
Airway resection for cT4b esophageal cancer: a single institution experience
Introduction
Esophageal carcinoma is an aggressive disease with devastating outcomes. Despite the improvement of surgical techniques and locoregional as well as systemic treatment modalities, 5-year survival remains poor. Locally advanced esophageal cancer with airway invasion represents a particularly challenging clinical scenario. The eighth edition TNM staging system of the combined American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) for esophageal cancer, classifies esophageal cancer with airway invasion as clinical T4b (cT4b) (1). However, in the Japanese literature cT4b (airway) has been defined either as tumour extension into the lumen or deformity of the airway (2,3). CT4b esophageal tumours have been associated with a dismal prognosis and median survival of only 1–6 weeks in patients with a fistula and 3–6 months in patients without a fistula (4,5). Although surgical resection remains the cornerstone of curative-intent treatment for most locally advanced esophageal cancers, there is no consensus on the optimal management approach for cT4b tumours with airway invasion in the current body of literature (4,6).
Locally advanced esophageal tumours with airway invasion have traditionally been considered “unresectable” and have therefore largely not been offered curative-intent surgery in most centers. Rather, surgery exclusive approaches have been advocated, such as palliative endoscopic stenting or definitive/palliative radiotherapy (± chemotherapy). However, endoscopic palliative treatment options including airway and esophageal stenting, although effective at alleviating dysphagia/stridor and controlling fistulae, have been associated with very poor outcomes, with a median survival less than 1 month (7). Radiotherapy, with or without chemotherapy, is another frequently employed approach for cT4b (airway) patients (8). However, radiation may further exacerbate the situation in patients with significant airway compression due to post-treatment edema. Even in the absence of an overt esophageal-airway fistula, radiation therapy has been associated with several adverse outcomes in cT4b (airway) patients, including the development of fistulas, worsening of existing fistulas as well as treatment-related fatal outcomes (4,9,10). More recently, induction chemotherapy followed by conversion surgical resection in responders has been proposed for all cT4b lesions (e.g., aorta, spine, and airway) following the results of a phase II trial from Japan (2,11). However, if the tumour was still adherent to the trachea or bronchus, airway resection was not performed and the esophagectomy was aborted in this study. Thus, in the absence of distant metastasis, esophagectomy with airway resection represents the best opportunity for curative-intent treatment for patients with confirmed cT4b (airway). However, the data on the outcomes of this highly selected patient population remains scarce.
Recognizing the controversy surrounding the optimal management of cT4b esophageal cancer with airway invasion, we sought to shed light on this topic by reviewing our surgical experience with the approach to cT4b (airway) malignancies. The objective of this study is not to delineate the optimal treatment of patients with cT4b (airway) esophageal cancer, but rather to describe the outcomes of a highly selected group of patients with esophageal carcinoma and airway invasion who have undergone esophagectomy with combined including tracheal or bronchial resection and reconstruction. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/aoe-20-65).
Methods
Data source and study population
A retrospective review of a prospective cohort study of all adult patients with locally advanced esophageal carcinoma with airway invasion (cT4b) who underwent curative-intent esophagectomy with airway resection and reconstruction between 2005 and 2018 was performed. Of the 657 patients in the esophagectomy database, we identified 14 in whom a combined airway and esophagectomy were performed. As this is a surgical database, we were not able to capture patients with cT4b tumours with airway invasion that had non-surgical treatment. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). After approval from the institutional review board (protocol number 2007-856), we accessed the prospectively collected database from the Thoracic Surgery Department at McGill University Health Center, Canada.
Variables and outcome measures
Patient characteristics included in the database consisted of patient demographics (age, gender, comorbidities), tumour characteristics, clinical staging, induction treatment modalities, operative procedures and postoperative complications. Postoperative complications were recorded using the thoracic morbidity and mortality (TM&M) classification system, a thoracic surgery specific adaptation of the Clavien-Dindo classification schema that has been internally and externally validated (12-14).
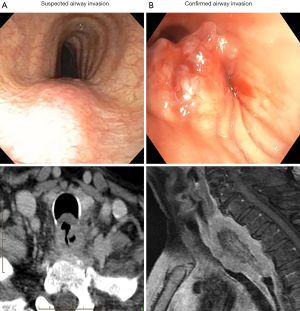
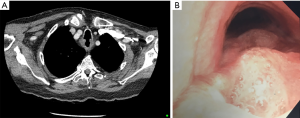
The data were abstracted and entered into an Excel spreadsheet (Microsoft Corporation, Way Redmond, WA, USA) by the study team. The eighth edition TNM staging system of the combined AJCC/UICC for esophageal cancer was used to determine clinical staging (1). CT4b was defined either as tumour extension into the lumen or deformity of the airway (2,3). We further classified airway invasion into suspected or confirmed cT4b: suspected cT4b was defined as a bulging of the tumour on bronchoscopy, and confirmed cT4b defined as clear tumour (by biopsy) or fistula present on endoscopic airway examination (Figure 1).
Airway resection
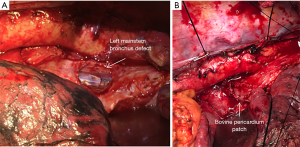
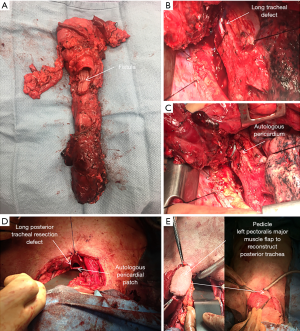
The extent of airway resection varied depending on the size, location, and depth of invasion of the tumour. If the membranous part of the trachea was adherent and clinically invaded at the time of surgery, the involved portion was resected, and margins revised according to intra-operative pathology consultation results until a negative margin was obtained. The resection was either extended to the muscular layer of the trachea, leaving the mucosa intact or, more commonly, the whole membranous layer was resected. With smaller, more distal, defects, the reconstruction was usually performed using a bovine or autologous pericardial patch tailored to the defect with a tendency to undersize transversely so as to avoid postoperative tracheo- or broncho-malacia. The patch was secured to the tracheal defect with absorbable suture (4-0 PDS) (Figure 2) and reinforced with the omentum accompanying the conduit into the chest. However, for more aggressive proximal esophageal tumours involving a significant portion of the cartilaginous rings, the proximal trachea, or cricoid cartilage, a pharyngo-laryngo-esophagectomy (PLE) was undertaken (Figure 3A). In some patients with significant tracheal resection and defect, a pectoralis major myocutaneous flap was used to reconstruct the posterior tracheal defect (Figure 3B,C,D,E).
Statistical analysis
Data are represented as n (%) for categorical variables and median [interquartile range (IQR)] for continuous variables. Kaplan-Meier curves were used to describe 5-year OS and log-rank tests to compare the cumulative survival distributions. All analyses were performed using STATA 12.1 (StataCorp., College Station, TX, USA).
Results
A total of 657 patients with esophageal carcinoma underwent esophageal resection at our institution between 2005 and 2018, of which 14 patients met our inclusion criteria of curative-intent esophagectomy with combined airway resection and reconstruction. Patient demographics and clinical characteristics are summarized in Table 1. The median age of resected patients was 65 years, and just over half were male. As expected, the majority of tumours were proximal. Most patients (57%) had confirmed tumour extension into the airway lumen with direct extension of tumour in the trachea/bronchus as evidenced by bronchoscopy (Figure 1B). The vast majority had some form of induction therapy (11/14), two had prior extensive radiation for a previous head and neck cancer precluding any further radiation as there would have been a significant overlap of treatment fields, and one patient was treated with up-front surgery and planned adjuvant therapy (Table 1). Three patients with confirmed significant airway invasion with high risk of developing a fistula underwent induction chemotherapy alone without radiation. Two of these patients developed an esophago-tracheal fistula during chemotherapy (Figure 4). Operative characteristics including type of esophageal resection, airway resection and reconstruction are presented in Table 2. Half of the cases had a pharyngo-laryngectomy as part of the resection for proximal intra-thoracic or cervical esophageal malignancies. The airway was reconstructed with a bovine pericardial patch in seven patients, and pedicled pectoralis major muscle flap in three patients. One patient had a combined pneumonectomy for right bronchial invasion. We were able to exteriorize the trachea in the remaining four patients with a mediastinal tracheostomy. Postoperative outcomes are summarized in Table 3. The majority of patients (86%) developed postoperative complications with 71% experiencing a major complication (complication grade >2). Pulmonary complications were common (78.6%) with a high rate of pneumonia (50%). The median length of stay for the cohort was 37 (IQR, 6–185) days. Failure of the trachea-bronchial repair arose in two patients, both of whom had significant prior radiotherapy (>60 Gy), either as induction therapy or for a prior head and neck cancer. These two patients were also the only patients who experienced in-hospital mortality (14%). One patient, whom had received extensive radiotherapy (over 75 Gy to the thoracic inlet), developed complete dehiscence of the esophageal anastomosis and tracheal repair. He initially was managed with a delto-pectoral rotation flap to control sepsis and divert saliva. Despite this maneuver, this patient suffered a delayed left carotid artery rupture which was ligated. Subsequent to this he developed further tracheal necrosis and eventually, 6 weeks after the original index operation, a ruptured innominate artery from which he expired. The other patient had a salvage PLE with extended tracheal resection and bovine pericardium reconstruction after definitive chemoradiation (60 Gy). Postoperatively, he developed multiple complications including anastomotic leak and failure of the tracheobronchial repair which was repaired using pectoralis muscle flap. He had a prolonged ICU stay and passed away secondary to hypoxic respiratory failure.
Full table
Full table
Full table
All the patients had esophageal squamous cell carcinoma on final pathology and 14% had a complete pathological response after neoadjuvant chemoradiotherapy but a residual mass adherent to the trachea. Amongst those without complete pathological response, one patient had pT2, 5 had pT3, and 6 had persistent pT4 tumours. In addition, 6 patients (43%) had a positive nodal disease. One patient who developed a large esophago-tracheal fistula after one cycle of induction chemotherapy had a near-complete pathological response with microscopic residual disease in the muscularis propria. Complete (R0) resection was achieved in the majority of the patients 13/14 (93%) (Table 4), one patient had microscopically positive circumferential margins.
Full table
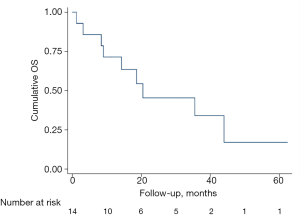
The median overall survival (OS) for this cohort was 20.4 months (95% confidence interval: 8.4–44.0). At 1 year, the estimated cumulative OS was 71.4% (40.6–88.2).
The estimated OS was 45.4% (17.8–70.0) and 34% (9.6–60.1) at 2 and 3 years respectively. The two patients with confirmed tracheal fistulas, whom had undergone induction chemotherapy followed by resection and pectoralis muscle flap remain alive and disease free at 44 and 51 months. The Kaplan-Meier survival curve is shown in Figure 5.
Discussion
The management of cT4b (airway) esophageal cancer, remains controversial, and this can, in part, be due to the different spectra of cT4b. In both the AJCC and Japanese Esophageal Society (JES) classification systems, cT4b is defined as suspected (e.g., bulging of the trachea due to extrinsic compression of the trachea) or confirmed (e.g., histologically proven biopsy at bronchoscopy) invasion of an unresectable structure (e.g., airway, aorta, spine). However, at the two ends of this spectrum there exist two very different disease entities, and perhaps they should be approached differently in terms of management. In this present study, we have attempted to highlight the distinction between the two different clinical scenarios of suspected or confirmed airway invasion. Whilst definitive chemoradiation may be entertained for patients with “suspected” airway invasion, only the most brazen radiation oncologist would consider radiotherapy in patients with “confirmed” airway disease with an evident fistula. Indeed, in patients who presented to our institution at diagnosis, a course of induction chemotherapy, without radiation, was preferentially employed in patients with confirmed tumour invading into the airway.
Several recent studies from Japan suggest that surgical resection, after stepwise multimodal approach, may be feasible for patients with cT4b esophageal cancers and airway invasion. Yamaguchi and colleagues from the Kyushu Cancer Centre compared patients with suspected or confirmed cT4b (airway) esophageal squamous cell carcinoma undergoing definitive chemoradiation versus induction chemoradiation and surgery (15). Of the 13 patients who underwent surgery, 3 had tumours with persistent invasion of the trachea. None of these underwent airway resection and they were left with a grossly positive margin. Irrespective, a very respectable 5-year survival of 26% was obtained in this cohort of patients. Another multi-institutional phase two trial sponsored by the Japanese Clinical Oncology Group (COSMOS trial) proposed an alternative approach employing induction chemotherapy prior to conversion surgery for locally advanced “unresectable” esophageal cancer including both cT4b (airway, aorta, or spine) or supraclavicular lymph node metastasis (2). The recently published long-term results of the COSMOS trial (11) reported an impressive overall 3-year survival rate of more than 45% for this heterogeneous group of locally advanced esophageal cancer patients. Although the authors were able to convert 20/48 (40%) of the patients to undergo esophagectomy, in those with persistent airway invasion surgery was abandoned and none had airway resection. However, by avoiding resection with persistent airway invasion these prior studies do not address the central theme in this present study, namely the feasibility, safety, and effectiveness of combined esophagectomy and airway resection. A bronchoscopic biopsy confirmed airway invasion offers a significantly more complex therapeutic challenge due to the risk of fistula formation than a suspected cT4b. A tumour with only extrinsic compression of the trachea, an accepted AJCC and JES definition, but no frank tracheal/bronchial mucosal invasion, is at risk of being over-staged as T4b, especially given the fact that the accuracy of CT/EUS for T4b is low (16).
In the current cohort of 14 patients with combined esophagectomy and airway resection for cT4b cancer, we reported eight with confirmed airway invasion and the rest suspected invasion or persistent adherence to the trachea or bronchus at the time of resection. By this definition, the patient population in the present study is distinctly different than the previously reported outcomes for surgical resection of cT4b esophageal cancers. With this aggressive surgical approach with en bloc airway excision complete R0 resection was achieved in over 93% of the patients with cT4b and yielded good oncological outcomes as good as or better than that previously in the two Japanese series, despite likely more locally invasive tumours. In the present study, we reported a 1-, 2-, and 3-year survival of 71.4%, 45.4%, and 34% respectively, which compare favorably to those published in the literature in similar, but not identical, patient populations. In a trial comparing definitive chemoradiation versus chemoradiation + surgery in locally advanced esophageal squamous cell carcinoma (cxT3-4, N0-1), Stahl and colleagues reported OS in both arms (14.9 and 16.4 months) lower than what we report herein a highly selected group of cT4b tumours requiring airway resection (20 months) (17). However, it is more appropriate to compare the present report’s survival outcomes to other studies with resection of cT4b lesions. Our survival rates are comparable to the surgical cohort in the COSMOS trial from Japan (67.9% and 46.6%) (2,11), a study in which there were no airway resections thus potentially represented earlier stage disease than those that we present in the current study. These results thus add to the body of literature advocating for curative-intent surgery following induction therapy in select patients with T4b disease.
However, this comes at a cost, as the perioperative morbidity we’ve reported of esophagectomy with combined airway resection is understandably very high. The majority of patients experience a complication, with a very high rate of pulmonary complications (79%). This rate of complications is clearly and understandably higher than what is reported from prior non-airway resection esophagectomy series (18). Balancing oncologic benefits with risks of adverse perioperative outcomes is key and future studies assessing patient quality of life may help tailor patient selection and treatment approach. Although the number of cases is low, given the rarity of cT4b airway tumours without metastatic disease, our data seems to suggest that the avoidance of radiation therapy in patients whom an airway resection is planned may be a wise approach: both patients with significant prior radiotherapy (>60 Gy) died postoperatively due to a complete breakdown of all airway and enteric repairs. Although this 14% mortality rate is high, it is equivalent to the surgical arm (13.2%) in the above-mentioned Stahl trial (17) in which the airway was not resected. Conversely the two patients with confirmed T4b and fistula’s pre-operatively that were managed with induction chemotherapy alone remain alive and disease free at over 3 years.
The present study has some limitations that warrant consideration. First of all, we are unable to comment on the ideal treatment of all patients with cT4b cancers with airway invasion. Rather we must acknowledge that the focus of this study was to describe the surgical approach, feasibility, and short- and long-term outcomes of patients undergoing combined esophagectomy and airway resection for locally invasive cancer. We were limited by the database available to us, a surgical database, and were not able to capture all patients with cT4b airway that presented to our institution treated with non-surgical approaches. Additionally, our results should be interpreted with some caution given our small sample size. Irrespective, given the extreme rarity of this condition, the current study represents one of the largest surgical series of combined esophagectomy and airway resection in the literature.
In conclusion, as improved systemic therapies, both conventional cytotoxic and biologic/immunotherapy, become more readily available for esophageal carcinoma, the opportunities to offer curative-intent surgical treatment will continue to expand. We have demonstrated, in a highly selected study population, that combined esophagectomy and airway resection are feasible, albeit with not insignificant morbidity, and that long-term survival is achievable with this locally aggressive malignancy. This study reinforces the notion that surgeons need to be engaged at all stages of esophageal cancer, and continually re-engaged during the management trajectory, to ensure that patients who could potentially benefit from curative-intent surgery are offered this approach.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/aoe-20-65
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoe-20-65
Peer Review File: Available at http://dx.doi.org/10.21037/aoe-20-65
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-65). LF serves as an unpaid editorial board member of Annals of Esophagus from Apr. 2020 to Mar. 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of McGill University Health Center (protocol number 2007-856) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amin MB, Edge SB, Greene FL, et al. editors. AJCC cancer staging manual. 8th ed. New York: Springer, 2017.
- Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer 2016;115:1328-34. [Crossref] [PubMed]
- Kawakami T, Tsushima T, Omae K, et al. Risk factors for esophageal fistula in thoracic esophageal squamous cell carcinoma invading adjacent organs treated with definitive chemoradiotherapy: a monocentric case-control study. BMC Cancer 2018;18:573. [Crossref] [PubMed]
- Burt M, Diehl W, Martini N, et al. Malignant esophagorespiratory fistula: management options and survival. Ann Thorac Surg 1991;52:1222-8; discussion 1228-9. [Crossref] [PubMed]
- Ku GY, Goodman KA, Rusch VW, et al. Successful treatment of esophageal cancer with airway invasion with induction chemotherapy and concurrent chemoradiotherapy. J Thorac Oncol 2009;4:432-4. [Crossref] [PubMed]
- Altorki NK, Migliore M, Skinner DB. Esophageal carcinoma with airway invasion. Evolution and choices of therapy. Chest 1994;106:742-5. [Crossref] [PubMed]
- Nasir BS, Tahiri M, Kazakov J, et al. Palliation of concomitant tracheobronchial and esophageal disease using a combined airway and esophageal approach. Ann Thorac Surg 2016;102:400-6. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Kim H, Oh D, Ahn YC, et al. Clinical outcomes of radiation therapy for clinical T4b oesophageal cancer with airway invasion. Radiat Oncol 2018;13:245. [Crossref] [PubMed]
- Nishimura Y, Suzuki M, Nakamatsu K, et al. Prospective trial of concurrent chemoradiotherapy with protracted infusion of 5-fluorouracil and cisplatin for T4 esophageal cancer with or without fistula. Int J Radiat Oncol Biol Phys 2002;53:134-9. [Crossref] [PubMed]
- Yokota T, Kato K, Hamamoto Y, et al. A 3-year overall survival update from a phase 2 study of chemoselection with DCF and subsequent conversion surgery for locally advanced unresectable esophageal cancer. Ann Surg Oncol 2020;27:460-7. [Crossref] [PubMed]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [Crossref] [PubMed]
- Ivanovic J, Seely AJ, Anstee C, et al. Measuring surgical quality: comparison of postoperative adverse events with the american college of surgeons NSQIP and the Thoracic Morbidity and Mortality classification system. J Am Coll Surg 2014;218:1024-31. [Crossref] [PubMed]
- Ivanovic J, Al-Hussaini A, Al-Shehab D, et al. Evaluating the reliability and reproducibility of the Ottawa Thoracic Morbidity and Mortality classification system. Ann Thorac Surg 2011;91:387-93. [Crossref] [PubMed]
- Yamaguchi S, Morita M, Yamamoto M, et al. Long-term outcome of definitive chemoradiotherapy and induction chemoradiotherapy followed by surgery for T4 esophageal cancer with tracheobronchial invasion. Ann Surg Oncol 2018;25:3280-7. [Crossref] [PubMed]
- van Zoonen M, van Oijen MG, van Leeuwen MS, et al. Low impact of staging EUS for determining surgical resectability in esophageal cancer. Surg Endosc 2012;26:2828-34. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
Cite this article as: Alkaaki A, Renaud S, Trépanier M, Cools-Lartigue J, Spicer J, Mueller C, Ferri L. Airway resection for cT4b esophageal cancer: a single institution experience. Ann Esophagus 2021;4:3.


