Endoscopic therapy for Barrett’s esophagus: a narrative review of potential complications and their management
Introduction
Barrett’s esophagus (BE) occurs when the normal squamous epithelium in the distal esophagus is replaced by specialized columnar epithelium with intestinal metaplasia (IM) (1,2). This change occurs from chronic exposure to acid reflux. BE is widely recognized as a pre-malignant entity with an increased risk of developing esophageal adenocarcinoma (EAC) (1). The progression from BE to EAC is felt to occur in a stepwise fashion over a number of years, with a small percentage of patients developing histopathologic changes of low-grade dysplasia (LGD), then high-grade dysplasia (HGD), which may progress to intramucosal adenocarcinoma (IMCA) and ultimately invasive adenocarcinoma (2,3). EAC has a dismal prognosis, with 5-year survival rate of <20% (2). For this reason, screening for BE is recommended in patients with multiple risk factors, such as chronic gastroesophageal reflux disease, central obesity, Caucasian race, male gender, older age, smoking and a family history of either BE or EAC (4-7).
For patients with an established diagnosis of BE, current guideline recommendations include surveillance with Seattle protocol forceps biopsies (FB) every 3–5 years (4,5,7). Aside from chronic acid suppression therapy, treatment of non-dysplastic BE is generally not recommended due to the low probability of malignant transformation. The exception to this would be for patients with a family history of BE or EAC, in which endoscopic ablation could be considered on a case-by-case basis.
Current national guidelines recommend endoscopic eradication therapy (EET) for all patients with HGD and mucosal adenocarcinoma (T1a) based on high success rates and relatively lower morbidity compared to esophagectomy. Recent data also suggests EET may be a reasonable option for select patients with submucosal adenocarcinoma (T1b) with favorable features [well or moderately well differentiated lesions with superficial disease (sm1) and no lymphovascular invasion], or for those patients who are poor surgical candidates (2-4). There is some controversary between surveillance versus EET for patients with LGD. The American College of Gastroenterology (ACG) and the American Gastroenterological Association (AGA) recommend consideration for EET, particularly if there is multifocal LGD in a patient with long-segment BE, while the British Society of Gastroenterology suggests endoscopic surveillance (2,4,6). Patients with invasive EAC should be referred for multidisciplinary evaluation with oncology and surgery.
Although generally well tolerated, EET carries a low but real potential for complications including sedation related events, bleeding, perforation, and esophageal stricture (Table 1). This review will briefly discuss the various endoscopic treatment options for BE and focus on the complications associated with these therapeutic procedures and their optimal management. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-96/rc).
Table 1
| Intraprocedural complications | Post-procedural complications |
|---|---|
| Sedation (cardiopulmonary) events | Chest pain |
| Bleeding | Throat discomfort |
| Perforation | Delayed perforation |
| Delayed bleeding | |
| Esophageal strictures |
Assessment of risks of EET for BE
Prior to embarking on endoscopic therapy for BE, a discussion should occur between the physician and the patient (and ideally also the patient’s family/support persons) detailing the rationale, risks, benefits of (and alternatives to) the treatment plan. Patients should also be aware that multiple EET sessions are typically necessary and long-term endoscopic surveillance will be required to ensure durability of treatment response.
Technical aspects of the procedure should also be considered, including anatomical concerns (i.e., significant cervical osteophytes, presence of Zenker’s diverticulum, esophageal webs, rings, and strictures) which may make the passage of endoscopic equipment more challenging and increase the risk of complications, such as bleeding and perforation. Although endoscopic therapy is typically well tolerated, the rate of cardiopulmonary events in upper endoscopy has been reported to range between 1 in 170 and 1 in 10,000 (8). A patient’s overall health, co-morbidities, quality of life and life expectancy should be considered prior to embarking on EET for BE (9).
Endoscopic ablation therapies are low risk for bleeding and therefore antithrombotic guidelines do not recommend interruption in antithrombotic regimen. Endoscopic resection [endoscopic mucosal resection (EMR)/endoscopic submucosal dissection (ESD)] are higher risk for intra-procedural and post-procedural (delayed) bleeding and holding the antithrombotic agent prior to those interventions is recommended if cardiovascular risk is acceptable (10).
Sedation and anesthesia related complications
In general, the most common complications associated with all endoscopic procedures are those related to sedation and anesthesia (8). Endoscopic therapy for BE can be performed using moderate sedation or general anesthesia, depending on the complexity of the procedure, patient comorbidity and available resources and practice patterns. In a retrospective analysis of 120 patients undergoing initial radiofrequency ablation (RFA) treatment under either monitored anesthesia care (MAC) or general endotracheal intubation (GET), sedation-related adverse events (SRAEs) occurred in 32% of patients (11). The most frequent SRAEs were hypotension (23%), hypoxia (7.5%) and arrhythmia (3%). Hypotension and cardiac events occurred more frequently in the GET cases (60% vs. 16%, P<0.001 and 10% vs. 2%, P<0.01, respectively) and hypoxia occurred more frequently in patients undergoing MAC. All of the SRAEs were minor and transient, most commonly treated with fluid bolus, airway maneuvers, and oxygen supplementation. The authors also reported an association between SRAE and increased number of RFA sessions needed to achieve complete endoscopic eradication (11).
EET
Endoscopic therapy for BE has evolved significantly over the past two decades. Several different devices, technologies and techniques are used to perform EET in BE patients (1). In many patients, more than one modality of treatment may be used in the same or sequential treatment sessions. Table 2 illustrates the most commonly used EET platforms in BE patients. Whereas photodynamic therapy (PDT) was one of the original treatment modalities several years ago, it has mostly gone out of favor in modern practice due to its side effects, cost and the evolution of newer, better tolerated and highly effective interventions (12,13).
Table 2
| Endoscopic resection | Endoscopic ablation |
|---|---|
| Endoscopic mucosal resection (EMR) | Radiofrequency ablation (RFA) |
| Endoscopic submucosal dissection (ESD) | Cryoablation |
| Argon plasma coagulation (APC) | |
| Hybrid-APC |
Endoscopic resection
Endoscopic resection is recommended for focal lesions in the BE segment due to an association with higher likelihood of malignancy (2,4). Histopathologic assessment of the resected specimen, including grade of dysplasia and depth of invasion, is important information to guide further therapy.
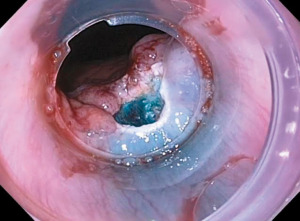
The two most common endoscopic therapies for initial resection of nodular BE are EMR and ESD. EMR is the most frequently used resection technique and can be performed with a banding device or cap-assisted. In band-EMR, the lesion is sucked into the banding device creating a pseudopolyp. Then, using electrocautery and a dedicated snare, the lesion is resected above or below the band. Cap-EMR involves submucosal injection to lift the lesion away from the muscularis propria following which the lesion is sucked into the EMR cap and then resected with a flexible snare using electrocautery (Figure 1).
The safety of EMR has been proven in several studies. In a large retrospective study of 681 patients (2,513 EMRs), no perforations occurred. The rate of bleeding was 1.2% and the rate of stricture was 1.0% (14). Another large study of 1,000 patients showed complete remission in early EAC was achieved in 96.3% of patients who underwent EMR. Major adverse events (AEs) occurred in 1.5% of patients, including bleeding (1.4%) and perforation (0.1%). Thirteen patients (1.3%) developed stenosis requiring dilation (15). The rate of developing post-EMR stricture is significantly higher in patients who undergo circumferential or complete EMR of the BE segment compared to focal EMR followed by endoscopic ablative therapy (16).
ESD has recently emerged as an option for en bloc resection of larger visible lesions (>1.5 cm in size). It is performed by lifting the lesion with submucosal injection, then dissecting along the submucosal plane. Compared to EMR, ESD results in longer procedure times, relatively higher rate of complications (bleeding, perforation, and stricture formation), and is more technically challenging with a steeper learning curve. A recent systematic review and meta-analysis of 11 studies (524 lesions), reported rate of post-ESD bleeding of 1.8% (95% CI, 0.6–3.4%) (17). All cases were managed with endoscopic therapy. The pooled incidence for perforation was 1.5% (95% CI, 0.4–3.0%). No patients required surgery for management of perforation. Nine studies reported rates of esophageal stricture after ESD; the pooled rate was 11.6% (95% CI, 0.9–29.6%). All cases were managed successfully with endoscopic dilation (17).
A meta-analysis of 8 studies comparing EMR versus ESD for superficial esophageal cancer showed significantly higher en bloc and curative resection rates, however the rate of perforation with ESD was higher compared to EMR (OR: 2.19, 95% CI: 1.08–4.47, P=0.03) (18). The rates of esophageal stricture (OR: 1.14, 95% CI: 0.71–1.84, P=0.59) and bleeding (OR: 0.74, 95% CI: 0.20–2.74, P=0.65) were not significantly different in this study (18).
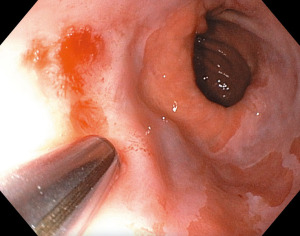
A third novel resection option is the EndoRotor powered endoscopic debridement device (Figure 2). This instrument utilizes a catheter with a rotating inner blade and fixed outer shell to precisely resect targeted tissue. The resected tissue is collected in a tissue trap which can then be analyzed histologically. It is not an en bloc resection and therefore is not ideal for staging purposes, however in a recent study it has been shown to effectively remove fibrotic and difficult to resect lesions in BE and provide meaningful histological samples (19). This study evaluated the use of the EndoRotor device in 8 patients with BE, with or without dysplasia. The most common AE was intraprocedural bleeding (62.5%). Hemostasis was achieved with standard endoscopic interventions in all patients. No delayed bleeding or procedure-related perforations were recorded. One patient reported mild post-procedure chest pain (12.5%) which was self-limited (19). The EndoRotor device was also evaluated for use in 14 patients with early EAC who underwent EMR and then EndoRotor resection for residual BE (20). Nine patients complained of moderate esophageal pain post-procedure, managed with analgesics. Six patients (37.5%) developed intra-procedural bleeding managed with endoscopic therapy. No post-treatment stenosis was seen on 3 months follow-up endoscopy (20).
Endoscopic ablation
Current consensus guidelines recommend endoscopic ablation therapy for patients with dysplastic BE and in patients with early adenocarcinoma after endoscopic resection of the nodular lesion(s) (2,4). Endoscopic ablation has also been used for palliative treatment of advanced esophageal cancer (21).
The earliest modality for endoscopic ablation was PDT. This was performed by intravenously injecting a photosensitizing medication then performing endoscopy with a diffuser/balloon to deliver laser light targeted at the BE mucosa (12). Although this was an effective therapy for ablating dysplastic BE and reducing the incidence of esophageal carcinoma, it is no longer used secondary to high cost, limited availability, and high rate of complications, most notably photosensitivity and post-treatment esophageal strictures (12,13,22). Current options for endoscopic ablative therapy include RFA, cryoablation, argon plasma coagulation (APC), and hybrid-APC.
RFA
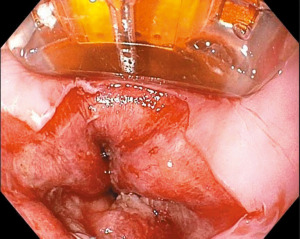
RFA is recommended as first-line therapy for flat dysplastic BE or for BE after resection of nodular lesions based on its ease of use and its efficacy at achieving complete eradiation of dysplasia (CE-D) and complete eradiation of intestinal metaplasia (CE-IM) (2,4,6,7). RFA directly applies radiofrequency energy at high frequency (350–500 kHz) to the targeted mucosa (Figure 3). It can be performed circumferentially with the balloon device (Barrx 360 RFA Balloon catheter, Medtronic, Sunnyvale, CA, USA) or more focally with the Barrx 90 RFA Focal Catheter (Medtronic, Sunnyvale, CA, USA) (3). In 2009, Shaheen et al. published a landmark study known as the AIM Dysplasia study which reported 91% of patients with LGD and 81% of patients with HGD achieved CE-D with RFA, and 78% of patients achieved CE-IM (23).
In addition to treatment efficacy, RFA has a favorable safety profile. The most common AE is stricture (5–10%) (23-25). Post-procedure chest pain, transient dysphagia, and mucosal laceration may also occur. Bleeding and perforation are rare (23). Post-RFA mediastinitis has also been reported (26).
In a recent systematic review and meta-analysis of 37 studies (9,200 patients), the pooled rate of overall AEs after RFA (with or without EMR) was 8.8%. The most common AE was stricture formation with pooled rate of 5.6%. Post-procedure chest pain was reported in 16 studies; pooled rate was 3.8%. Pooled rate of bleeding was 1%. The pooled rate of perforation after RFA ± EMR was 0.6%. The majority of the studies in this meta-analysis included EMR (25). AEs occurred more commonly in patients who received RFA + EMR compared with RFA alone (10.3% vs. 7.5%, P=0.28). Also of note, the rate of stricture in patients who underwent EMR + RFA was 2.5-fold higher versus patients who were treated with RFA alone (25).
Cryoablation
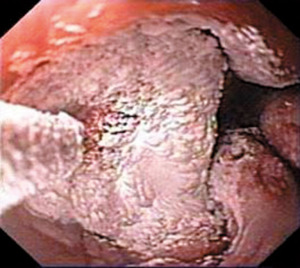
Cryoablation is a relatively newer option available for ablative therapy in BE. Two platforms for delivery are available, spray cryotherapy (CSA Medical, Lexington, MA) and the newer balloon-based cryotherapy (Pentax, Tokyo, Japan). Spray cryotherapy delivers nitrogen at –196 ℃ (–320 ℉) through a catheter which is inserted through the working channel of a standard endoscope (Figure 4). Balloon-based cryotherapy uses nitrous oxide to deliver direct contact ablation of the targeted tissue. Cycles of rapid freezing and slow thawing are performed to achieve adequate ablation.
Cryotherapy is typically well tolerated, with low rate of serious AEs (0–3%). The most common complication is post-treatment strictures, reported in 0–12.5%. Bleeding and perforation are rare (≤1%) (27-31). A recent multicenter prospective study on cryoballoon ablation reported self-limited bleeding in 1 patient on clopidogrel (0.8%). No perforation occurred as a direct result of cryoballoon ablation (31).
In a safety and tolerability study of 77 patients, 22 reported no side effects. In 323 procedures, 17.6% reported chest pain, 13.3% dysphagia, 12.1% odynophagia, and 9.6% sore throat. Esophageal stricture developed in 3 patients, successfully managed with endoscopic dilatation in all cases. Gastric perforation was reported in 1 patient (32). A recent meta-analysis evaluating the safety of balloon cryoablation included 7 studies (548 ablation sessions in 272 patients) and reported stricture rate of 5.8%, mucosal laceration (0.7%), perforation (0.4%) and bleeding (0.4%) (33).
Pain related to cryotherapy is minimal, typically short-lasting, and controlled with oral analgesics. In a recent multicenter, prospective study of 94 patients (35 underwent liquid nitrogen cryotherapy, 59 underwent RFA), cryotherapy was associated with significantly less postprocedural pain compared to RFA (34).
APC
APC is a non-contact technology which uses argon gas to uniformly deliver thermal energy to the tissue adjacent to the probe. It is readily available, low cost, and has been shown in several studies to be effective at ablating Barrett’s mucosa (35,36).
A randomized controlled trial comparing APC with surveillance for management of residual BE after endoscopic resection demonstrated patients who received APC treatment were significantly less likely to develop recurrent neoplasia (P=0.005) (35). AEs including stricture formation requiring bougie dilation (9%), retrosternal pain after APC ablation (12%), dysphagia (18%), and odynophagia (18%). They also reported three patients developed post-procedure fever, one lasting >24 hours (35).
A recent randomized pilot study compared RFA to APC for EET after endoscopic resection of BE with HGD or stage T1 adenocarcinoma (36). Patients were randomized to either receive APC (40 patients) or RFA (36 patients) therapy after undergoing endoscopic resection. Two patients in the APC group developed esophageal stricture post-endoscopic resection (prior to ablation). The rate of post-ablation stricture formation was similar in both groups, (RFA 8.3% vs. APC 8.1%) (36).
Hybrid-APC
Hybrid-APC is a technique which combines submucosal injection of the Barrett’s tissue with APC. In contrast to the high rate of stricture formation after thermal ablation, hybrid-APC has a reported stricture rate of only 2% (37). Other potential complications include retrosternal pressure/pain (12%), heartburn (4%), and odynophagia (4%) (37).
Managing complications of BE endotherapy
Intraprocedural
It has been reported that up to 60% of all upper endoscopy AEs are cardiopulmonary (38). The most common cardiopulmonary events are transient hypoxia and hypotension. When these events occur, the patient should be closely monitored, placed on supplemental oxygen, and stimulated/aroused. If unresponsive, airway protection, administration of a reversal agent, and implementation of a rapid response team with anesthesia support may be necessary. Other complications include respiratory distress, chest pain, wheezing, pulmonary edema, arrhythmias, aspiration pneumonia, and vasovagal reactions (39).
Intraprocedural complications of EET include bleeding and perforation. Both of these occur more commonly with endoscopic resection, but they can also occur during ablation procedures. Bleeding can often be controlled with standard endoscopic therapy. Minor bleeding may be treated with epinephrine injection or APC, whereas spurting arterial bleeds may require use of coagulation grasper and/or endoscopic clips. It is important to note that endoscopic clips may not be preferable as they can make sampling mucosa and subsequent ablation more challenging if they don’t spontaneously fall off. Hemospray (Cook Medical, NC, USA) is a newer modality which may be used for intraprocedural post-endoscopic therapy bleeding. A recent prospective, multicenter study of patients who developed bleeding following endotherapy (not limited to BE) reported 100% rate of immediate hemostasis and a low re-bleed rate of 4% after treatment with hemospray (40).
Gastric and esophageal perforations present with acute onset severe chest pain and/or epigastric pain which may radiate to the back or the left shoulder. Pain may be accompanied by dyspnea, fever, tachycardia, hypotension, and abdominal distension. Imaging including chest (posteroanterior and lateral) and abdominal plain X-rays should be immediately obtained. If X-ray imaging is inconclusive and clinical suspicion for perforation is high, appropriate CT scan should be obtained to confirm perforation and further delineate the extent and nature of the perforation, including whether there is contrast extravasation versus no contrast extravasation (i.e., microperforation). An esophagogram using gastrografin, a water-soluble contrast agent, may also be helpful in the diagnosis of esophageal perforation. Gastrografin is preferred to barium contrast to prevent mediastinitis, a known complication from barium-related contrast extravasation.
Gastric and esophageal perforations can be managed endoscopically (non-surgically) if recognized early, the defect is small and the patient is hemodynamically stable. Through-the-scope (TTS) clips can be considered for defects <1 cm, and over-the-scope clips (OTSC) can be considered for defects up to 3 cm. Endoscopic suturing and esophageal stents have also been used successfully as well (8,41). Delayed presentation/recognition of perforation, large perforations, and/or hemodynamic instability requires broad-spectrum antibiotics, intravenous proton pump inhibitor, fluid resuscitation, radiographic evaluation and multidisciplinary discussion. Teamwork and quick medical decision making are critical in these events. A multidisciplinary approach is often helpful in achieving the best outcomes.
Post-procedural
Esophageal strictures resulting from endoluminal therapy are the most common complication, as outlined above (Figure 5). Patients often present with complaint of dysphagia. Asymptomatic patients may have a post-treatment stricture found on repeat endoscopy and require dilation in order to safely and effectively perform subsequent ablation therapy. Post-treatment esophageal strictures are usually amenable to Bougie or balloon dilation, although multiple sessions may be required for complete resolution (8). Local steroid injection may be used during dilation for persistent strictures despite multiple attempts at dilation (42). Mitomycin C, an antineoplastic agent, has also been used in conjunction with, or as an alternative to, steroid injections for refractory strictures, however data regarding the efficacy and safety of this treatment modality is limited. Occasionally, fully covered removable esophageal stents may be needed to remodel the stricture. Biodegradable stents may also represent a treatment option. Availability and lack of robust data currently limits its use; however these stents maintain radial force for 6–8 weeks and then slowly disintegrate within 12 weeks, potentially reducing the need for repeat endoscopic treatments. Incisional therapy has also been described to help remodel short (1–2 cm) esophageal strictures which do not respond to traditional therapy. Finally, if all these therapies fail to provide durable relief, surgery can be considered, although this is rarely necessary (42).
Delayed presentation of perforation is a rare but serious complication. Multidisciplinary evaluation is recommended with most cases requiring surgical intervention. Delayed bleeding may occur, especially after endoscopic resection. This can usually be treated with endoscopic hemostasis as previously described.
Chest pain and throat discomfort can be managed with liquid acetaminophen, with or without codeine, and a lidocaine slurry. Patients with severe chest pain and fever post-procedure require close observation. Further diagnostic evaluation may be necessary to rule-out severe complications, such as esophageal or gastric perforation.
There is concern that residual BE could be buried beneath neosquamous epithelium following ETT. The clinical significance of this is not clear as there are also reports of buried metaplasia in patients who have not undergone ablation, and in fact some studies suggest buried metaplasia is found less often in patients after RFA (43,44).
Summary/take home points
EET is highly effective at treating dysplastic and early neoplastic BE, especially when multimodal therapy is used and individualized to the patient. While the rate of serious complications is low, there is a potential for bleeding, perforation, esophageal stricture formation, and cardiopulmonary events. Risk assessment including patient’s co-morbidities, anatomic considerations, and antithrombotic therapy should be performed and considered as part of this complex decision-making process.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Madhav Desai) for the series “Endoscopic Therapy for Barrett’s Esophagus” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-96/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-96/coif). The series “Endoscopic Therapy for Barrett’s Esophagus” was commissioned by the editorial office without any funding or sponsorship. VK reports serving as consultant with Steris, Inc. and has received personal payments within the last 36 months. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iyer PG, Kaul V. Barrett esophagus. Mayo Clin Proc 2019;94:1888-901. [Crossref] [PubMed]
- Wani S, Qumseya B, Sultan S, et al. Endoscopic eradication therapy for patients with Barrett's esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907-31.e9. [Crossref] [PubMed]
- Sharma P, Shaheen NJ, Katzka D, et al. AGA clinical practice update on endoscopic treatment of Barrett's esophagus with dysplasia and/or early cancer: expert review. Gastroenterology 2020;158:760-9. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol 2016;111:30-50; quiz 1. [Crossref] [PubMed]
- Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc 2019;90:335-59.e2. [Crossref] [PubMed]
- Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7-42. [Crossref] [PubMed]
- Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011;140:1084-91. [Crossref] [PubMed]
- Ben-Menachem T, Decker GA, Early DS, et al. Adverse events of upper GI endoscopy. Gastrointest Endosc 2012;76:707-18. [Crossref] [PubMed]
- Enslin S, Kaul V. Barrett's esophagus management in the elderly: principles and best practice. Curr Gastroenterol Rep 2020;22:37. [Crossref] [PubMed]
- Acosta RD, Abraham NS, Chandrasekhara V, et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 2016;83:3-16. [Crossref] [PubMed]
- Mizrahi M, Sengupta N, Pleskow DK, et al. Minor anesthesia-related events during radiofrequency ablation for Barrett's esophagus are associated with an increased number of treatment sessions. Dig Dis Sci 2016;61:1591-6. [Crossref] [PubMed]
- Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62:488-98. [Crossref] [PubMed]
- Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc 2007;66:460-8. [Crossref] [PubMed]
- Tomizawa Y, Iyer PG, Wong Kee Song LM, et al. Safety of endoscopic mucosal resection for Barrett's esophagus. Am J Gastroenterol 2013;108:1440-7; quiz 8. [Crossref] [PubMed]
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-60.e1. [Crossref] [PubMed]
- Gerke H, Siddiqui J, Nasr I, et al. Efficacy and safety of EMR to completely remove Barrett's esophagus: experience in 41 patients. Gastrointest Endosc 2011;74:761-71. [Crossref] [PubMed]
- Yang D, Zou F, Xiong S, et al. Endoscopic submucosal dissection for early Barrett's neoplasia: a meta-analysis. Gastrointest Endosc 2018;87:1383-93. [Crossref] [PubMed]
- Guo HM, Zhang XQ, Chen M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol 2014;20:5540-7. [Crossref] [PubMed]
- Kaul V, Diehl D, Enslin S, et al. Safety and efficacy of a novel powered endoscopic debridement tissue resection device for management of difficult colon and foregut lesions: first multicenter U.S. experience. Gastrointest Endosc 2021;93:640-6.
- Knabe M, Blosser S, Wetzka J, et al. Non-thermal ablation of non-neoplastic Barrett's esophagus with the novel EndoRotor(R) resection device. United European Gastroenterol J 2018;6:678-83. [Crossref] [PubMed]
- Kachaamy T, Prakash R, Kundranda M, et al. Liquid nitrogen spray cryotherapy for dysphagia palliation in patients with inoperable esophageal cancer. Gastrointest Endosc 2018;88:447-55. [Crossref] [PubMed]
- Eluri S, Shaheen NJ. Barrett's esophagus: diagnosis and management. Gastrointest Endosc 2017;85:889-903. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Visrodia K, Zakko L, Wang KK. Radiofrequency ablation of Barrett's esophagus: efficacy, complications, and durability. Gastrointest Endosc Clin N Am 2017;27:491-501. [Crossref] [PubMed]
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett's esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:1086-95.e6. [Crossref] [PubMed]
- Yoon SS, Rivera R, Antignano L, et al. A case of mediastinitis after radiofrequency ablation for Barrett's esophagus. Gastrointest Endosc 2011;74:1407-8. [Crossref] [PubMed]
- Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett's esophagus. Endoscopy 2015;47:591. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Gosain S, Mercer K, Twaddell WS, et al. Liquid nitrogen spray cryotherapy in Barrett's esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc 2013;78:260-5. [Crossref] [PubMed]
- Dumot JA, Vargo JJ 2nd, Falk GW, et al. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc 2009;70:635-44. [Crossref] [PubMed]
- Canto MI, Trindade AJ, Abrams J, et al. Multifocal cryoballoon ablation for eradication of Barrett's esophagus-related neoplasia: a prospective multicenter clinical trial. Am J Gastroenterol 2020;115:1879-90. [Crossref] [PubMed]
- Greenwald BD, Dumot JA, Horwhat JD, et al. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus 2010;23:13-9. [Crossref] [PubMed]
- Westerveld DR, Nguyen K, Banerjee D, et al. Safety and effectiveness of balloon cryoablation for treatment of Barrett's associated neoplasia: systematic review and meta-analysis. Endosc Int Open 2020;8:E172-8. [Crossref] [PubMed]
- Solomon SS, Kothari S, Smallfield GB, et al. Liquid nitrogen spray cryotherapy is associated with less postprocedural pain than radiofrequency ablation in Barrett's esophagus: a multicenter prospective study. J Clin Gastroenterol 2019;53:e84-90. [Crossref] [PubMed]
- Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett's epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6-12. [Crossref] [PubMed]
- Peerally MF, Bhandari P, Ragunath K, et al. Radiofrequency ablation compared with argon plasma coagulation after endoscopic resection of high-grade dysplasia or stage T1 adenocarcinoma in Barrett's esophagus: a randomized pilot study (BRIDE). Gastrointest Endosc 2019;89:680-9. [Crossref] [PubMed]
- Manner H, May A, Kouti I, et al. Efficacy and safety of Hybrid-APC for the ablation of Barrett's esophagus. Surg Endosc 2016;30:1364-70. [Crossref] [PubMed]
- Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol 2016;30:705-18. [Crossref] [PubMed]
- Sharma VK, Nguyen CC, Crowell MD, et al. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc 2007;66:27-34. [Crossref] [PubMed]
- Hussein M, Alzoubaidi D, Serna A, et al. Outcomes of Hemospray therapy in the treatment of intraprocedural upper gastrointestinal bleeding post-endoscopic therapy. United European Gastroenterol J 2020;8:1155-62. [Crossref] [PubMed]
- Paspatis GA, Dumonceau JM, Barthet M, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2014;46:693-711. [Crossref] [PubMed]
- Siersema PD. How to approach a patient with refractory or recurrent benign esophageal stricture. Gastroenterology 2019;156:7-10.
- Gray NA, Odze RD, Spechler SJ. Buried metaplasia after endoscopic ablation of Barrett's esophagus: a systematic review. Am J Gastroenterol 2011;106:1899-908; quiz 909. [Crossref] [PubMed]
- Zhou C, Tsai TH, Lee HC, et al. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos). Gastrointest Endosc 2012;76:32-40. [Crossref] [PubMed]
Cite this article as: Enslin S, Tariq R, Kaul V. Endoscopic therapy for Barrett’s esophagus: a narrative review of potential complications and their management. Ann Esophagus 2023;6:9.