
Electrical neuromodulation of the lower esophageal sphincter for the treatment of gastroesophageal reflux disease
Introduction
Gastroesophageal reflux disease (GERD) is a frequent and difficult to treat medical condition (1).
Inadequate symptom control has been cited as one of the main reasons to drive to surgical therapies for GERD (2), even for patients with mild symptoms. This unmet medical need has led to new attempts at developing less-invasive therapies for the treatment of GERD (1). Lower esophageal sphincter (LES) electrical neuromodulation therapy (LES-ENT), using an electrical current application in a power and frequency that modulates the local neural plexus in and around the LES has been shown to improve outcome in GERD patients (3,4) and may represent and alternative surgical option for patients that are not satisfied with their medical therapy. Moreover, in a particular subset of patients where fundoplication is not the best option, such as esophageal dysmotility, sleeve gastrectomy or lung transplants, LES-ENT may appear as a valid alternative. In this review, we address the state-of-the-art technique for LES-ENT and summarize the latest results available on clinical studies including original data from an international prospective registry.
Methods
A concise review of literature was performed through PubMed. We searched to identify published studies reporting on subjective and objective GERD after LES-ENT. All relevant articles and abstracts of all potentially relevant studies were evaluated. Data evaluated included GERD-health-related quality of life (HRQL) (5), extra esophageal symptoms, PPI discontinuation and patient satisfaction rates, pH study metrics, severe adverse effects (AEs), and treatment failures.
LES-ENT stimulation system
The LES-ENT Endostim device (Endostim Inc., the Netherlands) has three components: a bipolar stimulation lead with two stitch electrodes implanted by laparoscopy in the LES, a pulse generator implantable in a subcutaneous pocket and an external programmer.
Electrical neuromodulation to the LES is generated by the implantable pulse generator (IPG), sending electrical pulses of 5 mA at a rate of 20 Hz via the bipolar lead to the 2 stitch electrodes, implanted in the LES muscle. The IPG contains a non-rechargeable battery with a lifetime of 7–10 years. Electrical neuromodulation increases LES resting pressure and control reflux. The external programmer unit initiates therapy by starting 30-min stimulation cycles 6–12 times a day, with intensity and duration adapted to patient characteristics.
Surgical technique
Implantation of the IPG and bipolar lead is performed using laparoscopic techniques, which were described previously (6). In case of a hiatal hernia, this is repaired by standard surgical technique.
Upper gastrointestinal endoscopy is performed to identify the Z-line and to avoid penetration of the mucosa during electrode placement.
The electrodes at the proximal end of the lead are inserted and secured into the esophageal muscle wall. The distal end of the bipolar lead is retracted through the abdominal wall and connected to the IPG. This is tested before it is placed into the subcutaneous pocket, and then is programmed for electrical neuromodulation therapy.
Patients usually stay in the hospital for one night for control and are advised to wear an elastic compression bandage over the subcutaneous pocket and the IPG for 10 days to prevent formation of a seroma.
Results
Safety and efficacy
Several clinical studies investigating the efficacy and safety of LES-ENT therapy were initiated and showed promising efficacy results with both short and long-term follow-up (3,7,8).
The results of the current studies demonstrate the safety of LES stimulation with an excellent side effect profile. Among AEs reported, none of them were unanticipated and were classified as device, procedure or therapy related. These included asymptomatic lead erosion at the 6-month endoscopy in a patient implanted with an investigational lead with a 5-mm electrode; treatment adverse events consisted of explant of the IPG and lead, followed by a Toupet fundoplication performed during the same procedure. Six events of pain and 2 of gastroparesis were reported (both gastroparesis events were of the same patient). Mild or moderate dysphagia occurred in four patients, which resolved without intervention.
Other isolated events included a patient with decreased cardiac output, persisting GERD, lead dislodgment, palpitation, troponin raise, vomiting. All events resolved and four patients had the device explanted. No GI side effects such as diarrhea, bloating and inability to belch were reported. There were no unanticipated device- or stimulation-related adverse events or untoward sensation reported during the 2- to 3-year follow-up of the open label trial (9-11).
A recent prospective, international, and multi-center study, conducted at 10 sites in 8 countries evaluated efficacy of LES-therapy in GERD patients. With a 6 months follow-up, efficacy was evaluated by GERD symptoms and quality of life, esophageal acid exposure, LES pressure, and PPI usage and general quality of life measurements (8). GERD-HRQL improved from 31.0 (IQR, 26.2–36.8) off-PPI and 16.5 (IQR, 9.0–22.8) on-PPI to 4 (IQR, 1.0–8.0) at 3-month and 5 (IQR, 3.0–9.0) at 6-month follow-up (P<0.0001 vs. on- and off-PPI). Oesophageal acid exposure (pH <4.0) improved from 10.0% (IQR, 7.5–12.9%) to 3.8% (IQR, 1.9–12.3%) at 3 months (P=0.0027) and 4.4% (IQR, 2.2–7.2%) at 6 months (P<0.0001) (Table 1).
Table 1
| Variable | Visit interval | N | Median (IQR) | Change from baseline on-PPI | Change from baseline off-PPI | |||
|---|---|---|---|---|---|---|---|---|
| IQR | P value | IQR | P value | |||||
| GERD-HRQL scores | Baseline on-PPI | 42 | 16.5 (9–22.8) | |||||
| Baseline off-PPI | 42 | 31 (26.2–36.8) | ||||||
| 6 months | 41 | 5 (3.0–9.0) | −8 (−16 to −1) | <0.0001 | −22 (−32 to −17) | <0.0001 | ||
| Median % 24-hour esophageal pH <4.0 | Baseline | 42 | 9.9 (7.5–12.9) | |||||
| 6 months | 40 | 4.4 (2.2–7.2) | −5.5 (−10.2 to −2.5) | <0.0001 | ||||
| DeMeester score | Baseline | 42 | 35.1 (27.1–51.9) | |||||
| 6 months | 40 | 17.5 (10.9–23.4) | −19.7 (−37 to −6.9) | <0.0001 | ||||
pH monitoring as % time pH below 4.0. IQR, interquartile range; PPI, proton pump inhibitor; GERD, gastroesophageal reflux disease; HRQL, health-related quality of life.
The details of the open-label trial with 2 and 3-year results have been reported (9-11). At 3 years, there was a significant improvement in their median (IQR) GERD-HRQL on electrical stimulation compared to both their on PPI [9 (6.0–10.0) vs. 1 (0–2.0), P=0.001] and off PPI [22 (21.0–24.0) vs. 1 (0–2.0), P=0.001). Median 24-h distal esophageal acid exposure was significantly reduced from [10.3% (7.5–11.6%) at baseline vs. 3% (1.9–4.5%), P=0.001] at 3 years. Seventy-three % (11/15) patients had normalized their distal esophageal acid exposure at 3 years. Remaining four patients had improved their distal esophageal acid exposure by 39–48% from baseline. Four year follow up for a subset of these patients has shown similar levels of efficacy with median (IQR) GERD-HRQL of 3 [1–3], significantly improved from 9 [8–10] at baseline on-PPI (P=0.004) and 24 [21–25] at baseline off-PPI (p50% of days with PPI use) (11,12).
The updated data are originals of this manuscript and are represented in Figure 1.
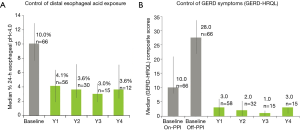
In 2017, 5-year data was published (13), 72 patients with 6 months post-op follow-up and 42 patients with 12 months follow up. Ninety % (56/62) patients showed an improvement in their GERD-HRQL score on ES at 6 months and 93% (31/33) showed an improvement at 12 months compared to baseline. The median (IQR) composite GERD-HRQL score improved from 22 (17.0–27.0) preoperatively to 7.5 (4.0–12.8) at 6 months (P<0.01) and from 21.0 (17.0–24.0) to 5.0 (2.0–7.0) at 12 months (P=0.03).
A Web-based international multicenter registry allowed physicians to track the outcomes of their patients treated with LES stimulation in their clinical practice outside of clinical trials.
Currently, this prospective multi-center registry of LES-ENT for GERD has enrolled 223 patients from 16 sites in Europe and Latin America with an extended 5-year-follow up. Results were encouraging regarding symptoms control, acid exposure and medication use. Symptoms; Heartburn and regurgitation were evaluated with GERD-HRQL, at baseline [on and off proton pump inhibitor (PPI) therapy] and during follow-up of electrical neuromodulation therapy. GERD-HRQL scores at 3 and 6 months after initiation of LES-therapy had improved, statistically significantly compared with baseline scores. At baseline, median GERD-HRQL score was 23.0 (N=244). At 12-month follow-up it had dropped to 6.5, and at 36 months-follow up it was 3. These final values were within normal range (Figure 2) (11).

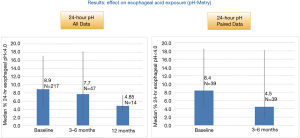
Post-implant esophageal pH testing was performed by a few sites either as standard of care or in patients with residual symptoms. In patients with pre-implant, and 3–6 months (n=32) and 12 months (n=7) post-op pH, median 24-hour esophageal acid exposure improved from 8.0% pre-implant to 4.5% at 3–6 months (P=NS) and from 6.2% pre-implant to 4.5% at 12 months (P=NS), respectively (Figure 3).
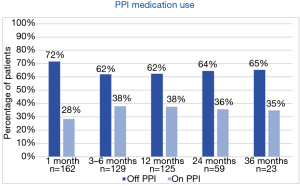
All patients on baseline had either persisting symptoms while on PPI, complain on side effects or did not wish to take medication lifelong. In total, 60–70% patients were able to completely stop PPI usage (Figure 4).
Results in specific patient populations
Laparoscopic sleeve gastrectomy (LSG)
This is now the most commonly performed bariatric procedure. However, LSG can result in new-onset GERD and may worsen pre-existing GERD. LSG patients with GERD not well controlled with PPI do not have good treatment options because the fundus is excised during the sleeve resection.
Preliminary results on patients with LSG and GERD with bothersome symptoms despite maximal medical therapy, treated with LES-ENT, revealed that LES-ENT is safe and results in a significant improvement in GERD symptoms and esophageal acid exposure with elimination for need for daily medication in the majority of subjects.
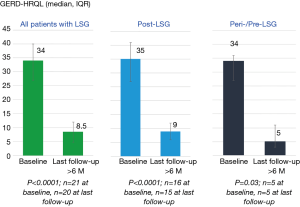
A recent study has shown the results on 10 patients with post-LSG GERD and underwent LES-ENT more than 1 year after LSG (14). All patients were using daily double-dose or higher dose PPIs before electrical neuromodulation. All patients reported improvement in their GERD symptoms shortly after initiation of LES-ENT. Median GERD-HRQL scores at baseline on-PPI were 25 (IQR, 18–31) which improved to 4 (IQR, 3–10) at their last follow-up at least 6 months on stimulation (Figure 5). Median distal esophageal acid exposure at baseline was 11.7% (IQR, 8.5–22.4%), which improved to 1.93% (IQR, 0.4–2.2%) at their last follow-up at least 6 months on stimulation This is within the normal range. At their last follow-up (median =12 months), 75% were off-PPIs and one each was using PPIs on <50% of days and standard dose once a day. The latter was on daily PPI as GI prophylaxis for chronic steroid therapy for kidney transplants and not GERD symptoms.
Discussion
In this paper, we have discussed the technique and latest findings on safety and efficacy of LES-ENT for GERD.
GERD has become one of the most frequent diseases of the upper GI tract (5).
Nearly 40% of patients are refractory to standard medical intervention, which typically begins with a PPI. The most severe cases with an impaired LES function as well as important anatomical disruptions are normally referred to surgery. Laparoscopic fundoplication remains to be the gold standard surgical option, with adequate long-term outcomes but at the expense of de novo symptoms such as bloating, inability to belch, diarrhea or constipation. In this scenario, 30% to 40% of the patients with partial symptom control under PPI, are not willing to undergo a laparoscopic fundoplication because of potential post fundoplication’s syndromes (15). Therefore, a “treatment gap” exists for these patients where new alternatives may play an important role.
Electrical neuromodulation of the LES has recently shown to improve outcomes in GERD in clinical trials up to 4 years, and may represent an alternative for this group of patients. Both clinical trials and registry studies have shown adequate efficacy in symptom control, medication use and acid exposure control (9,16). Although solid long-term data are not available yet, in patient series of up to 5 years excellent outcomes were reported. The main attractive feature of LES-ENT when compared to other surgical therapies is the lack of commonly reported side effects such as bloating, diarrhea or dysphagia. This can lead to an expanded indication of surgical therapies for those patients with unmet needs for GERD that are not satisfied under PPI therapy but would be reluctant to undergo side effects to avoid that. This is a common gap that is considered to include up to 20% of GERD patients (15). In contrast to endoscopic therapies, this technique allows for correction of hiatal hernia, if present, while still being a minimally invasive procedure. Other important beneficial feature is the ability of the device to allow optimization of the neuromodulation parameters after implant. Patients that not respond immediately after surgery can have different settings established and improve during the postoperative course. In the data presented from two different studies here we can observe that acid exposure control in the largest registry is improved but not significantly different to baseline as compared to the initial registry with fewer patients. This data is also contrasting with the excellent acid exposure control in the initial clinical trials. This is in partly explained by the fact that patients in the larger registry group are not undergoing pH-monitoring as standard of care. Moreover, patients who normally agree to repeat the study during the postoperative follow-up are normally those who are not finding a complete resolution of their symptoms creating a negative selection bias. Even within that scenario the trend is towards an improvement in acid exposure time which is not common in other alternative therapies for GERD.
Patients with GERD and other conditions where current surgical therapies are not feasible like LSG, or not advised like patients with severe esophageal dysmotility can benefit from this technology and appear to be good candidates for LES-ENT.
Longer term data and randomized trials are required to confirm these findings and expand the adoption of this novel medical therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luigi Bonavina) for the series “Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2018.10.03). The series “Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. AN serves as an unpaid editorial board member of Annals of Esophagus from Feb. 2018 to Jan. 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nicolau AE, Lobonåiu A, Constantinoiu S. New Minimally Invasive Endoscopic and Surgical Therapies for Gastroesophageal Reflux Disease (GERD). Chirurgia (Bucur) 2018;113:70-82. [Crossref] [PubMed]
- Vakil N, Shaw M, Kirby R. Clinical effectiveness of laparoscopic fundoplication in a US community. Am J Med 2003;114:1-5. [Crossref] [PubMed]
- Rodríguez L, Rodriguez P, Gómez B, et al. Long-term results of electrical stimulation of the lower esophageal sphincter for the treatment of gastroesophageal reflux disease. Endoscopy 2013;45:595-604. [Crossref] [PubMed]
- Soffer E, Rodríguez L, Rodriguez P, et al. Effect of electrical stimulation of the lower esophageal sphincter in gastroesophageal reflux disease patients refractory to proton pump inhibitors. World J Gastrointest Pharmacol Ther 2016;7:145-55. [Crossref] [PubMed]
- Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus 2007;20:130-4. [Crossref] [PubMed]
- Rodríguez L, Rodriguez P, Gómez B, et al. Electrical stimulation therapy of the lower esophageal sphincter is successful in treating GERD: final results of open-label prospective trial. Surg Endosc 2013;27:1083-92. [Crossref] [PubMed]
- Siersema PD, Smout AJ, Conchillo JM, et al. Electrical stimulation therapy (EST) of the lower esophageal sphincter (LES) - an effective therapy for refractory GERD - interim results of an international multicenter trial. United European Gastroenterol J 2013;1:A411.
- Kappelle WFW, Bredenoord AJ, Conchillo JM, et al. Electrical stimulation therapy of the lower oesophageal sphincter for refractory gastro-oesophageal reflux disease - interim results of an international multicentre trial. Aliment Pharmacol Ther 2015;42:614-25. [Crossref] [PubMed]
- Rodríguez L, Rodriguez PA, Gomez B, et al. Electrical stimulation therapy of the lower esophageal sphincter is successful in treating GERD: long-term 3-year results. Surg Endosc 2016;30:2666-72. [Crossref] [PubMed]
- Rodríguez L, Rodriguez P, Gomez B. Two-year results of intermittent electrical stimulation of the lower esophageal sphincter treatment of gastroesophageal reflux disease. Surgery 2015;157:556-67. [Crossref] [PubMed]
- Labenz O, Thattamparambil P, Nieponice A, et al. Interim Results of a Prospective Multi-Center Registry of Lower Esophageal Sphincter Stimulation for Gerd: The Lessgerd Registry. Gastroenterology 2018;154:S101. [Crossref]
- Rodriguez L, Rodriguez P, Gomez B, et al. Electrical Stimulation Therapy (EST) of the Lower Esophageal Sphincter (LES) is Successful in Treating GERD - Long-term 4 Year Results. Gastroenterology 2016;150:S476. [Crossref]
- Labenz J, Schulz H, Leodolter A, et al. Interim Results of a Prospective Multicenter Registry of Lower Esophageal Sphincter Stimulation for GERD. Surg Endosc 2017;31:S1-60.
- Borbély Y, Bouvy N, Schulz HG, et al. Electrical stimulation of the lower esophageal sphincter to address gastroesophageal reflux disease after sleeve gastrectomy. Surg Obes Relat Dis 2018;14:611-5. [Crossref] [PubMed]
- Labenz J, Labenz G, Stephan D, et al. Insufficient symptom control under long-term treatment with PPI in GERD - fact or fiction? MMW Fortschr Med 2016;158:7-11. [Crossref] [PubMed]
- Kim SE, Soffer E. Electrical stimulation for gastroesophageal reflux disease: current state of the art. Clin Exp Gastroenterol 2016;9:11-9. [PubMed]
Cite this article as: Nieponice A, Ramirez M, Badaloni A, Renda P, Lovera R, Ruurda JP, van Berge Henegouwen MI. Electrical neuromodulation of the lower esophageal sphincter for the treatment of gastroesophageal reflux disease. Ann Esophagus 2018;1:18.


