
Development of an infant stomach model: validation of products targeting reflux in neonates and infants
Introduction
Gastroesophageal reflux (GER) can affect 50% of infants younger than three months old (1). Up to 50% of GER is due to increased abdominal pressure overcoming the esophageal sphincter pressure which is low in early birth infants (2-4). The consequences can lead to a pathological disease even in neonates and young infants (5) which is stressful for babies and infants as well as for the parents/carers.
This is not an area which is rich in pharmacological interventions, there has been a mixed response to the use of acid suppression with H2-receptor antagonists and proton pump inhibitors (PPIs) and there remains a concern over both usage and potential side effects (6,7). Antacids are also used and more frequently feed thickening products (8). Alginate preparation are well established and proven feed thickeners in babies and infants (9,10). The promotion of cross-linking by calcium ions and milk proteins present in the infant gastric environment, increasing viscosity which adds to gelling and feed thickening. Alginate products are especially important post feeding which is when babies and infants present with maximum symptoms.
There is a need for more safe and effective products for treating GER in babies and infants and importantly a need for in vitro models to screen and evaluate these products. This paper describes the development and validation of an in vitro infant stomach model to be used for characterising new feed thickening products for suppressing reflux in neonates and infants. Although the developed model was in vitro it was important to develop a model which was physiologically as accurate as possible. The new model was maintained in a 37±0.5 °C environment containing an artificial stomach with a volume of 85 mL which mimicked the volume of a baby’s stomach (11). There is little literature on the esophageal length between upper esophageal sphincter and the lower esophageal sphincter in babies. However, one study in pediatric patients suggested an esophagus length of 10 to 14 cm (12). The model which has been developed included a 12 cm esophagus marked in 1 cm increments with a reservoir to collect any refluxate above 12 cm. A controlled reflux event was created by applying 100 mL of air via a syringe using the force provided by a 0.3 kg weight which allowed for a pressure gradient to pass through the stomach and into the esophagus provoking an event which mimicked reflux. Any excess refluxate post the reflux event was collected in the reservoir above the esophagus and the volume (mL) recorded.
The baby/infant stomach model was validated by three independent operators comparing a control of Infant Formula Milk (Milk control) with a commercially available Infant Alginate Formulation. The validation method and the results are described later in this paper.
We present the following article in accordance with the TRIPOD reporting checklist (available at http://dx.doi.org/10.21037/aoe-20-78).
Methods
Model development
GER in infants is common and the development of new products to alleviate the distress and suffering in neonates and infants is important. The infant stomach model was developed to meet the demand for introducing effective new products to treat the condition. The development of the model went through varies guises until the model described here was finally developed and validated. The basis of the model uses an inverted egg incubator which allowed for an accurate temperature control to be maintained at 37±0.5 °C, see Figure 1. The interior of the incubator was removed to make room for a rig to suspend the artificial stomach with a total stomach volume of 85 mL. The artificial stomach was a paediatric urinary leg bag supplied by Great Bear Healthcare Limited, Cardiff, UK.
Within the temperature-controlled unit was tubing to allow for internal pressure to be applied which passed through the stomach and contents and in turn generated a volume of refluxate. The refluxate passed into tubing to mimic an infant esophagus at the top of the artificial stomach see Figure 2.
The artificial esophageal tubing was marked in 1 cm increments [0–12] to allow for the measurement of the volume and height (cm) of a reflux event with a collection vessel to collect reflux which occurs above 12 cm (12,13), see Figure 3.
A reflux event was simulated by applying an internal pressure which passed through the artificial stomach containing the 85 mL volume of meal and which in turn generated a volume of refluxate which passed into the artificial esophagus allowing the volume and height of the refluxate to be measured.
As this is an in vitro study, there were no patients or human tissue involved in this study and thus the requirement of ethical approval and informed consent were waived.
Model validation
The infant stomach model was validated by a series of experiments comparing the refluxate volumes of an Infant Formula Milk Control and Infant Gaviscon (positive control). Three operators conducted a series of experiments to demonstrate the model’s robustness, ease of use and reproducibility.
The total volume of the artificial stomach was 85 mL, the optimum dose response curves at a weight of 0.3 g and at a range of internal pressures are illustrated in Figure 4.
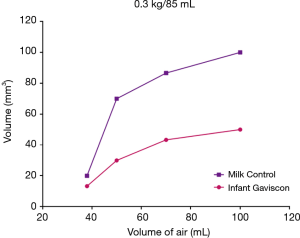
Each operator carried out 24 experiments including the Milk Control (Infant Formula Milk, SMA Pro) and the Infant Formula Milk (Milk Formulation) with the addition of Infant Gaviscon with five reflux events for each experiment. All experiments were carried out in a randomised order. The Milk Control formulation was prepared by adding 12.9 g (3 scoops) SMA Pro to 90 mL of water at 37 °C and shaking for one minute. 1M HCL was used to adjust to a pH of 4.80 with the temperature maintained at a constant 37±0.5 °C. Experiments using the Milk Control began by adding 35 mL Milk Control to the paediatric leg urinary bag used as the artificial stomach, followed by a further 15 mL of water at 37 °C and then a final 35 mL Milk Control was added, making a total volume of 85 mL. After five minutes a reflux event was created (applied by 100 mL air using the force provided by the 0.3 kg weight). The reflux event was measured and collected (g) along with measuring and recording the height the reflux travelled (mm) within the model. Any refluxed Milk Control was replenished. Each experiment was repeated five times and reflux events carried out at five-minute intervals.
The experiments using Infant Gaviscon began with addition of 35 mL Milk Formulation to the artificial stomach followed by adding a dose of the Infant Gaviscon (each sachet of Infant Gaviscon (0.65 g) was mixed with 5 mL water until a paste was formed (after approximately one minute) then 10 mL of water was added and well mixed (30 seconds), the 15 mL mix was then added to the artificial stomach by syringe. This was followed by a further 35 mL of the Milk Formulation added to the artificial stomach. After five minutes a reflux was created, and any reflux measured and collected as previously described. The experiment was repeated until five reflux events had been completed, reflux events were carried out at five-minute intervals. The artificial stomach and contents remained in situ at 37 °C for the duration of the experiment.
Statistical analysis
Throughout the study all analysis was performed using Analysis of Variance (ANOVA), and t-test analysis conducted using GraphPad Prism 8.3.0 (GraphPad Software, San Diego, CA 92018, USA).
Results
The validation study used three trained operators to investigate the differences in response between a Milk Control and Infant Gaviscon (positive control). In the study of 72 experiments there was no significant difference in the refluxate volumes for the Milk control within each reflux event when comparing between the three individual operators, see Figure 5. This demonstrated the infant stomach’s model robustness and reproducibility.
Similarly, there were no significant differences between the three operators within each reflux event during the Infant Gaviscon series of experiments, once again highlighting the model’s good reproducibility. For example, within reflux event 4, operator one recorded a mean refluxate volume of 1.26 g and operator two recorded a similar volume of refluxate 1.18 g. Figure 6 illustrates the mean volume of refluxate collected (g) after each reflux event following Infant Gaviscon.
The reflux suppression properties of Infant Gaviscon are illustrated in Figure 7. Operator three recorded an average of 6.67 g of refluxate for the Milk Control compared to only 2.80 g recorded for Infant Gaviscon (P=0.0102). A significant difference in the volume of refluxate collected when comparing the Milk Control with Infant Gaviscon was shown by all three operators.
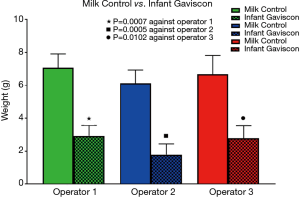
The Infant Stomach Model offered a validated and reliable in vitro method for differentiating between a Milk Control and a proven product for treating infant reflux.
Discussion
GER is common in newborn infants and alginate-based feed thickeners are frequently used as first line treatment. Other agents used as feed thickeners are sodium carboxymethyl cellulose, pectin, cellulose, bean gum and cereal rice. GER occurs more frequently in neonates compared to older infants and children and in premature neonates the occurrence is even higher, most commonly due to inappropriate relaxation of the lower esophageal sphincter (14,15).
The epidemiology of GER in the infant is interesting with regurgitation common and occurring at least once daily in 50% in infants up to three months of age. The prevalence of regurgitation peaks at four months of age with around 70% of infants regurgitating at least once daily (16). Regurgitation declines precipitously dropping to 14% by seven months of age and to less than 5% between 10 and 14 months of age (17). During year two a further decline in regurgitation is reported (18).
Neonate and infant GER is a growing market sector and the development of new products treating GER in neonates and infants is important and especially appropriate when GER changes from simply being mild and physiological to becoming pathological due to an increase in frequency and severity of reflux episodes. This can lead to insufficient caloric intake and a slowing of growth in the infant. The bigger concern is as the infant gets older the frequency of the GER can lead to more serious diseases later in life.
There are no infant stomach models currently available for screening new potential feed thickening products for suppressing reflux in neonate and infants. Any such model needs to be robust, reproducible and to be physiologically relevant. Such a model is described here along with the achievement of a full model validation demonstrating not only the model’s robustness and reproducibility but also the functionality of feed thickener agents.
There is a long history for the use of feed thickeners having been used to treat neonate and early infant GER for several decades with the theory being that increasing feed thickness will retain the feed in the stomach for longer and slow down or prevent reflux into the esophagus. The feed thickeners have been associated with decreasing GER symptoms including regurgitation and helping to improve sleep (19), a very important benefit for stressed parents and carers. As often seen with clinical evaluation studies there are some reports that feed thickeners make the GER symptoms worse for example Orenstein et al. [1992] (20) reported an increase in coughing episodes. However, the evidence is mainly supportive of infant feed thickeners being relatively free of major side effects although there was a report published stating acute bowel obstruction in neonates receiving feed thickeners containing cellulose and pectin (21).
In contrast to the negative report’s alginate preparations have proven efficacy in the treatment of GER in neonates and infants (22-24). Alginate reacts with gastric acid to produce alginic acid and forms a viscous gel that thickens the stomach contents which in turn makes reflux into the esophagus more difficult. This is increased even more by cross linking by calcium ions and milk proteins present in the feed within the infant gastric environment increasing viscosity and in turn adds to gelling and feed thickening. However, even with the demonstrable efficacy the guidelines for the use of alginates with GER are somewhat contradictory. The British National Institute for Health Care (NICE) guideline supports the use of alginate as a treatment option (25), whereas the guidelines of the European and North American Societies for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN, NASPGHAN) do not recommend the use of alginates (26). In a recent study (27) alginates significantly reduced GER related symptoms in infants decreasing both acid and non-acid GER symptom episodes.
What has been missing in neonate and infant product development was a viable physiological relevant model which mimicked how a product behaved in the stomach and a model where the height and volume of refluxate traveling up the esophagus could be recorded which could also measure total reflux suppression when appropriate. We know that most GER episodes are caused by transient relaxation of the lower esophageal sphincter triggered by postprandial gastric distention (22), so being able to monitor gastric refluxate was an important aspect of the model.
The model was fully validated by three trained independent operators as described in the method section of this paper, comparing a Milk control with Infant Gaviscon, a commercially available feed thickener. The study validators completed 72 experiments with 5 reflux events induced per experiment, the optimum pH was established at pH 4.8 and the pH along with the temperature at 37±0.5 °C were maintained throughout the studies. Naturally in vitro models can never truly replace what is happening in vivo. However, the validation study demonstrated how robust the model was with no significant differences between the three validation operators following studies with the Milk control. All three operators showed consistent and significant reflux suppression following the administration of Infant Gaviscon with no differences between operators.
Within the limitations of the infant stomach model the artificial stomach and the artificial esophagus mimic that of neonates and young infants. The internal pressure was pre-set and was used to mimic a reflux event, it would have been advantageous if this could have been more variable to represent changes that can happen although the majority of the GER events would be post-prandial.
The successful development of the Infant Stomach model has allowed for a well validated working model for screening new treatments for GER in neonates and infants. The model opens new opportunities in product development for this sector.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Epidemiology, Biomarkers and Modelling of Gastroesophageal Reflux Disease”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at http://dx.doi.org/10.21037/aoe-20-78
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoe-20-78
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-78). The series “Epidemiology, Biomarkers and Modelling of Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. PWD served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from Mar. 2020 to Feb. 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. As this is an in vitro study, there were no patients or human tissue involved in this study and thus the requirement of ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tighe M, Afzal NA, Bevan A, et al. Pharmacological treatment of children with gastro-oesophageal reflux. Paediatr Child Health 2017;22:30-32. [Crossref] [PubMed]
- Czinn SJ, Blanchard S. Gastroesophageal reflux disease in neonates and infants: when and how to treat. Paediatr Drugs 2013;15:19-27. [Crossref] [PubMed]
- Huang RC, Forbes D, Davis MW. Feed thickener for newborn infants with gastro-oesophageal reflux. Cochrane Database Syst Rev 2002.CD003211. [Crossref] [PubMed]
- Kwok TC, Ojha S, Dorling J. Feed thickener for infants up to six months of age with gastro-oesophageal reflux. Cochrane Database Syst Rev 2017;12:CD003211. [Crossref] [PubMed]
- Henry SM. Discerning differences: gastroesophageal reflux and gastroesophageal reflux disease in infants. Adv Neonatal Care 2004;4:235-47. [Crossref] [PubMed]
- Cohen S, Bueno de Mesquita M, Mimouni FB. Adverse effects reported in the use of gastroesophageal reflux disease treatments in children: a 10 years literature review. Br J Clin Pharmacol 2015;80:200-8. [Crossref] [PubMed]
- Mattos AZ, Marchese GM, Fonseca BB, et al. Antisecretory Treatment for Pediatric Gastroesophageal Reflux Disease - a Systematic Review. Arq Gastroenterol 2017;54:271-80. [Crossref] [PubMed]
- Orenstein SR, Magill HL, Brooks P. Thickening of infant feedings for therapy of gastroesophageal reflux. J Pediatr 1987;110:181-6. [Crossref] [PubMed]
- NICE. Gastro-oesophageal reflux in children and young people. 2016. Available online: https://www.nice.org.uk/guidance/qs112. Accessed 27 April 2020.
- Buts JP, Barudi C, Otte JB. Double-blind controlled study on the efficacy of sodium alginate (Gaviscon) in reducing gastroesophageal reflux assessed by 24 h continuous pH monitoring in infants and children. Eur J Pediatr 1987;146:156-8. [Crossref] [PubMed]
- Healthline. How Big Is Your Stomach. 2018. Available online: https://www.healthline.com/health/how-big-is-your-stomach. Accessed 31 October 2020.
- Yang GS, Bishop WP, Smith BJ, et al. Radiographic and endoscopic measurements of esophageal length in pediatric patients. Ann Otol Rhinol Laryngol 2005;114:587-92. [Crossref] [PubMed]
- Kalloor GJ, Deshpande AH, Collis JL. Observations on oesophageal length. Thorax 1976;31:284-8. [Crossref] [PubMed]
- Hillemeier AC. Gastroesophageal reflux. Diagnostic and therapeutic approaches. Pediatr Clin North Am 1996;43:197-212. [Crossref] [PubMed]
- Sutphen JL, Dillard VL. Effects of maturation and gastric acidity on gastroesophageal reflux in infants. Am J Dis Child 1986;140:1062-4. [PubMed]
- Martin AJ, Pratt N, Kennedy JD, et al. Natural history and familial relationships of infant spilling to 9 years of age. Pediatrics 2002;109:1061-7. [Crossref] [PubMed]
- Nelson SP, Chen EH, Syniar GM, et al. Prevalence of symptoms of gastroesophageal reflux during infancy. A pediatric practice-based survey. Pediatric Practice Research Group. Arch Pediatr Adolesc Med 1997;151:569-72. [Crossref] [PubMed]
- Campanozzi A, Boccia G, Pensabene L, et al. Prevalence and natural history of gastroesophageal reflux: pediatric prospective survey. Pediatrics 2009;123:779-83. [Crossref] [PubMed]
- Vandenplas Y, Sacre L. Milk-thickening agents as a treatment for gastroesophageal reflux. Clin Pediatr (Phila) 1987;26:66-8. [Crossref] [PubMed]
- Orenstein SR, Shalaby TM, Putnam PE. Thickened feedings as a cause of increased coughing when used as therapy for gastroesophageal reflux in infants. J Pediatr 1992;121:913-5. [Crossref] [PubMed]
- Montagne JP, Coussement A, Gruner M, et al. Neonatal intestinal obstruction. 2 cases in newborn infants fed pectin and cellulose food thickeners. J Radiol Electrol Med Nucl 1974;55:607-9. [PubMed]
- Baird DC, Harker DJ, Karmes AS. Diagnosis and Treatment of Gastroesophageal Reflux in Infants and Children. Am Fam Physician 2015;92:705-14. [PubMed]
- Del Buono R, Wenzl TG, Ball G, et al. Effect of Gaviscon Infant on gastro-oesophageal reflux in infants assessed by combined intraluminal impedance/pH. Arch Dis Child 2005;90:460-3. [Crossref] [PubMed]
- Miller S. Comparison of the efficacy and safety of a new aluminium-free paediatric alginate preparation and placebo in infants with recurrent gastro-oesophageal reflux. Curr Med Res Opin 1999;15:160-8. [Crossref] [PubMed]
- NICE. Gastro-oesophageal reflux disease in children and young people: diagnosis and management. 2015. Available online: https://www.nice.org.uk/guidance/NG1. Accessed 05 May 2020.
- Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2018;66:516-54. [Crossref] [PubMed]
- Salvatore S, Ripepi A, Huysentruyt K, et al. The Effect of Alginate in Gastroesophageal Reflux in Infants. Paediatr Drugs 2018;20:575-83. [Crossref] [PubMed]
Cite this article as: Fisher J, Boulton KHA, Dettmar PW. Development of an infant stomach model: validation of products targeting reflux in neonates and infants. Ann Esophagus 2021;4:4.







