Surgical treatment of Zenker diverticula
Introduction
The first description of a Zenker diverticulum (ZD) goes back to 1769 by Ludlow (1). In 1887, a German pathologist, Friedrich Albert Von Zenker recognized and better characterized the pathophysiology of this illness and that eponym has lasted ever since (2). Although a complete understanding of the pathogenesis of the ZD has not yet been achieved, it is generally accepted that the ZD is due to a disorder in the opening of the upper oesophageal sphincter. The onset of ZDs is related to an increase in intraluminal pressure at the oropharynx during swallowing and insufficient release of the cricopharyngeal muscle resulting in incomplete opening of the upper oesophageal sphincter, which causes the mucosa to protrude through an area of relative weakness of the posterior pharyngoesophageal wall (3). The incidence of Zenker diverticula is estimated between 0.01% and 0.11% (4) and classically occurs in males and the elderly, aged 70 to 80 years (5).
Therapeutic management of the patient with Zenker diverticulum is fundamentally influenced by the presence or absence of symptoms, the size and location of the diverticulum. For asymptomatic diverticula smaller than 1 cm, conservative treatment with periodic radiological checks using esophagograms is indicated (6). Operative treatment should be reserved only for symptomatic patients and for large diverticula (>2 cm), in order to improve the quality of life and avoid complications. Symptoms that would induce a surgical approach to Zenker diverticulum include episodes of aspiration pneumonia or inhalation of food material in the airways, regurgitation of food, dysphagia, dyspepsia, halitosis or a feeling of suffocation. As reported by Shahawy et al. (7), in almost half of patients, aspiration episodes are common.
Two main therapeutic approaches were described in the treatment of this type of diverticula: surgical or endoscopic. Historically, ZD was treated with open surgery (transcervical diverticulectomy, diverticulopexy or diverticular inversion) associated with a more or less extensive longitudinal myotomy of the cricopharyngeal muscle (8,9). Endoscopic approach can be performed using a rigid endoscope or using a flexible instrument to divide the cricopharyngeal muscle fibers forming the septum of the diverticulum and to improve dysphagia and regurgitation. The endoscopic technique was first used for patients in poor general condition, not fit for open surgery or for whom it was too difficult to obtain a good endoscopic exposure (10). Furthermore, a submucosal tunnelling technique similar to that used in per-oral endoscopic myotomy (POEM) has recently been introduced to optimize septal visualization and reduce complication rates. It has been called Z-POEM (POEM for Zenker diverticulum) (11) or STESD (Submucosal Tunnelling Endoscopic Septum Division) (12). A mucosal incision with muscular interruption also known as the “MIMI” approach has been proposed as a modification of the Z-POEM (13).
There is still an open debate on which of the two approaches is best for the patient and how each of them carries risks and benefits but, to our knowledge, no prospective comparative studies were reported. Most of the relevant data suggest that open surgery has a better clinical success rates and a higher complication rate than the endoscopic treatment. Major complications of surgery include damage to the recurrent laryngeal nerve with possible subsequent paralysis of the ipsilateral vocal cord and dysphonia (3%), leak or perforation (3%) and surgical site infection which can in rare cases lead to descending mediastinitis (<2%). The resolution of symptoms with the open approach is approximately 93–95% and the relapse rate of 2.9%. Compared with endoscopic treatments, the morbidity and mortality rates were higher (11% vs. 8.7% and 0.9% vs. 0.4% for the open and endoscopic approach respectively) (14,15).
In this paper we review current literature on surgical approach to Zenker’s diverticula in terms of clinical results and complications rate.
Preoperative evaluation
All patients with ZD must be subjected to a preoperative morphological and functional study.
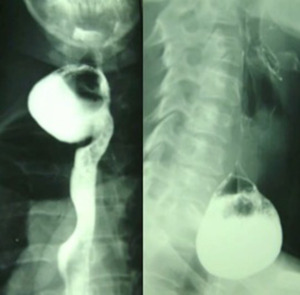
From the morphological point of view, a barium esophagogram and an upper GI endoscopic study are needed to, respectively, highlight the features of the diverticulum (size, neck, barium retention, Figure 1) and exclude the presence of ulcerations or possible neoplastic lesions located in the pouch.
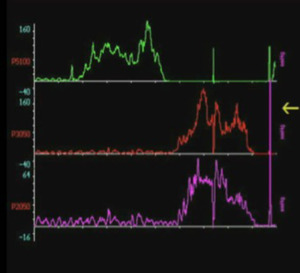
From a functional point of view, oesophageal manometry easily reveals hyper tonus of the upper oesophageal sphincter in all patients (Figure 2); however, the diverticular pouch anteriorly displaced the true oesophageal lumen, and it is not possible to perform oesophageal manometry in all patients, because the manometric tube remains in the pouch. Oesophageal scintigraphy with 99mTc, in these patients, is ideal for examining the motility and speed of peristalsis, stagnation of the tracer at the level of the diverticular sac and a hyper tonus and/or achalasia of the lower pharyngeal constrictor muscle.
The surgical approach
After induction of general anesthesia and endotracheal intubation, the patient’s neck shall be extended by placing a small rolled sheet beneath the shoulders, turning the head toward the right side.
Surgeons usually perform a J incision on the left side of the neck parallel and anterior to the sternocleidomastoid muscle and dissect the platysma and omohyoid muscles. Then sternocleidomastoid muscle and carotid sheath are retracted laterally and the trachea medially; in order to identify and protect the laryngeal nerve, the middle thyroid vein or inferior thyroid artery must be ligated and divided as required. At this point, the esophagus and the fundus of the diverticular pouch are visible.
Traditionally the surgical approach to the diverticulum of Zenker provides two distinct aspects: the treatment of the diverticular sac and the correction of the underlying motor disorder. These are two times of the same intervention, which are carried out based on anatomical, pathophysiological, and functional criteria.
When the diverticulum is larger than 4 cm, it is advisable to perform a resection, preferably after positioning a vascular TA-30 surgical stapler, according to Orringer’s technique (16). If diverticulum measures between 2 and 4 cm, can be suspended suturing it to the preverbal fascia (diverticulopexy), or can be resected as described above. When the pouch is smaller than 1 cm, there is no need for a resection or suspension, because after cricopharyngeal myotomy the small pouch disappears in the mucosal protrusion through the margins of myotomy. Usually, after resection, no reinforcement of the suture line is required.
Independent of the chosen treatment of the pouch, a correct surgical approach of the Zenker diverticulum always provides a complete myotomy of the crico-pharyngeal muscle.
The crico-pharyngeal muscle is easily identifiable from the endoscopic side, but its certain limits escape the surgeon’s eye, especially when patient is under general anestesia and cannot swallow. For this reason, Belsey (17), Orringer (16), and Duranceau (18) separately suggested to perform a myotomy up to 5.0 cm or longer, even if Hiebert (19), in his experience with patients sedated but awake and able to swallow, reported that a 2–3 cm myotomy is safe and very effective.
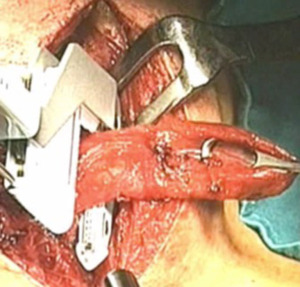
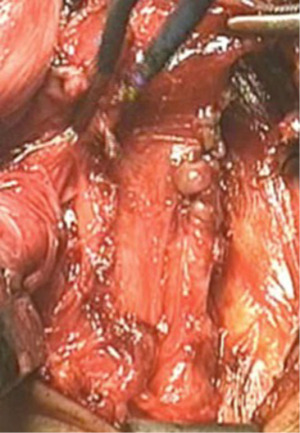
In our 15-year experience (2004 to 2018) at the “A. Gemelli” hospital (Fondazione A. Gemelli IRCCS, Catholic University of Rome), we resected the diverticulum after positioning a vascular TA-30 surgical stapler, according to Orringer’s technique (16) (Figure 3) in 41/45 (91%) patients. No reinforcement of the suture line was performed. The mean dimension of the diverticula was 5.1±1.76 cm. We didn’t perform a diverticulectomy in 4 cases (8,9%), because of the small dimension of the diverticula. Myotomy was performed in all patients and was extended for 5.57±1.56 cm on the left posterolateral face of the esophagus (Figure 4). A small tube is used to drain the wound. All diverticula subjected to surgical excision were analysed at histological examination and in 1 case (2%) an outbreak of carcinoma in situ was found within the diverticular sac.
Results of the surgical treatment
The main outcome of ZD surgical treatment is the resolution of symptoms, in particular of dysphagia. In their large reviews on treatment of ZD, Verdonk and Morton (20) compared the functional results obtained from open surgery (1,990 patients) and endoscopic approach (1,089 patients) and reported that transcervical surgery seemed to offer best results in terms of resolution of dysphagia (95.8%), with a significant lower rate of relapse than endoscopic procedures (4.2% vs. 18.4%, P<0.001). Similarly, Albers and colleagues (14) in their meta-analysis observed that in patients who underwent endoscopic procedure (300 patients), the success rate was 87%, but it was 96% when an open approach was performed (296 patients).
Bhatt et al. (21) in their recent Systematic Review and Network Meta-analysis studied a population of 903 patients arising from 9 cohort studies treated with either laser-assisted diverticulectomy (n=283), transcervical diverticulectomy (n=150), or stapler-assisted diverticulectomy (n=470), calculating the Odd Ratio (OD) for persistent or recurrent symptoms following surgery. Open diverticulectomy with cricopharyngeal myotomy had a statistically lower rate of relapse, persistent or recurrent symptoms following treatment.
In our series, we observed only one patient (2.2%) still complaining of a minimal dysphagia, which was resolved thanks to speech therapist. There were no others episodes of dysphagia or signs of relapse after 2 years. Therefore, the treatment was immediately effective in 97.8% of patients, according to papers previously mentioned.
On the other hand, from an endoscopic point of view, the incidence of post-procedure complications was referred to be higher for patients submitted to the open surgical technique.
The risks of transcervical treatment of Zenker’s diverticulum are partly inherent the more invasive surgical procedure itself, and partly reside in the fact that this disease often afflicts an elderly population. In these patients, even the less severe complication can turns into an important, adverse early or late event.
Mediastinitis, damage to the recurrent laryngeal nerve an esophageal perforation (22) are the most feared complications, but the most common are infections, probably originating from the transfixing stiches of divericulopexy or from the cutline at the neck of the pouch in diverticulectomy.
Moroco et al. (23), in their recent study of the NSQIP Database, analyzed 614 elderly patients submitted to open surgery for ZD, observing a complication rate of 6.7%, readmission rate of 7.2%, and reoperation rate of 6.4%, with a very low mortality rate of 0.3%. These data are significantly better than the overall complication rate of 11% reported in previous studies (20,24). In our series, we observed just one major complication (bleeding) in 45 surgical procedure (2.2%).
Despite the mean age of our patients (65.0±10.9 years) and the inevitable comorbidity of the third age of life, we did not observe any mortality and major complication in our experience. Many studies confirmed our data, demonstrating that peri- and postoperative outcomes are independent of chronological age alone (25-27).
Surgery-related mortality, in the Verdonk and Morton review (20), is low in either method (<0.9%); they reported a morbidity rate of 11% for the transcervical approach (especially hematomas, fistulas and recurrent laryngeal nerve palsy) and 7% for the endoscopic procedures (mediastinitis or subcutaneous emphysema mainly).
In a systematic review and meta-analysis, Howell et al. (28) analyzed 865 patients (106 submitted to open surgery, 310 endoscopic laser procedures, and 449 to endoscopic stapler-assisted technique) obtained from 11 studies. Endoscopic stapler-assisted diverticulectomy showed a lower complication rates but a higher reoperation rate.
Open approach after previous and unsatisfactory endoscopic approach can be inquisitive, but feasible. Contrariwise, endoscopic redo management can be particularly challenging (29,30). Diverticula bigger than 5 cm, in our opinion, need an open surgical approach. The European Society of Gastrointestinal Endoscopy (ESGE) also recommends that emerging treatments for Zenker’s diverticulum, such as Zenker’s peroral endoscopic myotomy (Z-POEM) and tunnelling, must be considered as experimental; these treatments should be offered in a research setting only (30).
Conclusions
There is still an open debate on the best approach for treatment of patients affected by ZD, in terms of risks and benefits.
Open approach seems to be a safe, feasible, and effective option to improve dysphagia and regurgitation, with a very small rate of complications, despite the mean age of the patients. Waiting for prospective comparative studies between surgery and endoscopic treatment, in our opinion, it is mandatory to perform the best choice of treatment according to clinical characteristics of each patient. For big diverticula o redo-surgery after endoscopic failure, open surgery still remains the first choice. In all the other cases, decision must be taken on the base of some factors: comorbidity of the patient, surgical risk, surgeon experience and endoscopist skills.
In the hands of experienced surgeons, major complication rates for this technique can be very low.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Management of Esophageal Perforations and Injuries and Other Benign Diseases”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-2020-25/coif). The series “Management of Esophageal Perforations and Injuries and Other Benign Diseases” was commissioned by the editorial office without any funding or sponsorship. VP served as an unpaid Guest Editor of the series. DN served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from October 2019 to September 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ludlow A. A case of obstructed deglutition from a preternatural dilatation of, and bag formed in, the pharynx. Med Observ Enq 1769;3:85-101.
- Zenker FA, Ziemssen H. Krankheiten des Oesophagus. In: Ziemssen H, editor. Handbuch der speciale Pathologie und Therapie. Leipzig, Germany: FC Vogel, 1877:1-87.
- Cook IJ, Gabb M, Panagopoulos V, et al. Pharyngeal (Zenker's) diverticulum is a disorder of upper esophageal sphincter opening. Gastroenterology 1992;103:1229-35. [Crossref] [PubMed]
- Watemberg S, Landau O, Avrahami R. Zenker's diverticulum: reappraisal. Am J Gastroenterol 1996;91:1494-8.
- Maran AGD, Wilson JA, Al Muhanna AH. Pharyngeal diverticula. Clin Otolaryngol 1986;11:219-25. [Crossref] [PubMed]
- Bizzotto A, Iacopini F, Landi R, et al. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013;33:219-29.
- Shahawy S, Janisiewicz AM, Annino D, Shapiro J. A comparative study of outcomes for endoscopic diverticulectomy versus external diverticulectomy. Otolaryngol Head Neck Surg 2014;151:646-51. [Crossref] [PubMed]
- Payne WS. The treatment of pharyngoesophageal diverticulum: the simple and complex. Hepatogastroenterology 1992;39:109-14.
- Aly A, Devitt PG, Jamieson GG. Evolution of surgical treatment for pharyngeal pouch. Br J Surg 2004;91:657-64. [Crossref] [PubMed]
- Yuan Y, Zhao YF, Hu Y, et al. Surgical treatment of Zenker’s Diverticulum. Dig Surg 2013;30:207-18. [Crossref] [PubMed]
- Ebrahim A, Leeds SG, Clothier JS, et al. Zenker’s diverticulum treated via per-oral endoscopic myotomy. Proc (Bayl Univ Med Cent) 2020;33:233-4. [Crossref] [PubMed]
- Li QL, Chen WF, Zhang XC, et al. Submucosal Tunneling Endoscopic Septum Division: A Novel Technique for Treating Zenker’s Diverticulum. Gastroenterology 2016;151:1071-4. [Crossref] [PubMed]
- Klingler MJ, Landreneau JP, Strong AT, et al. Endoscopic mucosal incision and muscle interruption (MIMI) for the treatment of Zenker’s diverticulum. Surg Endosc 2021;35:3896-904. [Crossref] [PubMed]
- Albers DV, Kondo A, Bernardo WM, et al. Endoscopic versus surgical approach in the treatment of Zenker’s diverticulum: systematic review and meta-analysis. Endosc Int Open 2016;4:E678-86. [Crossref] [PubMed]
- Ishaq S, Hassan C, Antonello A, et al. Flexible endoscopic treatment for Zenker’s diverticulum: A systematic review and meta-analysis. Gastrointest Endosc 2016;83:1076-89.e5. [Crossref] [PubMed]
- Orringer MB. Extended cervical esophagomyotomy for cricopharyngeal dysfunction. J Thorac Cardiovasc Surg 1980;80:669-78.
- Belsey R. Functional disease of the esophagus. J Thorac Cardiovasc Surg 1966;52:164-88.
- Duranceau AC, Jamieson GG, Beauchamp G. The technique of cricopharyngeal myotomy. Surg Clin North Am 1983;63:833-9. [Crossref] [PubMed]
- Hiebert CA. Surgery for cricopharyngeal dysfunction under local anesthesia. Am J Surg 1976;131:423-7. [Crossref] [PubMed]
- Verdonck J, Morton RP. Systematic review on treatment of Zenker’s diverticulum. Eur Arch Otorhinolaryngol 2015;272:3095-107. [Crossref] [PubMed]
- Bhatt NK, Mendoza J, Kaoolgjeri D, et al. Comparison of Surgical Treatments for Zenker Diverticulum - A Systematic Review and Network Meta-analysis. JAMA Otolaryngol Head Neck Surg 2021;147:190-6. [Crossref] [PubMed]
- Shaari CM, Buchbinder D, Costantino PD, et al. Complications of microvascular head and neck surgery in the elderly. Arch Otolaryngol Head Neck Surg 1998;124:407-11. [Crossref] [PubMed]
- Moroco AE, Saadi RA, Patel VA, et al. 30-Day Postoperative Outcomes Following Transcervical Zenker’s Diverticulectomy in the Elderly: Analysis of the NSQIP Database. Otolaryngol Head Neck Surg 2021;165:129-36. [Crossref] [PubMed]
- Mady LJ, Nilsen ML, Johnson JT. Head and neck cancer in the elderly: frailty, shared decisions, and avoidance of low value care. Clin Geriatr Med 2018;34:233-44. [Crossref] [PubMed]
- Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg 2012;215:453-66. [Crossref] [PubMed]
- Milet PR, Mallet Y, El Bedoui S, et al. Head and neck cancer surgery in the elderly—does age influence the postoperative course? Oral Oncol 2010;46:92-5. [Crossref] [PubMed]
- McGuirt WF, Davis SP. Demographic portrayal and outcome analysis of head and neck cancer surgery in the elderly. Arch Otolaryngol Head Neck Surg 1995;121:150-4. [Crossref] [PubMed]
- Howell RJ, Giliberto JP, Harmon J, et al. Open Versus Endoscopic Surgery of Zenker’s Diverticula: A Systematic Review and Meta‐analysis. Dysphagia 2019;34:930-8. [Crossref] [PubMed]
- Weusten BLAM, Barret M, Bredenoord AJ, et al. Endoscopic management of gastrointestinal motility disorders - part 2: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020;52:600-14. [Crossref] [PubMed]
- Costamagna G, Iacopini F, Bizzotto A, et al. Prognostic variables for the clinical success of flexible endoscopic septostomy of Zenker’s diverticulum. Gastrointest Endosc 2016;83:765-73. [Crossref] [PubMed]
Cite this article as: Porziella V, Zanfrini E, Tabacco D, Pogliani L, Vita ML, Petracca-Ciavarella L, Meacci E, Congedo MT, Chiappetta M, Margaritora S, Nachira D. Surgical treatment of Zenker diverticula. Ann Esophagus 2023;6:11.