv Radiat Oncol 2018;3:297-304. [Crossref] [PubMed]
- Saito T, Toya R, Yoshida N, et al. Spleen Dose-Volume Parameters as a Predictor of Treatment-related Lymphopenia During Definitive Chemoradiotherapy for Esophageal Cancer. In Vivo 2018;32:1519-25. [Crossref] [PubMed]
- Newman NB, Anderson JL, Sherry AD, et al. Dosimetric analysis of lymphopenia during chemoradiotherapy for esophageal cancer. J Thorac Dis 2020;12:2395-405. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- Noble F, Mellows T, McCormick Matthews LH, et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol Immunother 2016;65:651-62. [Crossref] [PubMed]
- Pivkin IV, Peng Z, Karniadakis GE, et al. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc Natl Acad Sci U S A 2016;113:7804-9. [Crossref] [PubMed]
Cite this article as: Giridhar P, Ramdulari AV, Mallick S, Upadhyay R, Elumalai T, Solipuram V, Venkatesulu P, Chiodo C, Hsieh CE, Venkatesulu B. Impact of radiation related lymphopenia on outcomes in esophageal cancer: a systematic review and meta-analysis of clinical studies. Ann Esophagus 2023;6:41.


