
Hand-sewn anastomosis for minimally invasive laparoscopic Ivor Lewis esophagectomy—how to do it: operative technique and short-term outcomes
Introduction
Esophageal cancer incidence is rapidly increasing in the western world at a rate greater than any other type of solid tumor.
For over a century, esophagectomy has been the mainstay of curative treatment for esophageal cancer and it is standard of care for patients with localized esophageal carcinoma staged as T1sm/N+ or higher.
The development of minimally invasive esophageal surgery has improved postoperative rehabilitation and reduced complications. Although current postoperative mortality has decreased in high-volume centers, complications related to anastomotic and respiratory failure are still significant. Anastomotic complications are one of the most important factors affecting morbidity after esophagectomy. Particularly in Ivor-Lewis minimally invasive esophagectomy (IL-MIE), a wide range of anastomosis techniques have been described, and multiple reports have compared anastomotic complications among different techniques. However, there is insufficient evidence in the literature to definitively recommend one anastomotic technique over another. The advent of robotic surgery and new articulated instruments has brought back attention to manual anastomosis.
In this paper, we describe our consecutive series of patients who underwent IL-MIE using a totally hand sewn anastomosis and we introduce the first use of an articulated needle holder for thoracoscopic suturing in the same technique. We present the following article in accordance with the STROBE reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-46/rc).
Methods
In this observational descriptive case series study, we have evaluated a cohort of 27 consecutive patients with distal esophageal lesions that were offered IL-MIE. The study was conducted in two tertiary care centers; “Favaloro Foundation University Hospital” and “Sanatorio Finocchietto” in Buenos Aires from June 2018 to November 2020 using a prospectively maintained clinical database. Demographic characteristics, pre-operative clinical measurements, perioperative outcomes and postoperative morbidity of the patients were also reported from the database (Table 1).
Table 1
| Characteristics | N | % |
|---|---|---|
| Gender | ||
| Male | 26 | 96.3 |
| Female | 1 | 3.7 |
| Age, years (mean, SD) | 60 (11.2) | – |
| Clinical T stage | ||
| T1 | 7 | 25.9 |
| T2 | 12 | 44.4 |
| T3 | 5 | 18.5 |
| T4 | 3 | 11.1 |
| Clinical N stage | ||
| N0 | 14 | 51.9 |
| N1 | 9 | 33.3 |
| N2 | 2 | 7.4 |
| N3 | 2 | 7.4 |
| Histology | ||
| Adenocarcinoma | 15 | 55.6 |
| Squamous cell carcinoma | 12 | 44.4 |
| Neoadjuvant therapy | ||
| None | 3 | 11.1 |
| Chemoradiotherapy | 17 | 63.0 |
| Chemotherapy | 7 | 25.9 |
Pre-operative tumor staging and nodal disease status were determined by dynamic enhanced computed tomography/positron emission tomography (CT/PET) scan or endoscopic ultrasound, to exclude locally advanced or metastatic disease. IL-MIE using a gastric tube was offered as the operation of choice to all these patients.
This technique is new in our group and represent the first 27 cases of our series. They were all performed by the same surgeon and results have a tendency to improve but are still at the early phase of the learning curve estimated for IL-MIE.
The primary endpoint was technical reproducibility of the anastomosis technique.
Secondary endpoints included leak rate, length of hospital stay, re-intervention rate, mortality rate, and postoperative strictures, all data from the medical record and surgical reports were recorded in a database. Complications related with anastomosis technique were graded according to the “Esophagectomy Complications Consensus Group” (1). Definitions are detailed in Table 2.
Table 2
| Complications | Definition |
|---|---|
| Anastomotic leak | Full thickness GI defect involving esophagus, anastomosis, staple line, or conduit irrespective of presentation or method of identification |
| Type I | Local defect requiring no change in therapy or treated medically or with dietary modification |
| Type II | Localized defect requiring interventional but not surgical therapy, for example, interventional radiology drain, stent or bedside opening, and packing of incision |
| Type III | Localized defect requiring surgical therapy |
| Conduit necrosis | |
| Type I | Focal necrosis of the conduit identified endoscopically |
| Type II | Focal necrosis of the conduit identified endoscopically and not associated with leak |
| Type III | Extensive conduit necrosis |
GI, gastrointestinal.
The study was conducted in accordance with the Declaration of Helsinki (as revises in 2013). The Ethics Committee of the Favaloro Fundation University Hospital approved the protocol [approval number: DDI (1301) 1515 CBE 546/15] and informed consent was waived due to the retrospective nature of the study.
Surgical technique
Under general anesthesia with selective left lung ventilation patients were positioned in prone decubitus. Three thoracoscopic chest ports were placed as follows: at the tip of the right scapula (10 mm, camera port), two intercostal spaces below the first port in the midline between the scapula and the spine (10 mm) and two spaces above the first port following the scapula dorsal edge (5 mm) (Figure 1).
The esophagus was resected en-bloc with extended lymphadenectomy and sectioned above the azygous vein. The gastric conduit was ascended from the abdomen and the conduit tip was resected with a linear 60 mm stapler between the last branch vessel of the right gastric and the first short gastric vessel. The length of the gastric conduit was estimated by the number of staplers used to create it.
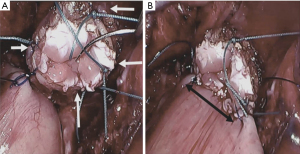
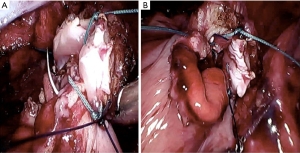
Prior to starting the anastomosis, four cardinal stitches of Ethibond 3.0 were used to consolidate all layers of the esophagus (Figure 2). Placement of the anastomosis in the gastric conduit was estimated by measuring 2 cm from both stapler lines (vertical and transversal) so that the spinal corner of the anastomosis lies next to the right gastric vessel which is the best vascularized portion of the conduit (Figure 3). The anastomosis was constructed with two posterior layers (one external binding both organs and one full thickness, Figure 4) and one anterior layer secured with 3 tension releasing U stitches. All layers were sutured with running pattern using 3.0 auto-adjustable PDS sutures (Stratafix, J&J, USA). Complete wrapping of the anastomosis with omental patch was achieved and secured with Ethibond 3.0 (Figure 5, Video 1).
Locorregional analgesia was instilled with a paravertebral catheter placed under direct vision at the end of the chest procedure. Two right chest drains were routinely placed at the anastomosis level and at the base on top of the right diaphragm. A decompressive nasogastric tube was left for at least 24 h. If there were no clinical signs of leakage feeding was started on PO day 5.
Use of solely articulating mechanical needle holder
The suturing of the anastomosis in the last case of this series was performed with the FlexDex® (FD). FD is a solely mechanical articulating device that combines the functionality of robotic surgery with the relative low cost and simplicity of laparoscopy (Figure 6). It consists of an articulated needle holder with 360 degrees of freedom that precisely translates the surgeon’s hand, wrist and arm movements from outside the patient into corresponding movements of an end-effector inside the chest.
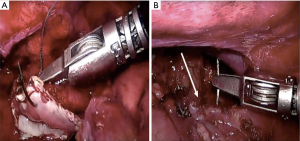
The use of FD does not change the concept of the anastomosis but gives better ergonomic to the surgeon and it should benefit the whole procedure in the long-term.
Statistical analysis
Continuous variables were reported as mean (with standard deviation) or median (with range), percentages were used for discrete characteristics. The hands sewn technique for MIE-IL anastomosis was the indicated treatment. All statistical analyses were performed using SPSS 25.0 (IBM) and Microsoft Excel 2018.
Results
Between June 2018 and November 2020, all 27 patients who underwent MIE with a hand-sewn anastomosis technique (100%) were male with a median age of 60 years (range, 46–75 years).
An end to side anastomosis was created in all patients as described above. Mean time for anastomosis completion including consolidating stitches in the esophagus and the omental wrap was 60 min (40–120 min).
Average number of linear staplers used to create the gastric conduit was 6 and the resected conduit tip length accounted for one stapler.
Anastomotic leakage occurred in 4 patients (14.8%). These included 1 patient (3.7%) with a type I, 2 patients (7.4%) with a type II anastomotic leak and one patient with a type III leak (3.7%). Two patients (8%) had type III necrosis of the conduit. Conservative management with endovac and stents was completed in 3 patients. Reoperation was required in 3 cases (12%). The mean length of stay was 9 days (7–28 days).
One serious complication (myocardial infarction and arrhythmia in the course of acute septic shock) resulting in postoperative mortality occurred in one patient (4%).
Five patients (19%) experienced dysphagia that turned out in anastomotic strictures and required endoscopic dilatation. Outcome are detailed in Table 3.
Table 3
| Variables | N | % |
|---|---|---|
| Leak-type I | 1 | 3.7 |
| Leak-type II | 2 | 7.4 |
| Leak-type III | 1 | 3.7 |
| Necrosis-type I | – | – |
| Necrosis-type II | – | – |
| Necrosis-type III | 2 | 7.4 |
| Reoperation | 2 | 7.4 |
| Length of stay, days, mean [range] | 9 [7–28] | – |
| Stricture | 5 | 18.5 |
| Mortality | 1 | 3.7 |
Discussion
In this study, we describe our initial experience with IL-MIE in patients treated for esophageal and gastroesophageal junction cancer using an intrathoracic totally hands sewn end-to-side anastomosis. We have found that constructing a laparoscopic hand sewn anastomosis is feasible and reproducible and has an acceptable leak and stricture rate even within the learning curve (2).
Even though mortality and morbidity from esophageal cancer surgery is decreasing, it still is a challenging procedure and the complications regarding all types of anastomoses are a source of significant concern (3,4).
Minimally invasive esophagectomy seem to contribute significantly to the recovery by reducing postoperative complications and improving quality of life (5).
Intrathoracic anastomosis reduces the tension on both the gastric conduit and the proximal esophagus, and is accompanied by a relatively well-nourished conduit tissue, which subsequently might lead to a reduced incidence of anastomotic dehiscence (6). Although prior studies have suggested that intrathoracic anastomotic leaks might be associated with greater morbidity and mortality than cervical anastomotic leaks (7), recent reports have shown similar related morbidity regardless of the location (8).
Ease of management of anastomotic leaks has many times driven the argument for location (cervical leaks are easier to manage when those are limited to the neck), but the rates of chest contamination that require surgical intervention after a cervical approach can be as high as 40% (9). With the advent of endovacuum therapy (10), conservative management of IL-MIE complications has increased with only a few of them requiring surgical intervention (11). This has also been the case in our series where two patients with anastomotic leaks were effectively treated using endovac and esophageal stents. On other aspects like stricture, mortality or 5-year survival rates, most approaches are comparable when performed in specialized units and regardless of the anastomosis site. Therefore, the ideal technique remains unclear.
Although we have experience with other anastomosis techniques, the comparison with our previous cases was not reliable because this series was followed prospectively and not all other data were available. Regarding technical details, in our experience the use of mechanical suture in the prone position is uncomfortable, with a lot of manipulation of the gastric tube and the need for a major thoracotomy in case of circular suture.
The leak rates reported with different anastomosis can range from 8% to 40% (8-12). Factors such as body habitus, peripheral vascular disease, neoadjuvant therapy, smoke habit and preop preparation may influence the esophago-gastric anastomotic leak rate. For this reason, evaluating the results in terms of efficacy of each anastomosis requires a complex multifactorial analysis that is beyond the scope of this manuscript.
While we believe that the results and standardization of this technique are promising, the leak rate reported in this manuscript is somewhat higher than the optimal results reported in the best high-volume centers but it still is within the acceptable range. We are aware that esophagectomy requires a learning curve and this can be highly variable according to the published literature. With which, we know that this technique can continue to improve. Considering that a learning curve in this procedure can entitle up to 100 patients, these numbers are likely to improve with time.
Anastomotic strictures are another important technical complication of esophago-gastric anastomoses. The stricture rate using different intrathoracic anastomotic techniques can be difficult to determine because there is no objective scoring system. Therefore, the results of studies comparing the stricture rates vary; there is no consistent trend favoring one technique over the other. In general, authors have reported a spectrum ranging from postoperative dysphagia (22–73%) to radiologically or endoscopically noted narrowing not requiring intervention, to strictures necessitating multiple dilations (13–40%) (13,14). In this study, we have reported a 19% stricture rate that falls in the lower reported range and at the time of final endoscopy, all patients were eating and drinking without limitations. Futures studies may seek whether the tailored construction allowed by the hand-sewn technique may account for these findings.
This series of cases introduce and reports to our knowledge the first world-wide use of FlexDex for an IL-MIE anastomosis. Initial reports in other surgical approaches and training activities demonstrated improved ergonomics and effectiveness suturing at difficult locations. They also have shown shorter operative times, and better ergonomic for the surgeon. Further studies in IL-MIE will compare time and outcomes to assess clear benefit of this novel technology for this specific purpose. While comparisons with standard robotic platforms are lacking, intuitively, cost and accessibility should be benefitted by this approach.
The wide range described for anastomosis options in IL-MIE probably marks how challenging this step becomes in an already challenging surgery. Pros and cons of each approach should be discussed but objective conclusions are hard to reach due to heterogenous sampling and data collection.
In our approach, we hypothesize that hand-sewn construction facilitates extra precision for anastomotic location with less handling and traction from the tissues. Submucosal vascular plexus are known to play an important role in conduit’s viability. Particularly with FlexDex but even with regular laparoscopic tools, the anastomosis could be completed with almost no touching of the conduit that helps to preserve the vascular network (15). Recent publications on robotic IL-MIE have reported the construction of hand-sewn anastomosis with excellent outcomes. Our approach embraces the same concept of the robotic approach but with a simpler, more accessible and cost-effective alternative (16).
Cons for this approach include a steeper learning curve than with mechanical staplers but this could be overcome in the near future with a wider adoption of solely articulating tools like FD. Reproducibility is always harder when surgeon’s skills play a bigger role in a technical performance. However, mechanical staplers also rely on an important background from the operating surgeon for anvil placement or gap suturing.
Limitations of this study include the low number of patients, the short-term follow-up and the lack of detailed timing in each step within the anastomosis. However, it still represents the largest series of patients reported with this technique. While longer follow-up will provide further insight on functionality of the anastomosis, the follow-up reported in this manuscript is acceptable for the proposed endpoints.
Finally, an important strength of this study is that it reports the first detailed technical parameters of a laparoscopic hand-sewn intrathoracic anastomosis. This information could be particularly helpful when training other surgeons.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-46/rc
Data Sharing Statement: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-46/dss
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-46/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-46/coif). The series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” was commissioned by the editorial office without any funding or sponsorship. AN served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from February 2020 to January 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revises in 2013). The Ethics Committee of the Favaloro Fundation University Hospital approved the protocol [approval number: DDI (1301) 1515 CBE 546/15] and informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Tam V, Zenati M, Novak S, et al. Robotic Pancreatoduodenectomy Biotissue Curriculum has Validity and Improves Technical Performance for Surgical Oncology Fellows. J Surg Educ 2017;74:1057-65. [Crossref] [PubMed]
- Sarela AI, Tolan DJ, Harris K, et al. Anastomotic leakage after esophagectomy for cancer: a mortality-free experience. J Am Coll Surg 2008;206:516-23. [Crossref] [PubMed]
- Viklund P, Lindblad M, Lu M, et al. Risk factors for complications after esophageal cancer resection: a prospective population-based study in Sweden. Ann Surg 2006;243:204-11. [Crossref] [PubMed]
- Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc 2012;26:1795-802. [Crossref] [PubMed]
- Gao HJ, Mu JW, Pan WM, et al. Totally mechanical linear stapled anastomosis for minimally invasive Ivor Lewis esophagectomy: Operative technique and short-term outcomes. Thorac Cancer 2020;11:769-76. [Crossref] [PubMed]
- Patil PK, Patel SG, Mistry RC, et al. Cancer of the esophagus: esophagogastric anastomotic leak--a retrospective study of predisposing factors. J Surg Oncol 1992;49:163-7. [Crossref] [PubMed]
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. 2011. In: Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. York (UK): Centre for Reviews and Dissemination (UK); 1995.
- van Rossum PSN, Haverkamp L, Carvello M, et al. Management and outcome of cervical versus intrathoracic manifestation of cervical anastomotic leakage after transthoracic esophagectomy for cancer. Dis Esophagus 2017;30:1-8. [PubMed]
- Goenka MK, Goenka U. Endotherapy of leaks and fistula. World J Gastrointest Endosc 2015;7:702-13. [Crossref] [PubMed]
- Heits N, Bernsmeier A, Reichert B, et al. Long-term quality of life after endovac-therapy in anastomotic leakages after esophagectomy. J Thorac Dis 2018;10:228-40. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Williams VA, Watson TJ, Zhovtis S, et al. Endoscopic and symptomatic assessment of anastomotic strictures following esophagectomy and cervical esophagogastrostomy. Surg Endosc 2008;22:1470-6. [Crossref] [PubMed]
- Campos GM, Jablons D, Brown LM, et al. A safe and reproducible anastomotic technique for minimally invasive Ivor Lewis oesophagectomy: the circular-stapled anastomosis with the trans-oral anvil. Eur J Cardiothorac Surg 2010;37:1421-6. [Crossref] [PubMed]
- Vetter D, Gutschow CA. Strategies to prevent anastomotic leakage after esophagectomy and gastric conduit reconstruction. Langenbecks Arch Surg 2020;405:1069-77. [Crossref] [PubMed]
- de Groot EM, Möller T, Kingma BF, et al. Technical details of the hand-sewn and circular-stapled anastomosis in robot-assisted minimally invasive esophagectomy. Dis Esophagus 2020;33:doaa055.
Cite this article as: Ramirez M, Turchi M, Llanos F, Badaloni A, Nieponice A. Hand-sewn anastomosis for minimally invasive laparoscopic Ivor Lewis esophagectomy—how to do it: operative technique and short-term outcomes. Ann Esophagus 2022;5:22.





