Endoscopic management of esophageal perforations and tears
Introduction
Oesophageal perforation is a rare, life-threatening condition occurring in 3 out of 100,000 people (1). It arises more commonly in the intra-thoracic oesophageal tract (54%), followed by cervical (27%) and abdominal tracts (19%) (1). Oesophageal perforations are burdened by high morbidity and mortality rate (19.7%, range, 3–67%) (2). The anatomical localization of the oesophagus and its contact with vital structures make the oesophageal injury a challenging scenario. The mediastinum involvement with consequent mediastinitis development, the onset of septic complications and the delay in therapeutic management are negative prognostic factors leading to an increased mortality rate (3). The aetiology can be divided into two main groups: non-iatrogenic and iatrogenic perforations. Among the non-iatrogenic injures, the most common is the spontaneous oesophageal rupture (also known as Boerhaave syndrome), accounting for 15% of all oesophageal perforations, caused by a sudden increase of the intraluminal pressure during vomiting (1). Other etiologies are the traumatic perforation caused by penetrating injuries, foreign bodies impaction on the oesophageal walls and malignancy (1). The occurrence of iatrogenic perforation is raising for the growing use of invasive diagnostic and therapeutic procedures, including both endoscopy and surgery (4). The risk for perforation during flexible endoscopy is low, occurring in 0.03% of diagnostic procedures and increasing during therapeutic endoscopy (5). After endoscopic pneumatic dilatation for achalasia, it ranges between 1% and 6%, the risk for perforation after strictures dilatation occurs in 0.09–2.2%, and 1–5% after sclerotherapy (4). The occurrence of perforation after variceal ligation, although rare, has been described when the mucosa is grasped between the overtube and the endoscope (6). Nowadays, the new advanced endoscopic therapeutic techniques available may increase the occurrence of oesophageal injuries. For example, per-oral-endoscopic-myotomy (POEM) is burdened by mucosal tears and perforation occurring in 4.8% of cases. However, the need for surgery after POEM is rare (0.3%) (7). Moreover, the placement of nasogastric tube, oesophageal stents and Sengstaken–Blakemore tube may else lead to oesophageal injury and perforation (8,9).
The risk for oesophageal perforation during surgery has also been reported. Orthopaedic surgery on the cervical spine is complicated by oesophageal perforation in up to 3.4% of cases (3). Pneumectomy, lung transplant and bronchial artery embolization are associated with thoracic tract oesophageal injuries (5). Anti-reflux surgery and Heller myotomy for achalasia are associated with abdominal tract oesophageal perforation (10).
The therapeutic approach in oesophageal perforation is multidisciplinary and involves radiologists, surgeons and endoscopists. Nowadays, endoscopy is emerging as the first-line treatment modality and offers multiple options applicable to the specific clinical settings. This study aims to review the literature to assess the diagnostic approach and the therapeutic role of endoscopy in oesophageal perforations and tears.
Perforation site and clinical presentation
The intra-thoracic oesophageal tract is the most common site of perforation, followed by cervical and abdominal tracts. In the Boerhaave syndrome, the lower third of the oesophagus is involved, the perforation being located in 80% of cases on the left border with a longitudinal shape (11). Foreign body impaction occurs at the oesophageal narrow tracks, particularly in the cervical tract (5). Iatrogenic perforation generally occurs at the Killian’s triangle, at the oesophageal narrowing nearby the aortic arch and the left bronchus, and at the esophagogastric junction (5). The clinical presentation differs depending on the perforation site. Cervical tract perforation can present with neck pain, subcutaneous emphysema, dysphagia, dysphonia, and odynophagia (5). Physical examination can show swelling of the cervical region, tenderness and crepitus during palpation (1). Thoracic tract perforation can present with vomiting, chest pain, dyspnea, epigastralgy, subcutaneous emphysema and dysphagia (5). The association between vomiting, chest pain and subcutaneous emphysema is called Meckel’s triad and is classically associated with the Boerhaave syndrome. However, it is present in less than 5% of cases (11). Physical examination may show mediastinal crackling on auscultation and signs of pleural effusion (1).
Abdominal tract perforation is associated with abdominal pain irradiating to the shoulder and vomiting (5). Abdominal examination can document signs of peritoneal irritation (1). In case of delayed diagnosis, the clinical presentation is mainly associated with septic complications such as mediastinitis, pleuritis, peritonitis, and sepsis (5).
Diagnostic approach
The clinical presentation is often atypical, requiring a high level of suspicion to perform a proper diagnosis (12). Vermeulen et al., in a metanalysis involving 576 patients, reported that an early diagnosis (defined as a diagnosis performed within 24 hours) decreases the overall mortality, the risk for reintervention and the length of hospital stay (13).
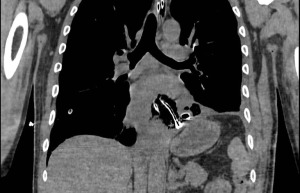
The initial radiological assessment consists of plain radiography, contrast esophagography and chest computed tomography (CT) scan (14). Plain radiography may show an indirect sign of oesophageal perforation. The presence of air in the prevertebral fascia, subcutaneous emphysema in the neck region and tracheal displacement is a sign of cervical perforation (15). Pneumomediastinum, pneumothorax, hydrothorax and pleural effusion are associated with thoracic oesophagus perforation (16,17). The presence of pneumoperitoneum can be detected with chest radiography as free air in the subdiaphragmatic area and can be associated with abdominal oesophagus perforation (14). Contrast esophagography is a useful tool to confirm the diagnosis and to localize the perforation (18). Barium sulphate is generally the most accurate contrast media to detect gastrointestinal perforation, particularly for cervical oesophagus (19). However, barium can be associated with an inflammatory response when spilling out the gastrointestinal lumen in oesophageal perforation that can result in a chemical mediastinitis (1). Moreover, the extravasation of barium can interfere with further radiological investigations (14). To avoid this complication, a water-soluble contrast media is generally preferred, although less accurate (1). The performance of a contrast CT scan is mandatory when an oesophageal perforation is suspected (20). The CT scan on one side allows the diagnosis and proper localization of the injury, on the other provides information on consequent complications such as fluid collections (21). The CT scan may report the presence of oesophageal communication with adjacent structures, oesophageal walls thickening, pneumomediastinum, fluid collections, pleural effusion, pneumothorax, a sign of mediastinitis, pneumoperitoneum (Figure 1) (20).
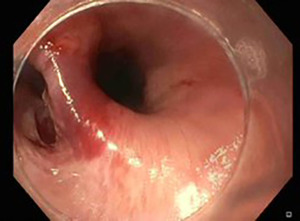
Invasive examination such as gastroscopy should be avoided in case of suspected oesophageal perforation because air insufflation may worsen the oesophageal injury (22). In this setting the diagnostic role of flexible endoscopy is limited to iatrogenic perforation: if during an endoscopic procedure there is the suspicion of oesophageal injury, the endoscopist should avoid air insufflation and should carefully examine the lumen to localize the mucosal damage before removing the gastroscope (Figure 2) (14-22).
Endoscopic management
Classically surgery has been considered the standard of care in oesophageal perforation. However, the surgical approach may be burdened by a high risk for reintervention, morbidity, and mortality (23). Nowadays, the technical advances make flexible endoscopy a useful tool to manage oesophageal injuries as an alternative to surgery or in combination with the percutaneous and the surgical approach.
The endoscopic options include the use of through-the-scope clips (TTSCs), over-the-scope clips (OTSCs), oesophageal stents, endoscopic suturing techniques, endoluminal vacuum therapy (EVT) and locoregional application of stem cells (24).
TTSCs
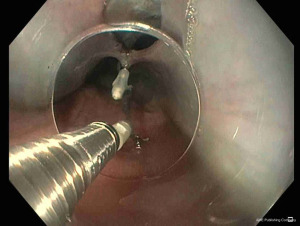
TTSCs were formally designed for hemostasis in gastrointestinal bleeding (25). However, their new designs allow their application in gastrointestinal perforation (26,27). Instinct clip (Cook Medical, Bloomington, IN, USA), QuickPro clip (Olympus, Center Valley, PA) and Resolution clip (Boston Scientific, Natick, MA, USA) are the most frequently applied (28). These different clips can vary for rotation ability, jaw opening capacity, strength, and deployment mechanism (29). In a comparative study, Daram et al. reported that QuickPro and Instinct clips have better rotation capacity when compared with Resolution clips (29). Moreover, Instinct clips are mechanically stronger and consequently associated with better therapeutic outcomes (29). For their limited jaw opening capacity (among 11 and 16 mm), TTSCs are indicated for small perforations and tears measuring few centimeters (30,31) (Figure 3). The first TTSCs should be deployed at the distal edge of the perforation to reduce the risk for accidental endoscope induced clip displacement (28). Further clips can be deployed with a sequential “zipper” technique avoiding the overlap of opposite edges (24) (Figure 4).
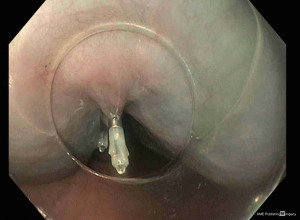
Several case reports and case series reported the efficacy of TTSCs in the management of small oesophageal perforations and tears, including both spontaneous and iatrogenic aetiology. Lázár et al. in a systematic review of the literature reported a success rate of TTSCs in oesophageal perforation healing of 88.8% (30).
Over-the-scope clips
Over-the-scope clips (OTSCs - Ovesco, Tubingen, Germany) were developed to manage full-thickness gastrointestinal defects (32). OTSCs are nitinol clips available in 11, 12 and 14 mm sizes (24). The clip is pre-loaded in a cap that is then mounted at the tip of the endoscope (28). The deployment procedure provides the suction of the defect into the cap, and a twin grasping forceps can be advanced to ensure the correct position of the defect into the cap, the OTSC is finally deployed (33). OTSCs can be used for larger defects (between 10 and 20 mm) compared with TTSCs (34). For injuries larger than 20 mm clips are generally not applicable, and other techniques should be attempted (31). In a multicentric retrospective study, Haito-Chavez et al. reported a total of 188 patients with gastrointestinal fistulae, perforations and leaks treated with OTSCs (35). The overall success rate reported among all GI perforations was 90%, particularly in oesophageal perforation, the defect closure was achieved in 100% of cases (35).
Esophageal stents
Oesophageal stents were developed for palliative management of malignant oesophageal stenosis (36). The development of fully covered stents allowed removability and expanded their application in benign conditions. The oesophageal stents available include fully-covered self-expandable metal stents (FC-SEMS), partially-covered self-expandable metal stents (PC-SEMS), uncovered self-expandable metal stents (U-SEMS) and plastic self-expandable stents (SEPS) (37). Esophageal perforation, SEMS are the most widely used stents (28). The main indications are perforations larger than 20 mm, not manageable with clips and perforation associated with malignancy (31). The placement of a stent allows the diversion of enteral content reducing the risk for contamination of adjacent structures, restarting the enteral nutrition, and promoting the oesophageal re-epithelization (28) (Figure 5). The FC-SEMS presents a plastic polymer around the metallic mesh reducing the risk for tissue ingrowth and allowing an easier removal (37). However, the major concern with FC-SEMS is the risk of stent migration (38). PC-SEMS have been developed to avoid these adverse events. PC-SEMS presents the plastic polymer in the central portion of the stent, but the proximal and distal ends are uncovered, allowing a stronger anchor (37). This design, on the one hand, reduces the risk for migration; on the other, it makes challenging the stent removal. In this setting, a “stent-in-stent” removal technique may be necessary (39). This procedure implies the deployment of FC-SEMS within the PC-SEMS to necrotize the tissue ingrowth, and two weeks later, removal of both stents (39). Other two techniques have been described to reduce the risk for FC-SEMS migration: clips stent fixation and suture stent fixation (40,41).
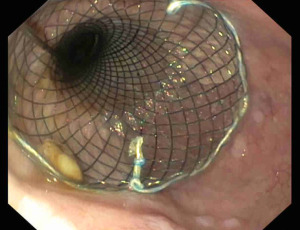
The stent deployment procedure is performed under fluoroscopic guidance to release the stent in the proper position and to reduce the risk for adverse events (24). After stent deployment, a close X-ray monitoring is required for early detection of stent migration (24). The stent is left in place for 2 or 3 weeks. After removal, a contrast esophagogram is mandatory to confirm the sealing of the perforation and to exclude the presence of fistulas (28).
The efficacy of oesophageal stents in perforation management has been demonstrated by several studies. van Boeckel et al., in a systematic review involving 25 studies and 267 patients with oesophageal perforation, reported a technical success rate of 99%, and overall success rate of 85% and a stent-related adverse events rate of 34% (42). Stent migration rate was higher in SEPS and FC-SEMS when compared with PC-SEMS (42). No differences among FC-SEMS, PC-SEMS and SEPS were documented in term of success rate and long-term outcomes (42). Dasari et al., in a meta-analysis of 27 studies, reported similar results with a technical success rate of 91% and the overall success rate of 81% (43). Moreover, they reported a higher risk for stent migration with SEPS and for post-procedural strictures with metallic stents (43). Johnsson et al., in a prospective controlled study, showed that oesophageal stent placement within 24 hours from perforation onset reduced the risk for septic complications (44).
Endoscopic suturing
Since their introduction in 1986, the suturing intraluminal devices faced rapid development and are nowadays available for the management of several gastrointestinal conditions (45). The most commonly used endoscopic suturing devices are OverStitch™ (Apollo Endosurgery, Austin, TX, USA) and OverStitch Sx™ (Apollo Endosurgery, Austin, TX, USA). The first one must be used with a double-channel endoscope, while the second one can be applied to a single-channel endoscope.
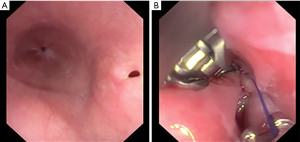
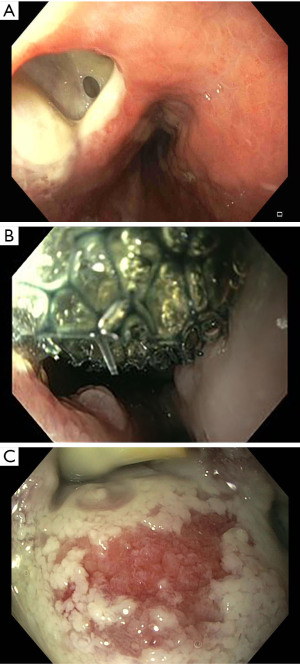
The endoscopic procedure involves the introduction of the device through the oesophageal lumen until the perforation, if necessary, the oesophageal tissue can be approximated with a Helix device (Apollo Endosurgery, Austin, TX, USA), and then a full-thickness suture is performed. Multiple and continuous sutures can be done without endoscope retrieval, allowing direct control and visualization of the oesophageal injury (Figure 6A,B).
In oesophageal perforation, the endoscopic suturing can have two applications: stents fixation and perforation primary closure (46,47).
As stated above, endoscopic suturing can be a useful tool to ensure SEMS fixation and to avoid complication related to stent migration. Sharaiha et al. in a multicentric retrospective study reported 47 patients that underwent endoscopic suturing oesophageal stent fixation with a clinical success rate of 91.4% (48). Ngamruengphong et al. in a retrospective comparative study reported a significantly lower incidence of stent migration in patients undergoing oesophageal stent suturing fixation when compared with not fixated FC-SEMS (33% vs. 16%) (49). Moreover, the endoscopic suturing can be used as a direct treatment performing a primary suture of the perforation. Henderson et al. reported a case series of 3 patients with oesophageal injuries of 15, 25 and 30 mm that were successfully treated by endoscopic suturing (47). In another case series, Sharaiha et al. reported 13 cases of oesophageal perforations, with a size ranging from 25 to 50 mm, treated by endoscopic suturing with a technical and clinical success rate of 100% (48).
EVT
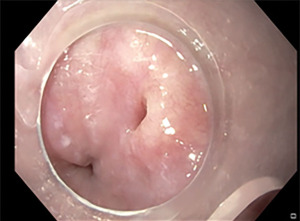
One of the most common complications following oesophageal perforations are fluid collections. EVT is nowadays available as an alternative or in combination with surgical or percutaneous drainage (50). EVT was first introduced to manage rectal post-surgical perforations (51). EVT consist of a polyurethane sponge that can be modelled according to the perforation shape (52). The endoscopic procedure involves the introduction of an overtube through the oesophagus, the connection of the sponge to a drain tube, the placement of the sponge into the cavity of the perforation, the connection of the drain tube to a vacuum pump system that creates a negative pressure and therefore a continuous suction (52) (Figure 7A-7C). The EVT is generally changed every 3–4 days until the perforation is sealed (52). The negative pressure ensures a continuous wound cleaning and promotes the development of granulation tissue (53).
Newton et al., in a systematic review involving 180 patients with oesophageal perforation, reported a success rate of EVT in wound healing of 91% (54). Rausa et al., in a meta-analysis of 4 studies (163 patients) comparing SEMS and EVT in the management of oesophageal perforations, documented that the perforation healing rate is higher in patients treated with EVT than SEMS (pooled odds ratio 5.51, 95% CI 2.11–14.88; P<0.001) (55). Moreover, EVT has a shorter treatment duration and a lower incidence of major complications and mortality when compared with SEMS (55).
Regenerative medicine
Regenerative medicine implies the use of autologous grafts to promote the regeneration of tissues and wounds healing (56). This method is emerging as a new opportunity in the management of gastrointestinal injuries. One of the most used technique is fat grafting that involves harvesting of autologous adipose tissue then injected locally under endoscopic control (57). Fat grafting has been applied in several conditions such as plastic surgery, neurosurgery, head and neck surgery, orthopaedic surgery, and fistulizing Crohn’s disease (58,59). Recently this technique has been described in the management of oesophageal injuries (60). Nachira et al. reported a case series of 5 patients with oesophageal injuries unresponsive to standard treatments, successfully managed by local injection of emulsified autologous adipose tissue (61) (Figure 8). The fistula healing was obtained in all patients within seven days, and the follow-up endoscopy (mean time of follow-up eight months) confirmed the resolution of the perforation in all of cases (61).
Discussion and conclusions
Oesophageal perforation is a challenging scenario that requires a high level of medical attention from the diagnosis to the final treatment. Spontaneous oesophageal injuries are often diagnosed late mostly due to their non-specific clinical presentation thus may be life-threatening. Differential diagnosis includes all conditions causing chest, neck and abdominal pain, subcutaneous emphysema, pleural effusion, vomiting and hematemesis. Clinical examples are myocardial infarction, aortic dissection, pericarditis, pleuritis, pneumonia, pulmonary embolism, Mallory-Weiss tears, and peptic ulcer (1). Iatrogenic perforations are generally diagnosed immediately and are often treated contextually. Once diagnosed, and the patient is stabilized and the perforations is closed, treatment of eventual septic complications and nutritional support are essential (62). Nutrition should be enteral or parenteral and a nasogastric tube should be placed to reduce the risk for contamination from the gastric content or saliva (14). In particular, total parenteral nutrition should be started if a long fasting period is predicted (4). Intravenous broad-spectrum antibiotics and antifungal should be promptly initiated and continued for at least 14 days to reduce the risk for an overinflection of tissues around the oesophageal perforation (1). High dose proton-pump-inhibitors may be useful, particularly in perforation involving the lower third of the oesophagus (1). Close monitoring of general conditions is mandatory for prompt detection of complications and clinical deterioration.
Formerly, surgery was considered the standard of care of oesophageal injuries (63). Currently, a conservative approach is preferred, and endoscopic management is more and more employed (63). Oesophageal perforation is a complex condition requiring a multidisciplinary approach to select the best treatment for the specific patient. Endoscopy offers several types of treatment that should be carefully chosen with specific indications. When the endoscopist faces the oesophageal perforation, few questions should be done in order to choose the proper endoscopic treatment: location, size and eventual presence of collections. The localization of the injury is crucial for the sealing technique to use, for example when the perforation is located in the upper tract of the oesophagus the application of an oesophageal stent may be challenging, and other approaches are generally preferred (64). The presence of a fluid collections may require the use of EVT to promote the peri-oesophageal tissues cleaning and to reduce the risk for systemic septic complications (54) or combined surgical treatment with drainage. The exact size of the perforation must be carefully defined during the esophagogastroscopy and/or radiological exams. Injuries <2 cm in diameter can be approached with endoscopic clips deployment. More specifically, in perforation <1 cm TTSCs can be the first choice, while in perforations with a size between 1cm al 2 cm TTSCs are generally not applicable and OTSCs should be preferred (30,31). In perforations larger than 2 cm clips are not applicable, and other techniques might be required. Oesophageal stents and endoscopic suturing alone or in combination can be used regardless of the perforation size and site (28).
In conclusion, oesophageal perforations for best outcomes require multidisciplinary approach. An internist should be involved to stabilize the patient’s general conditions, an infectivologist should define the proper antimicrobial therapy at the beginning and during the hospital stay, the radiologist should describe the localization of the injury and the eventual presence of local complications or to perform a percutaneous treatment, the surgeon is essential to define the need for more aggressive treatment, and finally the endoscopist is crucial for the final treatment in most of the cases. An early diagnosis, proper medical management and a careful choice of the appropriate treatment may be the key point to improve the success rate in such a complex and life-threatening condition.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Dania Nachira and Venanzio Porziella) for the series “Management of Esophageal Perforations and Injuries and Other Benign Diseases” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-2020-28/coif). The series “Management of Esophageal Perforations and Injuries and Other Benign Diseases” was commissioned by the editorial office without any funding or sponsorship. IB reports personal fees from Boston Scientific, from Endo Tools, personal fees from Apollo Endosurgery, from null, during the conduct of the study. GC reports personal fees from Cook Medical, personal fees from Boston Scientific, personal fees from Olympus, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of this work in ensuring that questions related to the accuracy or integrity of any part of this work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kassem MM, Wallen JM. Esophageal Perforation And Tears 2020 Aug 10. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
- Huber-Lang M, Henne-Bruns D, Schmitz B, et al. Esophageal perforation: principles of diagnosis and surgical management. Surg Today 2006;36:332-40. [Crossref] [PubMed]
- Vrouenraets BC, Been HD, Brouwer-Mladin R, et al. Esophageal perforation associated with cervical spine surgery: report of two cases and review of the literature. Dig Surg 2004;21:246-9. [Crossref] [PubMed]
- Chirica M, Champault A, Dray X, et al. Esophageal perforations. J Visc Surg 2010;147:e117-28. [Crossref] [PubMed]
- Brinster CJ, Singhal S, Lee L, et al. Evolving options in the management of esophageal perforation. Ann Thorac Surg 2004;77:1475-83. [Crossref] [PubMed]
- Lee JG, Lieberman DA. Complications related to endoscopic hemostasis techniques. Gastrointest Endosc Clin N Am 1996;6:305-21.
- Nabi Z, Reddy DN, Ramchandani M. Adverse events during and after per-oral endoscopic myotomy: prevention, diagnosis, and management. Gastrointest Endosc 2018;87:4-17. [Crossref] [PubMed]
- Ahmed A, Aggarwal M, Watson E. Esophageal perforation: a complication of nasogastric tube placement. Am J Emerg Med 1998;16:64-6. [Crossref] [PubMed]
- Kratz JM, Reed CE, Crawford FA, et al. A comparison of endoesophageal tubes. Improved results with the Atkinson tube. J Thorac Cardiovasc Surg 1989;97:19-23.
- Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 2009;249:45-57. [Crossref] [PubMed]
- Korn O, Onate JC, Lopez R. Anatomy of the Boerhaave syndrome. Surgery 2007;141:222-8. [Crossref] [PubMed]
- Wang N, Razzouk AJ, Safavi A, et al. Delayed primary repair of intrathoracic esophageal perforation: is it safe? J Thorac Cardiovasc Surg 1996;111:114-21. [Crossref] [PubMed]
- Vermeulen BD, van der Leeden B, Ali JT, et al. Early diagnosis is associated with improved clinical outcomes in benign esophageal perforation: an individual patient data meta-analysis. Surg Endosc 2021;35:3492-505. [Crossref] [PubMed]
- Lampridis S, Mitsos S, Hayward M, et al. The insidious presentation and challenging management of esophageal perforation following diagnostic and therapeutic interventions. J Thorac Dis 2020;12:2724-34. [Crossref] [PubMed]
- Sarr MG, Pemberton JH, Payne WS. Management of instrumental perforations of the esophagus. J Thorac Cardiovasc Surg 1982;84:211-8.
- Rubesin SE, Levine MS. Radiologic diagnosis of gastrointestinal perforation. Radiol Clin North Am 2003;41:1095-115. [Crossref] [PubMed]
- Okten I, Cangir AK, Ozdemir N, et al. Management of esophageal perforation. Surg Today 2001;31:36-9. [Crossref] [PubMed]
- Bladergroen MR, Lowe JE, Postlethwait RW. Diagnosis and recommended management of esophageal perforation and rupture. Ann Thorac Surg 1986;42:235-9. [Crossref] [PubMed]
- Foley MJ, Ghahremani GG, Rogers LF. Reappraisal of contrast media used to detect upper gastrointestinal perforations: comparison of ionic water-soluble media with barium sulfate. Radiology 1982;144:231-7. [Crossref] [PubMed]
- Maniatis V, Chryssikopoulos H, Roussakis A, et al. Perforation of the alimentary tract: evaluation with computed tomography. Abdom Imaging 2000;25:373-9. [Crossref] [PubMed]
- Backer CL, LoCicero J, Hartz RS, et al. Computed tomography in patients with esophageal perforation. Chest 1990;98:1078-80. [Crossref] [PubMed]
- Pasricha PJ, Fleischer DE, Kalloo AN. Endoscopic perforations of the upper digestive tract: a review of their pathogenesis, prevention, and management. Gastroenterology 1994;106:787-802. [Crossref] [PubMed]
- Sudarshan M, Elharram M, Spicer J, et al. Management of esophageal perforation in the endoscopic era: Is operative repair still relevant? Surgery 2016;160:1104-10. [Crossref] [PubMed]
- Gurwara S, Clayton S. Esophageal Perforations: An Endoscopic Approach to Management. Curr Gastroenterol Rep 2019;21:57. [Crossref] [PubMed]
- Xavier AT, Campos JF, Robinson L, et al. Endoscopic clipping for gastrointestinal bleeding: emergency and prophylactic indications. Ann Gastroenterol 2020;33:563-70. [Crossref] [PubMed]
- Shimizu Y, Kato M, Yamamoto J, et al. Endoscopic clip application for closure of esophageal perforations caused by EMR. Gastrointest Endosc 2004;60:636-9. [Crossref] [PubMed]
- Qadeer MA, Dumot JA, Vargo JJ, et al. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc 2007;66:605-11. [Crossref] [PubMed]
- Saxena P, Khashab MA. Endoscopic Management of Esophageal Perforations: Who, When, and How? Curr Treat Options Gastroenterol 2017;15:35-45. [Crossref] [PubMed]
- Daram SR, Tang SJ, Wu R, et al. Benchtop testing and comparisons among three types of through-the-scope endoscopic clipping devices. Surg Endosc 2013;27:1521-9. [Crossref] [PubMed]
- Lázár G, Paszt A, Man E. Role of endoscopic clipping in the treatment of oesophageal perforations. World J Gastrointest Endosc 2016;8:13-22. [Crossref] [PubMed]
- Paspatis GA, Arvanitakis M, Dumonceau JM, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement - Update 2020. Endoscopy 2020;52:792-810. [Crossref] [PubMed]
- Kocataş A, Somuncu E, Bozkurt MA. Over-the-scope clip application for severe gastrointestinal bleeding, leak, or perforation: A single-center experience. Ulus Travma Acil Cerrahi Derg 2021;27:146-50. [Crossref] [PubMed]
- Iabichino G, Eusebi LH, Palamara MA, et al. Performance of the over-the-scope clip system in the endoscopic closure of iatrogenic gastrointestinal perforations and post-surgical leaks and fistulas. Minerva Gastroenterol Dietol 2018;64:75-83. [Crossref] [PubMed]
- Stavropoulos SN, Modayil R, Friedel D. Closing perforations and postperforation management in endoscopy: esophagus and stomach. Gastrointest Endosc Clin N Am 2015;25:29-45. [Crossref] [PubMed]
- Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc 2014;80:610-22. [Crossref] [PubMed]
- Wang C, Wei H, Li Y. Comparison of fully-covered vs partially covered self-expanding metallic stents for palliative treatment of inoperable esophageal malignancy: a systematic review and meta-analysis. BMC Cancer 2020;20:73. [Crossref] [PubMed]
- Kang Y. A Review of Self-Expanding Esophageal Stents for the Palliation Therapy of Inoperable Esophageal Malignancies. Biomed Res Int 2019;2019:9265017. [Crossref] [PubMed]
- Liang DH, Hwang E, Meisenbach LM, et al. Clinical outcomes following self-expanding metal stent placement for esophageal salvage. J Thorac Cardiovasc Surg 2017;154:1145-50. [Crossref] [PubMed]
- El Hajj II, Imperiale TF, Rex DK, et al. Treatment of esophageal leaks, fistulae, and perforations with temporary stents: evaluation of efficacy, adverse events, and factors associated with successful outcomes. Gastrointest Endosc 2014;79:589-98. [Crossref] [PubMed]
- Mönkemüller K, Martinez-Alcala A, Schmidt AR, et al. The use of the over the scope clips beyond its standard use: a pictorial description. Gastrointest Endosc Clin N Am 2020;30:41-74.
- Wright A, Chang A, Bedi AO, et al. Endoscopic suture fixation is associated with reduced migration of esophageal fully covered self-expandable metal stents (FCSEMS). Surg Endosc 2017;31:3489-94. [Crossref] [PubMed]
- van Boeckel PG, Sijbring A, Vleggaar FP, et al. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 2011;33:1292-301. [Crossref] [PubMed]
- Dasari BV, Neely D, Kennedy A, et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 2014;259:852-60. [Crossref] [PubMed]
- Johnsson E, Lundell L, Liedman B. Sealing of esophageal perforation or ruptures with expandable metallic stents: a prospective controlled study on treatment efficacy and limitations. Dis Esophagus 2005;18:262-6. [Crossref] [PubMed]
- Swain CP, Mills TN. An endoscopic sewing machine. Gastrointest Endosc. 1986;32:36-8. [Crossref] [PubMed]
- Shah ED, Hosmer AE, Patel A, et al. Valuing innovative endoscopic techniques: endoscopic suturing to prevent stent migration for benign esophageal disease. Gastrointest Endosc 2020;91:278-85. [Crossref] [PubMed]
- Henderson JB, Sorser SA, Atia AN, et al. Repair of esophageal perforations using a novel endoscopic suturing system. Gastrointest Endosc 2014;80:535-7. [Crossref] [PubMed]
- Sharaiha RZ, Kumta NA, DeFilippis EM, et al. A large multicenter experience with endoscopic suturing for Management of Gastrointestinal Defects and Stent Anchorage in 122 patients: a retrospective review. J Clin Gastroenterol 2016;50:388-92. [Crossref] [PubMed]
- Ngamruengphong S, Sharaiha RZ, Sethi A, et al. Endoscopic suturing for the prevention of stent migration in benign upper gastrointestinal conditions: a comparative multicenter study. Endoscopy 2016;48:808. [Crossref] [PubMed]
- Schorsch T, Müller C, Loske G. Endoscopic vacuum therapy of anastomotic leakage and iatrogenic perforation in the esophagus. Surg Endosc 2013;27:2040-5. [Crossref] [PubMed]
- Weidenhagen R, Gruetzner K, Weilbach C, et al. Endoscopic vacuum assisted closure of anastomotic leakage after anterior resection of the rectum a new method. Surg Endosc 2008;22:1818-25. [Crossref] [PubMed]
- De Pasqual CA, Mengardo V, Tomba F, et al. Effectiveness of endoscopic vacuum therapy as rescue treatment in refractory leaks after gastro-esophageal surgery. Updates Surg 2021;73:607-14. [Crossref] [PubMed]
- Heits N, Stapel L, Reichert B, et al. Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann Thorac Surg 2014;97:1029-35. [Crossref] [PubMed]
- Newton NJ, Sharrock A, Rickard R, et al. Systematic review of the use of endo-luminal topical negative pressure in oesophageal leaks and perforations. Dis Esophagus 2017;30:1-5. [Crossref] [PubMed]
- Rausa E, Asti E, Aiolfi A, et al. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus 2018; [Crossref]
- Hsu VM, Stransky CA, Bucky LP, et al. Fat grafting’s past, present, and future: why adipose tissue is emerging as a critical link to the advancement of regenerative medicine. Aesthet Surg J 2012;32:892-9. [Crossref] [PubMed]
- Trivisonno A, Nachira D, Boškoski I, et al. Regenerative medicine approaches for the management of respiratory tract fistulas. Stem Cell Res Ther 2020;11:451. [Crossref] [PubMed]
- Trivisonno A, Nachira D, Boškoski I, et al. Autologous Fat Grafting Restores Soft-tissue Contour Deformities after Vascular Anomaly: Widening the Horizons of Employment of Autologous Fat Grafting. Plast Reconstr Surg Glob Open 2019;7:e2518. [Crossref] [PubMed]
- Panés J, García-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet 2016;388:1281-90. [Crossref] [PubMed]
- Porziella V, Nachira D, Boškoski I, et al. Emulsified stromal vascular fraction tissue grafting: a new frontier in the treatment of esophageal fistulas. Gastrointest Endosc 2020;92:1262-3. [Crossref] [PubMed]
- Nachira D, Trivisonno A, Costamagna G, et al. Successful therapy of esophageal fistulas by endoscopic injection of emulsified adipose tissue stromal vascular fraction. Gastroenterology 2021;160:1026-8. [Crossref] [PubMed]
- Bufkin BL, Miller JI Jr, Mansour KA. Esophageal perforation: emphasis on management. Ann Thorac Surg 1996;61:1447-51; discussion 1451-2. [Crossref] [PubMed]
- Voermans RP, Le Moine O, von Renteln DCLIPPER Study Group, et al. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol 2012;10:603-8. [Crossref] [PubMed]
- Gabr A. Sealing the hole: endoscopic management of acute gastrointestinal perforations. Frontline Gastroenterol 2020;11:55-61. [Crossref] [PubMed]
Cite this article as: Schepis T, Boškoski I, Bove V, Landi R, Pontecorvi V, Matteo MV, Mangiola F, Sessa L, Costamagna G. Endoscopic management of esophageal perforations and tears. Ann Esophagus 2023;6:13.