
Recurrent esophageal candidiasis: a case report of different complications
Introduction
Esophageal candidiasis is an infection with multiple known rare complications and is commonly encountered in immunosuppressed patients (1). However, symptomatic esophageal candidiasis is rarely encountered in patients with intact host defence mechanisms, despite the fact that Candida can colonize the esophagus in up to 20% of healthy adults (2,3). Due to the rare occurrence of symptomatic esophageal candidiasis in non-HIV patients, surveillance such as screening endoscopy is too costly and invasive to be used in this population group (4,5). As a result, asymptomatic esophageal candidiasis, which is easily treatable, can go unnoticed, resulting in inadequate treatment and increased complications such as esophageal perforations, fistula formations and development of mitotic lesions (6,7). Here we report a non-HIV patient with recurrent esophagitis from candidiasis infection, complicated by dysphagia, food bolus obstruction, benign stricture, ulceration, intramural pseudodiverticulosis and hyperkeratosis. To our knowledge, this is a first case report illustrating the progression of symptoms and disease process with various complications over many years. We aim to highlight the importance of developing a higher level of clinical suspicion for esophageal candidiasis and recognizing possible complications that may arise. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/aoe-20-29).
Case presentation
A currently retired 71-year-old Chinese male has a history of diabetes mellitus, hypertension, and ischemic heart disease. He is an ex-smoker and until recently, drinks 2 to 3 cans of beer per day. A summary of the case presentation is illustrated in Figure 1.

He had 2 previous episodes of impacted food bolus in the upper esophagus that were cleared with rigid esophagoscopy in 2007 and 2009. Barium Swallow and Meal study done in 2009 demonstrated a thin, incomplete membrane arising from the anterior wall of the cervical esophagus at the level of C6. There was no circumferential involvement seen. The finding was consistent with an esophageal web. There was no significant obstruction to the flow of contrast down the esophagus. The esophageal mucosa was otherwise of normal contour, with no intraluminal mass seen. Mild gastro-esophageal reflux into the distal esophagus was demonstrated. No intraluminal gastric mass, stricture or peptic ulcer disease was detected.
In September 2013, he was admitted to hospital for foreign body sensation at the base of throat after eating. Esophagogastroduodenoscopy (EGD) showed esophagitis with whitish plaques. The endoscope passed through the esophagus with some resistance. There was also pangastritis with erosions. Gastric antrum biopsy showed mild chronic inflammation with no evidence of atrophic gastritis. Esophageal biopsy showed mild lymphocytic infiltration, no dysplasia or carcinoma. Fungal testing proved negative. He was treated with a short course of proton pump inhibitor, Omeprazole 40 mg BD for 2 weeks.
In June 2017, he presented to hospital again with acute dysphagia. CT Neck showed a bolus of ingested material impacted in the upper esophagus. There was also diffuse mural thickening of the esophagus distal to the bolus, possibly a response to chronic inflammation. The food bolus cleared spontaneously before he underwent EGD. Endoscopy showed an irregular mucosa with whitish plaques at upper esophagus (Figure 2). Biopsy from the esophagus showed acute erosive esophagitis and returned positive for fungal organisms. Gastric biopsy from the antrum and incisura showed chronic gastritis with intestinal metaplasia. He was treated with Omeprazole 40 mg BD for 6 weeks and Fluconazole 200 mg OM for 3 weeks after a loading dose of 400 mg.
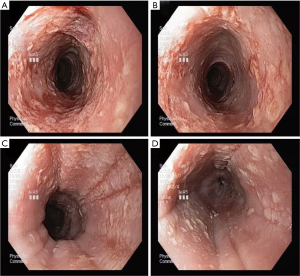
About 15 months later in September 2018, he presented with recurrent dysphagia to solids of 2 months duration. Repeat EGD showed inflammation and benign stricture at the upper esophagus. The gastroscope was unable to pass through the stricture. A pediatric gastroscope was used for the rest of the examination. There was esophagitis of entire esophagus likely from fungal infection. White and yellow plaques were seen at 33–34 cm from incisors (Figure 3). Severe chronic pangastritis of the stomach, worse at antrum. Histology of the distal esophagus biopsies demonstrated hyperkeratosis and reflux changes while the proximal biopsies showed esophagitis. No yeast spores or pseudohyphae were seen. The gastric biopsies showed mild to moderate chronic gastritis with intestinal metaplasia. He was treated with another 3 weeks course of oral Fluconazole 200 mg OM after a loading dose of 400 mg based on the endoscopic findings of suspected candidiasis.
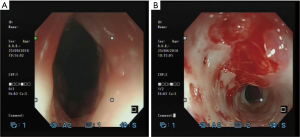
In August 2019, the patient underwent repeat EGD for recurrent dysphagia. A pediatric gastroscope was used. There were whitish plaques likely from candidiasis causing a short segment stenosis at 20–22 cm from the incisors. Hyperkeratosis was seen from 24–34 cm from incisors, while the distal 6cm of esophagus appeared relatively normal. Pangastritis with intestinal metaplasia changes were again seen in the distal stomach. In view of presence of active acute inflammation, esophageal dilatation for the symptomatic stenosis was not performed. Nasoendoscopy was also performed and it was completely normal. CT scan of the neck, thorax, abdomen, and pelvis in August 2019 showed diffuse mild mural thickening of the esophagus with small volume peri-esophageal reactive lymph nodes likely represent known fungal esophagitis. No discrete esophageal mass is identified. Serological screening for HIV infection was negative. Esophageal biopsy and fungal cultures subsequently returned positive results for Candida albicans and the patient received another 3 weeks course of oral Fluconazole 200 mg OM after a loading dose of 400 mg in September to October 2019.
Barium Swallow study performed in October 2019 demonstrated that the patient was able to swallow barium without any difficulty. There were multiple small ulcers seen at the upper half of the esophagus, in keeping with fungal esophagitis. However, no stricture or mass or stenosing lesion was seen and no hold up of the column of contrast was noted in the esophagus. No significant gastro-esophageal reflux was evident.
The patient was reviewed in October 2019 soon after the third treatment course for esophageal candidiasis. His dysphagia symptom had improved significantly. A repeat EGD was planned to check for resolution of the esophageal candidiasis. EGD showed multiple small pits in the esophageal wall from intramural diverticulae starting at 19 to 38 cm from the incisors. Esophagitis had much improved with no significant stricture but the hyperkeratosis of the esophagus persisted, starting at 24 cm to the cardio-esophageal junction at 40 cm, most prominent from 32 to 33 cm (Figure 4). Biopsies of the distal esophagus showed moderate reflux esophagitis and keratin while the proximal esophageal biopsy showed unremarkable squamous epithelium.
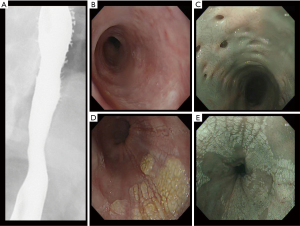
After pharmacological treatment of the candidiasis, the patient was reviewed twice in November 2019. He has been asymptomatic, with good appetite and no dysphagia. The candidiasis was considered to be in remission. The plan was to continue follow-up with surveillance EGD for the esophageal hyperkeratosis and chronic gastritis with intestinal metaplasia.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Centralised Institutional Review Board and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Esophageal candidiasis is the most common type of infectious esophagitis, accounting for up to 88% of cases (5). It is most common amongst immunosuppressed patients, such as HIV seropositive and AIDS patients, patients on chemotherapy, patients on long-term medications like antibiotics or steroids, and patients with advanced age or adrenal deficiency. However, it is an uncommon occurrence in non-immunosuppressed patients. A retrospective study detected a prevalence of only 0.32% of esophageal candidiasis in non-HIV patients (2). Despite the many known complications of esophageal candidiasis, there have been no prior reports of a single patient presenting with esophageal candidiasis complicated by all the following: dysphagia; food bolus obstruction; benign stricture; ulceration; intramural pseudodiverticulosis and hyperkeratosis.
Dysphagia is one of the commonest symptom of esophagitis which, in chronic disease, can be a manifestation of stricture formation and it may progress to cause food bolus obstruction. The most common cause of esophageal stricture is gastroesophageal reflux. For our patient, he did have mild gastro-esophageal reflux disease as evident from the earlier barium study and esophageal biopsies showed moderate reflux esophagitis. Whether the reflux disease contributed to candidiasis and the upper esophageal stricture remains unknown. Esophageal stricture is a rare complication of esophageal candidiasis with very few cases being reported in the literature. Kim et al. documented a case of a 57-year-old woman complaining of dysphagia, nausea and vomiting who was found to have esophageal candidiasis complicated by esophago-mediastinal fistula and strictures. Anti-fungal therapy resolved the fistula, whilst the stricture was dilated under fluoroscopy, and the patient improved and tolerated a solid food diet (8). Another report documented a case of a child with glycogen storage disease 1b and recurrent dysphagia that was diagnosed with esophageal candidiasis complicated by stricture. Similarly, anti-fungal treatment and esophageal dilatation resulted in resolution of the stricture (9).
Our patient had first presented with intermittent food bolus obstruction in 2007, some 10 years prior to the eventual diagnosis of esophageal candidiasis in 2017. He also had recurrent candidiasis and had received repeated courses of anti-fungal medication that each time resulted in improvement of his symptoms between episodes and so far had not required dilatation of the stricture.
Intramural pseudodiverticulosis is an interesting finding that is less commonly reported (10-12). The wall of the esophagus develops numerous small outpouchings. The outpouchings represent the ducts of submucosal glands of the esophagus. Pseudodiverticula may also be seen on barium swallow imaging of the esophagus as flask-shaped pseudodiverticula. While it is associated with certain chronic conditions, particularly alcoholism, diabetes and gastroesophageal reflux disease, the association with esophageal candidiasis is less well established, with very few case reports published (10,12). It has been hypothesised that pseudodiverticula are not a primary phenomenon, but rather are secondary to a chronic irritant to the esophagus, leading to compression of the submucosal ducts and the formation of pseudodiverticula (10). The most relevant complication is the development of esophageal stricture which is mainly localized in the upper esophagus (11,12).
Hyperkeratosis of the esophagus is a poorly understood phenomenon that has been documented rarely. It was first described by Starr in 1928 on 3 patients who presented with dysphagia, regurgitation, and weight loss. From the author’s observation, all 3 patients were fond of very hot tea (13). A prospective study conducted by Taggart et al. on esophageal hyperkeratosis revealed several important findings. Firstly, majority of the cases (62%) occurred in the setting of Barrett’s esophagus or adenocarcinoma, but typically was an incidental finding with low clinical significance and it showed no correlation with squamous dysplasia or squamous cell carcinoma. Secondly, esophageal hyperkeratosis in the non-Barrett’s esophagus setting had a higher association with concomitant or previous esophageal squamous dysplasia or squamous carcinoma, as well as head and neck mitotic lesions. Thirdly, esophageal candidiasis was common (22%) in patients with esophageal hyperkeratosis in the non-Barrett’s esophagus setting. However, the study did not differentiate correlation versus causation of esophageal candidiasis and hyperkeratosis. Other risk factors for esophageal hyperkeratosis include smoking and alcohol use and gastro-esophageal reflux disease (14).
There are case reports documenting clinical esophageal leucoplakia with histological hyperkeratosis in conjunction with esophageal mitotic lesions (15-17). While the malignant potential of esophageal leucoplakia is unknown, given the strong association between esophageal leucoplakia and squamous dysplasia, it is recommended for patients with esophageal leucoplakia to undergo close monitoring with treatment by endoscopic resection or ablation (7). Furthermore, studies have demonstrated evidence that esophageal candidiasis has a causative role in the development of esophageal cancer, through production of carcinogenic nitrosamines like nitroso-N-methylbenzamine (NBMA) and other less clear mechanisms (18). In light of such morbid sequelae for a treatable infection, aggressive treatment of candidiasis is recommended. According to the Infectious Diseases Society of America, their Clinical Practice Guideline for the Management of Candidiasis clearly outlines treatment recommendations for different patient groups (19). Our patient’s treatment was in-line with their recommendation, which resulted in resolution of his esophagitis and stricture.
There are some limitations of our case report. Firstly, we assumed the immune status of our patient to be non-immunosuppressed based on his negative HIV serological testing and clinical presentation. However, his age and medical history of diabetes mellitus may have contributed a degree of immunosuppression. HIV testing was only conducted in 2019, and although it was negative, HIV testing is well associated with false negatives in its latency phase, and ideally serial HIV testing should have been conducted given his recurrent candidiasis (20). Secondly, our patient was only formally diagnosed with esophageal candidiasis in 2017 based on fungal testing, and it is unclear if our patient was colonized with Candida or was symptomatic with esophageal candidiasis since the start of his symptoms in 2007. This makes it unclear as to the effect of possible long-standing esophageal candidiasis on his esophageal stricture and hyperkeratosis, and if earlier treatment could have prevented these complications.
In conclusion, clinicians should have a lower threshold for considering esophageal candidiasis even in non-immunosuppressed patients presenting with esophageal symptoms and have earlier consideration of esophageal biopsies and fungal testing and ensuing fungal eradication therapy if indicated. Our case report also serves to highlight the possible clinical presentation, disease progression and complications that may arise from chronic esophagitis and recurrent esophageal candidiasis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/aoe-20-29
Peer Review File: Available at http://dx.doi.org/10.21037/aoe-20-29
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-29). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Centralised Institutional Review Board and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Robertson KD, Nagra N, Mehta D. Esophageal Candidiasis. Treasure Island (FL): StatPearls Publishing, 2020.
- Choi JH, Lee CG, Lim YJ, et al. Prevalence and risk factors of esophageal candidiasis in healthy individuals: A single center experience in Korea. Yonsei Med J 2013;54:160-5. [Crossref] [PubMed]
- Vermeersch B, Rysselaere M, Dekeyser K, et al. Fungal colonization of the esophagus. Am J Gastroenterol 1989;84:1079-83. [PubMed]
- Nishimura S, Nagata N, Shimbo T, et al. Factors associated with esophageal candidiasis and its endoscopic severity in the era of antiretroviral therapy. PLoS One 2013;8:e58217. [Crossref] [PubMed]
- Takahashi Y, Nagata N, Shimbo T, et al. Long-term trends in esophageal candidiasis prevalence and associated risk factors with or without HIV infection: Lessons from an endoscopic study of 80,219 patients. PLoS One 2015;10:e0133589. [Crossref] [PubMed]
- Desai JP, Moustarah F. Esophageal stricture. Treasure Island (FL): StatPearls Publishing, 2020.
- Singhi AD, Arnold CA, Crowder CD, et al. Esophageal leucoplakia or epidermoid metaplasia: a clinocopathological study of 18 patients. Mod Pathol 2014;27:38-43. [Crossref] [PubMed]
- Rosołowski M, Kierzkiewicz M. Etiology, diagnosis and treatment of infectious esophagitis. Prz Gastroenterol 2013;8:333-7. [Crossref] [PubMed]
- Lee KJ, Choi SJ, Kim WS, et al. Esophageal stricture secondary to candidiasis in a child with glycogen storage disease 1b. Pediatr Gastroenterol Hepatol Nutr 2016;19:71-5. [Crossref] [PubMed]
- Starr FNG. Hyperkeratosis of the oesophagus. Can Med Assoc J 1928;18:22-4. [PubMed]
- Hahne M, Schilling D, Arnold JC, et al. Esophageal intramural pseudodiverticulosis: review of symptoms including upper gastrointestinal bleeding. J Clin Gastroenterol 2001;33:378-82. [Crossref] [PubMed]
- Halm U, Lamberts R, Knigge I, et al. Esophageal intramural pesudodiverticulosis: endoscopic diagnosis and therapy. Dis Esophagus 2014;27:230-4. [Crossref] [PubMed]
- Siba Y, Gorantla S, Gupta A, et al. Esophageal intramural pseudodiverticulosis, a rare cause of food impaction: case report and review of the literature. Gastroenterol Rep (Oxf) 2015;3:175-8. [Crossref] [PubMed]
- Taggart MW, Rashid A, Ross WA, et al. Oesophageal hyperkeratosis: clinicopathological associations. Histopathology 2013;63:463-73. [PubMed]
- Biemond P, ten Kate FJ, van Blankenstein M. Esophageal verrucous carcinoma: histologically a low-grade malignancy but clinically a fatal disease. J Clin Gastroenterol 1991;13:102-7. [Crossref] [PubMed]
- Westerterp M, Busch OR, Bergman JJ, et al. A “Crackleware” oesophagus. J Clin Pathol 2005;58:1325-7. [Crossref] [PubMed]
- Tonna J, Palefsky JM, Rabban J, et al. Esophageal verrucous carcinoma arising from hyperkeratotic plaques associated with human papilloma virus type 51. Dis Esophagus 2010;23:E17-20. [Crossref] [PubMed]
- Delsing CE, Bleeker-Rovers CP, van de Veerdonk FL, et al. Association of esophageal candidiasis and squamous cell carcinoma. Med Mycol Case Rep 2012;1:5-8. [Crossref] [PubMed]
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;62:e1-50. [Crossref] [PubMed]
- Taylor D, Durigon M, Davis H, et al. Probability of a false-negative HIV antibody test result during the window period: a tool for pre- and post-test counselling. Int J STD AIDS 2015;26:215-24. [Crossref] [PubMed]
Cite this article as: Ching SS, Lim TW, Ng YLA. Recurrent esophageal candidiasis: a case report of different complications. Ann Esophagus 2021;4:11.


