
Neoadjuvant versus adjuvant chemoradiotherapy for esophageal carcinoma: a systematic review and meta-analysis
Introduction
Esophageal carcinoma (EC) is characterized by easy metastasis to lymph nodes and the haematological system even at an early stage. The 5-year survival is approximately 20% for resectable EC with surgery alone (1,2). To improve the outcomes, multidisciplinary treatments have been studied worldwide. However, chemotherapy or radiotherapy alone combined with surgery failed to show a significant benefit on survival (3). Chemoradiotherapy was recommended for most cancers because chemotherapy can not only control systematic metastases but also exhibit a radio-sensitizing effect when concurrent chemoradiotherapy was used. Therefore, chemoradiotherapy combined with surgery should improve the chance of curative treatment. However, the optimal timing of chemoradiotherapy is still controversial for EC.
Neoadjuvant chemoradiotherapy (NCRT) followed by surgery (NCRT + S) has been studied for several decades and most randomized controlled trials (RCTs) have shown that there was no significant survival benefit before the 21st century. However, these trials were criticized for low samples, inadequate trial design and poor treatments. The successful Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) trial reported that there was a significant survival benefit in the NCRT + S group, with a hazard ratio (HR) of 0.657 (95% CI, 0.495–0.871; P=0.003) compared to surgery alone (4). The overall survival (OS) benefits were further confirmed after long-term follow-up for both squamous cell carcinoma (SCC) and adenocarcinoma (AC) subtypes (5). Most subsequent trials supported this result and NCRT + S is now recommended for locally advanced EC in many countries. In contrast, there were limited RCTs about adjuvant therapies (6). Many retrospective studies have shown that adjuvant chemoradiotherapy after upfront surgery (S + ACRT) can improve survival and decrease recurrences, especially for patients with pathological T3/4 or N1-3 stage, larger tumor size, poorly differentiated tumors, and R1/2 resections (7-9). A meta-analysis also demonstrated that S + ACRT yielded a significant survival benefit with tolerable toxicity for EC (10). Hence, S + ACRT remains another potential option for EC. The only published CRT comparing NCRT + S with S + ACRT also showed that there was no significant survival difference (11).
The debate about the optimal timing of chemoradiation combined with surgery will continue. This study reviewed all related studies comparing NCRT + S with S + ACRT and performed a meta-analysis to compare their efficacy and safety to identify more evidence for multimodal treatment of EC.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/aoe-20-46).
Methods
Identification of studies
The inclusion criteria for the literature search were defined using the PICOS approach (Population: patients diagnosed with resectable EC; Intervention: NCRT + S; Control: S + ACRT; Outcome: OS, progression-free survival, R0 resection rate and perioperative complications; Study Design: RCTs and non-RCTs). Electronic databases, including PubMed, Scopus, EMBASE, Web of Science and the Cochrane Library were searched for relevant studies until March 2019. The search terms were (“esophageal” OR “oesophageal” OR “esophagus” OR “oesophagus”) AND (“cancer” OR “carcinoma” OR “tumor” OR “neoplasm”) AND ((“neoadjuvant” OR “preoperative” OR “pre”) AND (“adjuvant” OR “postoperative” OR “post”)) OR (“perioperative” OR “peri”)) AND (“chemoradiotherapy”). All the retrieved studies were screened in Endnote X8.1 by two investigators. The reference lists of included studies, meta-analyses and systematic reviews were also manually searched to identify any eligible studies comparing the efficacy of EC between the NCRT + S group and the S + ACRT group directly.
The following study selection criteria were applied: (I) esophageal SCC or adenocarcinoma; (II) data on OS must be reported; (III) only articles in English were eligible.
Data extraction
The following information was extracted: first author; country; year of publication; data period; tumor stage; number of patients; treatment regimens; follow-up time; and outcomes including survival, R0 resection rate and complications. Data extraction was performed independently by two researchers (Mei Kang and Li Zhang). Yichun Wang resolved discrepancies.
Assessing the risk of bias and grading the quality of evidence
Methodological quality/risk of bias was assessed using the Cochrane Risk of Bias tool (12) and Newcastle-Ottawa Scale (NOS) (available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm). The quality of the RCT was assessed using the Cochrane handbook for systematic reviews of interventions. A value of ‘‘high’’, ‘‘low’’ or ‘‘unclear’’ to the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias. A trial with a high risk of bias for anyone or more key domains was considered at ‘‘high risk’’. A trial with a low risk of bias for all key domains was considered at ‘‘low risk’’. Otherwise, it was considered ‘‘unclear’’. The NOS was used to assess the quality of nonrandomized studies. There were eight items categorized into three dimensions: selection, comparability and outcome (cohort studies) or exposure (case-control studies). The results of the NOS range from zero to nine stars. The quality of individual studies was independently assessed by two reviewers (Mei Kang and Li Zhang) and discrepancies were resolved by Yichun Wang.
Statistical analysis
Meta-analysis was performed with STATA version 15.1 software. The primary outcome was OS, and the secondary outcomes were progression-free survival (PFS), R0 resection rate and perioperative complications. The summary statistics were estimated by the HRs or odds ratios (ORs) with their 95% CIs. When HR was not available, they were estimated using the method described by Parmar et al. (13). The statistical heterogeneity of each study was assessed by the chi-square (χ2) and I-square (I2) tests. Significant heterogeneity was confirmed if P≤0.1 or I2>50%. If there was no significant heterogeneity between the included studies, a fixed-effects model was adopted. Otherwise, a random-effects model was employed. A funnel plot was used to detect publication bias among the primary outcomes. P<0.05 was considered statistically significant.
Results
Study selection
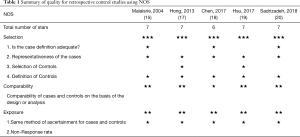
An overview of the literature selection shown in Figure 1. After the elimination of duplicates or irrelevant papers, twenty-one studies were eligible for final assessment. Among them, eleven studies were repeated data, two studies had no HRs of OS or survival curves, and one study did not meet the standard treatment regimens comparing NCRT + S with S + ACRT after reading the full-text paper (14). One study was found by searching the references of relevant reviews and meta-analyses (15). Finally, eight studies involving a total of 1601 patients (763 patients with NCRT + S and 838 patients with S + ACRT) were included in our meta-analysis (11,15-21). Two studies were not available to analyse the quality because they were published as abstracts (16,21). Five nonrandomized studies scored 6 stars or more according to the NOS (Table 1) (15,17-20) and the RCT had a low risk of bias (11). Hence, there was no study of low quality.
Full table
Characteristics of eligible studies
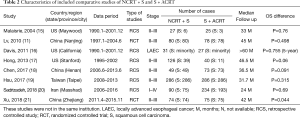
The included studies consisted of two prospective RCTs (11,21) and six retrospective control studies (15-20). Their characteristics are presented in Table 2. Most studies were conducted in Asian countries and region(s) (11,18-21), including three in China (11,18,21), one in Taiwan (19) and one in Iran (20). Four studies enrolled 1,001 patients with SCC only (11,18,19,21). For other studies, one enrolled 324 patients with SCC accounted for the majority (20) and three enrolled 276 patients with AC accounted for the majority (15-17).
Full table
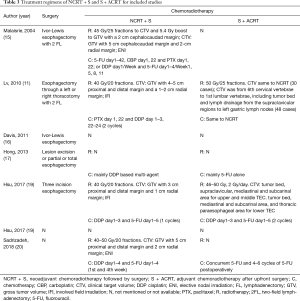
The chemoradiotherapy regimens of the eight studies are shown in Table 3. Cisplatin (DDP) plus fluorouracil (5-FU) was the most commonly used chemotherapy regimen. Other chemotherapy regimens included DDP plus paclitaxel (PTX), carboplatin (CBP) plus PTX or DDP plus 5-FU plus PTX. For radiotherapy in patients with NCRT, the clinical target volume encompassed the gross tumor with craniocaudal margins of 3–5 cm and transversal margins of 1–2 cm, and elective nodal irradiation or involved field irradiation of regional lymph nodes. A total dose of 40–50.4 Gy for 4–5 weeks was commonly used. For radiotherapy in patients with adjuvant chemoradiotherapy, there was no uniform clinical target volume. Elective irradiation of the supraclavicular region, mediastinal region, left gastric region and tumor bed with a total dose of approximately 50 Gy for 5 weeks was performed in these studies. Ivor-Lewis esophagectomy was the major surgical procedure. However, many details of the surgical procedure could not be found.
Full table
Overall survival
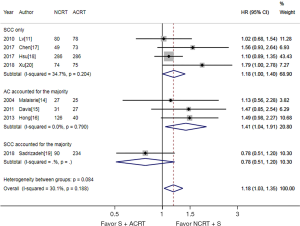
As shown in Figure 2, the meta-analysis suggested that NCRT + S was associated with a significantly better OS, with a pooled HR of 1.18 (95% CI, 1.03–1.35). There was no significant heterogeneity detected among studies (I2=30.1% and P=0.188). Survival benefits were also observed in both patients with SCC and SCC accounted for the majority, and patients with AC accounted for the majority. When we only focused on SCC, NCRT + S was also associated with a significantly better OS, with a pooled HR of 1.18 (95% CI, 1.00–1.40, I2=34.7% and P=0.204).
Progression-free survival
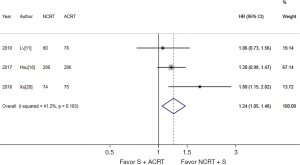
Three studies (879 patients) were available for PFS analysis (11,19,21). As shown in Figure 3, NCRT + S was associated with a significantly better PFS. The pooled HR was 1.24 (95% CI, 1.05–1.46). There was no significant heterogeneity detected among studies (I2=41.2% and P=0.183).
Complication and R0 resection rate
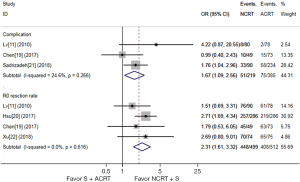
As shown in Figure 4, 3 studies (604 patients) (11,18,20) reported complications, and 4 studies (1,011 patients) (11,18,19,21) reported the R0 resection rate. Though NCRT + S was associated with a higher R0 resection rate (OR, 2.31, 95% CI, 1.61–3.32), it was also associated with a higher incidence of complications (OR, 1.67, 95% CI, 1.09–2.56). There was no significant heterogeneity observed among studies (I2=0.0% and 24.6%, P=0.616 and 0.266 respectively).
Publication bias and sensitivity analysis
We used the leave-one-out approach to evaluate whether any single study had a remarkable impact on the pooled HRs for OS. The results of the sensitivity analysis demonstrated a robust conclusion (Figure 5). The funnel plots and the analysis with Egger’s test (P=0.361) implied no significant publication bias based on the pooled HRs of OS (Figure 6).
Discussion
Surgery remains the cornerstone of curative treatment for resectable EC. To improve the poor survival of surgery alone for EC, the treatment has evolved into multidisciplinary therapy. Due to the unsuccessful or even poor results of adjuvant radiotherapy after surgery (22-26), the focus of radiotherapy or chemoradiotherapy shifted to neoadjuvant therapies from the late 20th centuries. Neoadjuvant chemotherapy or chemoradiotherapy followed by surgery was associated with a survival benefit compared to surgery alone (27-29), and NCRT + S seemed to provide superior OS compared to neoadjuvant chemotherapy (29). Therefore, NCRT + S is accepted in many countries. Though the role of adjuvant therapies is not recommended, they should be considered for patients with pathological upstaged clinical early EC who did not receive neoadjuvant therapies or patients with resectable locally advanced EC who received upfront surgery. A meta-analysis confirmed that S + ACRT yielded a significant survival benefit compared to surgery alone (10). The published RCT also suggested that S + ACRT can provide a benefit in PFS and OS in patients with EC compared to surgery alone (11). Moreover, previous poor radiotherapy technologies, nonstandard irradiation fields and lack of safe and effective chemotherapy regimens should also be taken into consideration for the poor efficacy or serious complications of adjuvant therapies (22-26). Therefore, S + ACRT should be another potential combined modality therapy for selected EC.
Most retrospective studies (15-20) and the published RCT (11) suggested that there were no significant differences in OS between NCRT + S and S + ACRT. However, most studies showed a trend towards survival benefits in NCRT + S, except for one study (20). Only one study showed significant survival benefits in NCRT + S (21). Our meta-analysis confirmed that NCRT + S can significantly improve OS (HR, 1.23, 95% CI, 1.09–1.40) and PFS (HR, 1.38, 95% CI, 1.19–1.60) compared to S + ACRT. Subgroup analysis also showed that NCRT + S may be better than S + ACRT for both SCC and AC. However, the number of patients with AC in these studies was low. The studies that enrolled patients with AC accounted for the majority were conducted in Western countries where lower thoracic EC and carcinoma of the esophagogastric junction was prominent. Hence, the treatment principle of esophageal AC may be similar to that of AC of esophagogastric junction (30). There was only one pooled study reporting the treatment of NCRT + S versus S + ACRT for clinical stage II and stage III EC separately, thus we could not perform subgroup analysis of stage (18).
The R0 resection rate can be improved for EC after NRCT due to an apparent downstage and a pathological complete response rate in almost one-third of patients. Additionally, NCRT may eliminate potential micrometastasis at an earlier time. These advantages may account for the survival benefit of NCRT + S. However, NCRT may also delay the time of surgery for patients who are not sensitive to NCRT. Another possible advantage for NCRT is the unchanged anatomy of the esophagus and adjacent tissues and organs, which can facilitate the delineation of the radiotherapy target volume. However, it is still difficult to design a suitable radiotherapy plan for EC because of the complex lymphatic drainage of the esophagus (31). Involved-field irradiation is commonly used for NCRT, which means that radiotherapy is mainly used to control visible lesions to facilitate surgery. To these points, preoperative radiotherapy may have little benefit for some early stage EC (stage I and II). For clinical T2N0 EC, NCRT + S did not significantly improve outcomes compared with surgery alone (32). One RCT also demonstrated that NCRT + S cannot improve the R0 resection rate or survival in patients with stage I or II EC (33). Taken together, NCRT + S may be more suitable for clinical stage III EC. It was found that NCRT + S can improve the OS of patients with stage III EC but cannot improve the OS of patients with stage II EC compared to S + ACRT (18,34). Adjuvant therapies may be suitable for some clinical early stage EC with high risk or patients with upstaged EC after surgery, therefore avoiding overtreatment.
Treatment-related complications are also important factors in making our multidisciplinary treatment decisions. The impact of NCRT on postoperative mortality and morbidity is still a conflicting topic. A multicentre study found that NCRT + S was associated with more chylothorax and a trend towards more cardiovascular and thromboembolic events (35). A meta-analysis found that NCRT + S tended to have a significantly higher rate of postoperative mortality and cardiopulmonary complications (36). In our meta-analysis, the perioperative complications in NCRT + S were higher than those in S + ACRT (OR, 1.67, 95% CI, 1.09–2.56). However, many factors may affect the incidence of perioperative complications, including patient selection, preoperative treatment regimen, and surgical procedure. The difficulty of the operation may also increase as the location of the tumor shifts from the lower thoracic part to the upper thoracic part of the esophagus, thus increasing perioperative complications. S + ACRT may increase the risk of chemo-radiotherapy related toxicity due to the poor physical condition after surgery. Previous poor results and serious complications of postoperative radiotherapy lead to little use of ACRT (22-26). However, previous poor radiotherapy technologies and nonstandard irradiation fields of these studies should be taken into consideration to reevaluate the efficacy. Retrospective studies suggested that postoperative radiotherapy should be focused on some high recurrence regions after radical surgery, such as the lower neck, upper mediastinum, and paraaortic regions, where it is not cleared up or difficult for complete clearing up during surgery (37,38). Only one pooled study reported that there were no significant differences in severe haematologic toxicities, radiation-induced pneumonitis, anastomotic leakage and anastomotic stenosis between NCRT + S and S + ACRT (18). A meta-analysis demonstrated that S + ACRT did not increase the risk of pneumonitis, anastomotic stenosis or severe hematologic toxicities (10). Additionally, new chemotherapy regimens should also be evaluated in neoadjuvant or adjuvant therapies.
This meta-analysis included eight studies concerning NCRT + S versus S + ACRT in the treatment of EC. Most of the studies were carried out in Asia and the histological type was SCC. Therefore, these results may be suitable for guiding the treatment of patients with esophageal SCC. Since the gene expression, pathogenetic mechanism and pathobiological behavior of esophageal AC and SCC are different (39), the treatment of esophageal AC may have some differences. There were many limitations to our meta-analysis. First, given the scarcity of RCTs, our meta-analysis included the results of all RCTs and non-RCTs and they were mainly non-RCTs. As a result, there may be some selection bias. In retrospective studies, NCRT + S may usually be chosen for patients with better condition, younger age and fewer complications. Second, the treatment regimens were not well controlled in different studies. The quality of surgical procedures and the target volumes and dosages of radiotherapy may affect the final results. Third, we could not identify and select the appropriate population most likely to benefit from NCRT + S. Except for the pathological type, many factors may affect the choice of treatment, such as stage, location of the tumor, tumor size, body condition, and age. Future studies will be needed to address the optimal subgroup populations for different treatment regimens. With the development of different treatment techniques, we need to reevaluate their merits and demerits using multidisciplinary therapy.
Conclusions
Our meta-analysis showed that NCRT + S was associated with better OS and PFS, a higher R0 resection rate and more perioperative complications for EC compared to S + ACRT. Because most of the patients had esophageal SCC, these results might be more suitable for esophageal SCC. More prospective RCTs are desperately needed to confirm these results and address the optimal subgroup populations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/aoe-20-46
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-46). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;390:2383-96. [Crossref] [PubMed]
- Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers 2017;3:17048. [Crossref] [PubMed]
- Pasquali S, Yim G, Vohra RS, et al. Survival After Neoadjuvant and Adjuvant Treatments Compared to Surgery Alone for Resectable Esophageal Carcinoma: A Network Meta-analysis. Ann Surg 2017;265:481-91. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Okines A, Sharma B, Cunningham D. Perioperative management of esophageal cancer. Nat Rev Clin Oncol 2010;7:231-8. [Crossref] [PubMed]
- Hwang JY, Chen HS, Hsu PK, et al. A Propensity-matched Analysis Comparing Survival After Esophagectomy Followed by Adjuvant Chemoradiation to Surgery Alone for Esophageal Squamous Cell Carcinoma. Ann Surg 2016;264:100-6. [Crossref] [PubMed]
- Hsu PK, Huang CS, Wang BY, et al. Survival benefits of postoperative chemoradiation for lymph node-positive esophageal squamous cell carcinoma. Ann Thorac Surg 2014;97:1734-41. [Crossref] [PubMed]
- Rice TW, Adelstein DJ, Chidel MA, et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg 2003;126:1590-6. [Crossref] [PubMed]
- Kang J, Chang JY, Sun X, et al. Role of Postoperative Concurrent Chemoradiotherapy for Esophageal Carcinoma: A meta-analysis of 2165 Patients. J Cancer 2018;9:584-93. [Crossref] [PubMed]
- Lv J, Cao XF, Zhu B, et al. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 2010;16:1649-54. [Crossref] [PubMed]
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Fujita H, Sueyoshi S, Tanaka T, et al. Prospective non-randomized trial comparing esophagectomy-followed-by-chemoradiotherapy versus chemoradiotherapy-followed-by-esophagectomy for T4 esophageal cancers. J Surg Oncol 2005;90:209-19. [Crossref] [PubMed]
- Malaisrie SC, Untch B, Aranha GV, et al. Neoadjuvant chemoradiotherapy for locally advanced esophageal cancer: experience at a single institution. Arch Surg 2004;139:532-8; discussion 538-9. [Crossref] [PubMed]
- Davis CS, Johns JR, Abood GJ, et al. Locally advanced esophageal cancer: Actual 5-year survival comparing neoadjuvant and adjuvant chemoradiation. J Clin Oncol 2011;29:149. [Crossref]
- Hong JC, Murphy JD, Wang SJ, et al. Chemoradiotherapy before and after surgery for locally advanced esophageal cancer: a SEER-Medicare analysis. Ann Surg Oncol 2013;20:3999-4007. [Crossref] [PubMed]
- Chen Y, Hao D, Wu X, et al. Neoadjuvant versus adjuvant chemoradiation for stage II-III esophageal squamous cell carcinoma: a single institution experience. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Hsu PK, Chen HS, Liu CC, et al. Pre- versus postoperative chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2017;154:732-40.e2. [Crossref] [PubMed]
- Sadrizadeh A, Bagheri R, Soltani E, et al. The Comparison of the Advantages of Neoadjuvant Chemoradiotherapy versus Postoperative Chemoradiotherapy: Outcomes in Esophageal Cancer Patients. J Gastrointest Cancer 2018;49:50-6. [Crossref] [PubMed]
- Xu Y, Chen Q, Sun X, et al. Phase III Randomized Study of Preoperative Versus Postoperative Chemoradiotherapy in Resectable Locally Advanced Esophageal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys 2018;102:S30. [Crossref]
- Fok M, Sham JST, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993;113:138-47. [PubMed]
- Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg 2003;75:331-6. [Crossref] [PubMed]
- Zieren HU, Müller JM, Jacobi CA, et al. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World J Surg 1995;19:444-9. [Crossref] [PubMed]
- Ténière P, Hay JM, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991;173:123-30. [PubMed]
- Kunath U, Fischer P. Radical nature and life expectancy in the surgical treatment of esophageal and cardial carcinoma. Dtsch Med Wochenschr 1984;109:450-3. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [Crossref] [PubMed]
- Chan KKW, Saluja R, Delos Santos K, et al. Neoadjuvant treatments for locally advanced, resectable esophageal cancer: A network meta-analysis. Int J Cancer 2018;143:430-7. [Crossref] [PubMed]
- Cheng J, Cai M, Shuai X, et al. Multimodal treatments for resectable esophagogastric junction cancer: a systematic review and network meta-analysis. Ther Adv Med Oncol 2019;11:1758835919838963. [Crossref] [PubMed]
- Wang Y, Zhu L, Xia W, et al. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag Res 2018;10:6295-303. [Crossref] [PubMed]
- Chen WH, Chao YK, Chang HK, et al. Long-term outcomes following neoadjuvant chemoradiotherapy in patients with clinical T2N0 esophageal squamous cell carcinoma. Dis Esophagus 2012;25:250-5. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery Alone Versus Chemoradiotherapy Followed by Surgery for Stage I and II Esophageal Cancer: Final Analysis of Randomized Controlled Phase III Trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Hsu PK, Chen HS, Liu CC, et al. Neoadjuvant Chemoradiation Versus Upfront Esophagectomy in Clinical Stage II and III Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2019;26:506-13. [Crossref] [PubMed]
- Gronnier C, Trechot B, Duhamel A, et al. Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: results of a European multicenter study. Ann Surg 2014;260:764-70; discussion 770-1. [Crossref] [PubMed]
- Sathornviriyapong S, Matsuda A, Miyashita M, et al. Impact of Neoadjuvant Chemoradiation on Short-Term Outcomes for Esophageal Squamous Cell Carcinoma Patients: A Meta-analysis. Ann Surg Oncol 2016;23:3632-40. [Crossref] [PubMed]
- Zhang X, Yang X, Ni J, et al. Recommendation for the definition of postoperative radiotherapy target volume based on a pooled analysis of patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma. Radiat Oncol 2018;13:255. [Crossref] [PubMed]
- Wang Y, Zhang L, Ye D, et al. A retrospective study of pattern of recurrence after radical surgery for thoracic esophageal carcinoma with or without postoperative radiotherapy. Oncol Lett 2018;15:4033-39. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Analysis Working Group. Asan University; BC Cancer Agency. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
Cite this article as: Zhang L, Wang Y, Xia W, Kang N, Kang M, Zhu L, Chen D, Niu L, Gao Y, Yang M, Wu L. Neoadjuvant versus adjuvant chemoradiotherapy for esophageal carcinoma: a systematic review and meta-analysis. Ann Esophagus 2021;4:5.




