射频消融对Barrett食管治疗的作用
引言
在西方人群中,胃食管反流病发生率为30%~40%[1-3],其典型或非典型症状(烧心、反酸、咳嗽、气喘、哮喘、鼻窦炎)影响着人们健康和生活质量[1-3]。在30%的GERD阳性患者中,从鳞状上皮和柱状上皮交界处(SCJ)活检可以诊断Barrett食管(BE)[1,4]。BE增加了食管腺癌发病的风险(平均每年增加0.5%)[1,5]。
在病理生理上,GERD因食管下括约肌(LES)功能障碍及其膈食管裂孔异常(如食管裂孔疝)而引起[6],这促使胃内容物(胃酸、胆汁、食物)反流到食管。反流引起食管鳞状上皮被柱状上皮取代(CLE)[4,6,7]。与Chandrasoma关于CLE的分类一致,CLE是由反流引起的,影响到正常食管鳞状上皮和近端胃的正常黏膜(泌酸黏膜)交界线[7]。组织病理学对CLE做出分型:贲门黏膜(CM;上皮黏膜;浅表型;小凹型;腺型);近胃端黏膜(由黏液上皮组织和CLE中央凹下区域内的壁细胞腺体);肠化生(IM,CM以杯状细胞代替壁细胞);BE[4,6,7]。胃泌酸黏膜由直管状腺体组成,CLE腺体的小凹下区域呈分叶状和分支状[7]。因此,正常组织病理学可以区分正常(鳞状上皮黏膜;胃泌酸黏膜)和反流导致的CLE[CM,贲门黏膜(OCM),IM][7]。
随着时间的推移,反流可能会引起不伴有发育异常的BE向低级(LGD)、高级(HGD)和癌症(CA)发展。进展的危险因素包括长期GERD(>10年)、使用质子泵抑制药(PPI)、食管裂孔疝、食管炎、食管癌和GERD阳性家族史[1,7,8]。
射频消融(RFA)是一种新的内镜治疗,可有效、持久地消除BE(±发育不良)[1,5,8]。因此,与对照组相比,RFA已被证实可预防LGD和HGD患者的癌症发展[1,5,8]。尽管可能存在个体差异,无异型增生的BE的RFA有助于预防癌症,根据该理论,BE的RFA治疗应该有助于预防癌症[1,5,6]。但仍需要大样本量对照研究进一步证实。
本综述旨在评估我们目前对RFA治疗伴或不伴异型增生的BE的效果。最后,我们讨论了生活方式和营养(饮食)对BE和GERD发展和治疗的影响。
方法
使用PubMed、Google和Springer LINK,我们的检索包括以下关键词:GERD、Barrett's食管、内窥镜检查、组织病理学、Chandrasoma分类、RFA、抗反流手术和饮食/GERD,以及BE的营养。没有应用统计学。使用Storz技术获得内窥镜图像,组织病理学幻灯片由Fritz Wrba博士(维也纳)慷慨提供。
结果
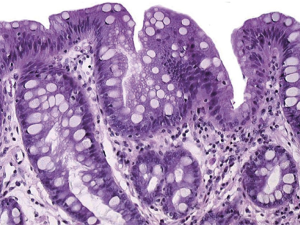
BE的诊断是通过SCJ活检获得的组织病理确定的[1,3,7](图1~图2)。如果SCJ活检病理包含带有杯状细胞的CLE(例如CM),即是无发育不良的BE[1,7](图2)。通过是否存在不规则形态的细胞和腺体来区分BE与LGD和HGD[7,8]。食管腺癌(T1a、T2b)定义是HGD超出CLE固有肌层和(或)向血液、淋巴管和周围神经侵犯[7]。病理学家对于LGD、HGD和癌症的诊断起到重要作用[1]。一项有趣的研究发现证实,嗜酸性粒细胞性食管炎(EoE)的存在可以排除BE的诊断[9]。由此可见EoE似乎阻止了BE发生,但这潜在的机制尚未阐明。
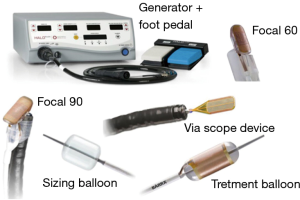
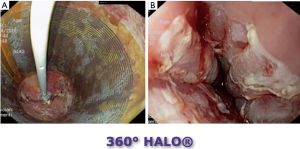
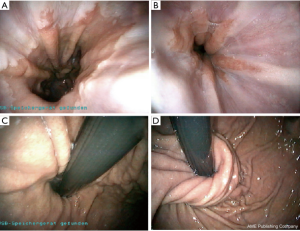
由于癌症风险显著增加,LGD、HGD和早期食管癌(T1a)的治疗应包括内镜(亚)黏膜切除术(EMR)和存在CLE的RFA[1,10-15]。RFA采用一种新的球囊导管,内镜尖端安装或通过合适的电极系统,将RF能量传递到黏膜,进而消融BE病变黏膜[1,10-15](图3~图4)。可能需要3~4次治疗才能完全消除伴有异型增生和无异型增生BE[1]。在有经验的医疗中心进行时,早期癌症/发育不良(LGD、HGD)和非发育不良BE(NDBE)的清除干净率分别达到80%以上和90%以上[1,10-15]。在黏膜切除和RFA治疗之后,推荐PPI治疗以促进伤口愈合并防止复发[1,10-15]。对于NDBE癌症风险较高(即GERD>10年,CLE长度>2.0 cm)的患者,以及有食管炎、食管裂孔疝、食管癌和胃癌阳性家族史、不典型增生和NDBE病史的患者,建议对NDBE进行RFA[1,16,17]。目前,对于NDBE的RFA治疗后癌症预防效果需进一步在对照试验中验证[1]。
建议进展期的食管癌(>T1a)进行手术切除±肿瘤综合治疗。GERD、BE、发育不良和癌症的病因仍然存在疑问,需进一步研究[1,7]。
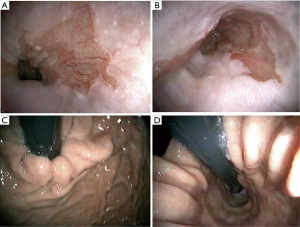
从定义上看,胃酸分泌、胃食管反流、CLE和BE(±LGD,HGD)的发展不是病因,只是病因的表现。GERD和BE的病因是LES的功能障碍及其在膈食管裂孔发育异常[7]。PPI治疗仅改变反流的pH值,但不能减少反流,PPI治疗不能恢复LES的功能障碍,PPI治疗不能修复扩大的膈肌裂孔(食管裂孔疝)[1,5,7,18,19]。因此,抗反流手术和食管裂孔修补术应考虑用于消除存在反酸或无反酸的GERD阳性症状的患者[18,19]。(图5)。无症状BE±不典型增生的抗反流手术预防癌症发展的效果需进一步研究证实。然而,最近的证据表明,与药物(PPI)治疗相比,有效的抗反流手术(+裂孔修补术)可能对癌症预防更有效 [1,8,20]。因此,在抗反流手术前,建议应用食管功能检查(高分辨阻抗测压;pH阻抗回流监测)评估食管功能并排除食管运动障碍(贲门失弛缓症、食管痉挛、Jackhammer食管)[21-25]。这有助于手术治疗方案的优化。内镜治疗后的后续内镜检查在HGD、LGD和无发育不良BE治疗后3、3和6个月进行[1,26,27]。BE阴性CLE应每12个月随访一次[1],BE阳性CLE应接受重复内镜治疗(RFA±EMR),直至切除干净[1]。RFA后的并发症包括狭窄(4%)、出血和穿孔(<1.0%)[1]。发生RFA±EMR后并发症可能与疾病进展有关(存在发育不良、食管炎、食管裂孔疝,GERD持续时间)[27,28]。
最近有文献研究表明,营养在BE、发育异常和食管癌方面起到重要作用[29]。因此,已证实高糖饮食会造成BE的进展,以及使食管腺癌发病风险提高7~10倍[29]。因此,在治疗GERD和BE饮食方案中推荐低碳水化合物饮食。
讨论
我们发现,与对照组相比,RFA±EMR可以预防BE阳性患者进展为LGD、HGD和早期癌症[10-15,30,31]。因此,发育不良的BE和早期食管癌应该安排RFA治疗。对于进展期食管癌治疗包括手术切除±肿瘤治疗[1]。
对于无发育不良的BE治疗仍存在争议。目前,在对照组中,对于食管癌风险较高的NDBE应予以RFA[1,4]。这些风险因素包括:长期GERD(>10年)、食管炎、食管裂孔疝、GERD阳性家族史、BE和贲门癌,BE±不典型增生病史[1,4]。对BE阳性患者的GERD治疗采用PPI治疗仍有待研究[19]。
从定义上讲,LES的功能障碍和解剖结构异常是疾病发生的原因[7,8]。然而,胃内容物反流到食管引起食管炎,又促进了BE和食管癌的发展[7,8]。虽然PPI疗法会改变反流液的pH值,但并不能修复LES的功能障碍,也无法缩小已经扩大的膈肌裂孔[20,22]。因此,PPI治疗本身并不能减少反流,只是让酸性反流液变为非酸性的。证据研究表明,PPI治疗引起的碱性反流会增加食管癌的发病[20]。相比之下,最近研究表明,有效的抗反流手术是修复病因,消除已经出现的反流和症状,并有助于修复BE进展的问题[1,8,22]。因此,在对照试验中考虑抗反流手术(+食管裂孔修补)治疗BE±发育不良和早期癌症是合理的。抗反流手术(+裂孔修补)包括胃底折叠术、磁括约肌增强术(LINX)和食管下括约肌电刺激疗法[32,33-35]。建议通过食管功能检查帮助制订个体化治疗方案[21,23,24]。
RFA治疗后(±EMR,抗反流手术)通过精准的内镜随访检查可排除BE(±发育不良)复发,并排除存在所谓隐匿性腺体(<1.0%)[1]。因此,BE治疗应在诊断和治疗GERD和BE方面有足够经验的中心进行。随访间隔正如结果描述那样,根据组织病理学基线水平,时间范围从3~12个月不等。
我们发现了一个有趣的研究结果,营养对于BE和食管癌的发展具有重要意义和相关性。正如最近的研究表明,增加含浓缩糖的食品和饮料,以及天然和合成糖的摄入,与BE和食管癌的风险增加有关[29]。因此,应将营养摄入方式管理纳入BE和GERD的治疗过程中。
综上所述,BE治疗管理(诊断、治疗、随访)需协调多学科完成,包括内窥镜检查、组织病理学、生理学、营养学(饮食)和手术,通过正在进行和未来研究的结果来证明这种新方法的有效性,否则“北欧神话”将继续存在,反流仍是BE发展的根本原因和病因所在[35,36]。正确的理解到这点,为患有GERD和BE的人有效预防癌症的提供可行性[1,7,8]。
Acknowledgments
We thank the patients from whom we learnt to watch out and listen to the sound and specific orchestration of GERD and BE. In addition, we thank our families, friends and teachers, who supported our search for a better understanding of the disease. May the paper contribute to foster childlike curiosity of the readers, who are interested in GERD and BE.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luigi Bonavina) for the series “Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2018.07.04). The series “Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. SFS serves as an unpaid editorial board member of Annals of Esophagus from Aug. 2018 to Jul. 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Riegler M, Kristo I, Nikolic M, et al. Update on the management of Barrett’s esophagus in Austria. Eur Surg 2017;49:282-7. [Crossref] [PubMed]
- Falk GL, Vivian SJ. Laryngopharyngeal reflux: diagnosis, treatment and latest research. Eur Surg 2016;48:74-91. [Crossref]
- Koch OO, Antoniou SA. Advances in diagnosing GERD: Which examinations should be performed before interventional therapy? Eur Surg 2016;48:203-8. [Crossref]
- Chandrasoma P, DeMeester T. A new pathologic assessment of gastroesophageal reflux disease: the squamo-oxyntic gap. Adv Exp Med Biol 2016;908:41-78. [Crossref] [PubMed]
- Kristo I, Riegler M, Schoppmann SF. Endoscopic therapy for Barrett’s esophagus: who and how? In: Simic AP, Bonavina L, DeMeester SR. editors. Surgery for benign oesophageal disorders. London WC2H 9HE: World Scientific, 2018:105-32.
- Chandrasoma PT. The effect of damage to the abdominal segment of the LES: the dilated distal esophagus. In: Chandrasoma PT. GERD: a new understanding. Academic Press Elsevier, 2018:255-96.
- Chandrasoma PT. Histologic definition and diagnosis of epithelia in the esophagus and proximal stomach. In: Chandrasoma PT. GERD: a new understanding. Academic Press Elsevier, 2018:73-107.
- Kristo I, Asari R, Rieder E, et al. Treatment of Barrett's esophagus: update on new endoscopic surgical modalities. Minerva Chir 2015;70:107-18. [PubMed]
- Saboorian MH, Genta RM, Marcus PB, et al. Inverse association of esophageal eosinophilia and Barrett’s esophagus. J Clin Gastroenterol 2012;46:752-57. [Crossref] [PubMed]
- Lipman G, Haldry RJ. Endoscopic management of Barrett’s and early oesophageal neoplasia. Frontline Gastroenterol 2017;8:138-42. [Crossref] [PubMed]
- Costamagna G, Battaglia G, Repici A, et al. Diagnosis and endoscopic management of Barrett’s esophagus: an Italian expert’s opinion based document. Dig Liver Dis 2017;49:1306-13. [Crossref] [PubMed]
- Muñoz-Largacha JA, Litle VR. Endoscopic mucosal ablation and resection of Barrett’s esophagus and related diseases. J Vis Surg 2017;3:128. [Crossref] [PubMed]
- Visrodia K, Zakko L, Wang KK. Radiofrequency Ablation of Barrett's Esophagus: Efficacy, Complications, and Durability. Gastrointest Endosc Clin N Am 2017;27:491-501. [Crossref] [PubMed]
- Yang D, Zou F, Xiong S, et al. Endoscopic submucosal dissection for early Barrett's neoplasia: a meta-analysis. Gastrointest Endosc 2018;87:1383-93. [Crossref] [PubMed]
- Belghazi K, Bergman JJGHM, Pouw RE. Management of Nodular Neoplasia in Barrett's Esophagus: Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection. Gastrointest Endosc Clin N Am 2017;27:461-70. [Crossref] [PubMed]
- Jomrich G, Schoppmann SF. Targeted therapy in gastric cancer. Eur Surg 2016;48:278-84. [Crossref] [PubMed]
- Derici ZS, Sokmen S. Gastric carcinoma presenting with severe rectal stenosis: “Schnitzler’s metastasis”: care report and review of the literature. Eur Surg 2016;48:246-9. [Crossref]
- Hatlebakk JG, Zerbib F, Bruley des Varannes S, et al. Gastroesophageal acid reflux control 5 years after anti reflux surgery, compared with long term esomeprazole therapy. Clin gastroenterol Hepatol 2016;14:678-85.e3. [Crossref] [PubMed]
- Kristo I, Riegler M, Schoppmann SF. Should anti-reflux surgery be considered after successful endoscopic treatment of Barrett’s esophagus with dysplasia and early cancer? Endoscopy 2016;48:92. [PubMed]
- Greene CL, Worrell SG, DeMeester TR. Rat reflux model of esophageal cancer and its implication in human disease. Ann Surg 2015;262:910-24. [Crossref] [PubMed]
- Ringhofer C, Lenglinger J, Riegler M, et al. Waist to hip ratio is a better predictor of esophageal acid exposure than body mass index. Neurogastroenterol Motil 2017;29: [Crossref] [PubMed]
- Gurski RR, Mazzini GdS, Campos VJ. Fundoplication for gastroesophageal reflux disease. In: Simic AP, Bonavina L, DeMeester SR. editors. Surgery for benign oesophageal disorders. London WC2H 9HE: World Scientific, 2018:11-33.
- Andolfi C, Bonavina L, Kavitt RT, et al. Importance of esophageal manometry and pH monitoring in the evaluation of patients with refractory gastroesophageal reflux disease: a multicenter study. J Laparoendosc Adv Surg Tech A 2016;26:548-50. [Crossref] [PubMed]
- Weitzendorfer M, Köhler G, Antoniou SA, et al. Preoperative diagnosis of hiatal hernia: barium swallow X ray, high resolution manometry, or endoscopy? Eur Surg 2017;49:210-7. [Crossref] [PubMed]
- Johnson CS, Louie BE, Wille A, et al. The durability of endoscopic therapy for treatment of Barrett’s metaplasia, dysplasia and mucosal cancer after Nissen fundoplication. J Gastrointest Surg 2015;19:799-805. [Crossref] [PubMed]
- Saxena N, Inadomi JM. Effectiveness and cost-effectiveness of endoscopic screening and surveillance. Gastrointest Endosc Clin N Am 2017;27:397-421. [Crossref] [PubMed]
- Fujii-Lau LL, Cinnor B, Shaheen N, et al. Recurrence of intestinal metaplasia and early neoplasia after endoscopic eradication therapy for Barrett’s esophagus: a systematic review and meta-analysis. Endosc Int Open 2017;5:E430-49. [Crossref] [PubMed]
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors Associated With Progression of Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:1046-55.e8. [Crossref] [PubMed]
- Riegler M, Kristo I, Asari R, et al. Dietary sugar and Barrett’s esophagus. Eur Surg 2017;49:279-81. [Crossref] [PubMed]
- Lee SW, Lien HC, Chang CS, et al. Health-related quality of life of subjects with Barrett's esophagus in a Chinese population. PLoS One 2017;12:e0190201 [Crossref] [PubMed]
- Hendy P. Radiofrequency ablation is associated with decreased neoplastic progression in patients with Barrett’s oesophagus and confirmed low-grade dysplasia. Frontline Gastroenterol 2016;7:156-7. [Crossref] [PubMed]
- Schwameis K, Nikolic M, Morales Castellano DG, et al. Results of Magnetic Sphincter Augmentation for Gastroesophageal Reflux Disease. World J Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Louie BE, Smith CD, Smith CC, et al. Objective evidence of reflux control after magnetic sphincter augmentation: one-year results from a post approval study. Ann Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Prusa AM, Kristo I, Rieder E, et al. Tension-Free Inlay Repair of Large Hiatal Hernias Using Dual-Sided Composite PTFE/ePTFE Meshes in Laparoscopic Surgery for Gastroesophageal Reflux Disease. J Laparoendosc Adv Surg Tech A 2017;27:710-4. [Crossref] [PubMed]
- Riegler M. Delphi comes to Milan to stop oracles on GERD and Barrett‘s esophagus. Eur Surg 2018;50:1-3. [Crossref]
- Kousoulis AA, Mylonas KS, Economopoulos KP. Violent death and trauma in norse mythology: a systematic reading of the Prose Edda. Eur Surg 2016;48:304-10. [Crossref]

程净革
医学硕士,河北医科大学第四医院胸外主治医师,长期从事胸外科常见病诊疗工作,主要研究方向:食管癌及肺癌的综合治疗。近3年发表论文3篇,主要集中在食管癌和肺癌的综合治疗方面。(更新时间:2021/8/7)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Riegler M, Jomrich G, Schoppmann SF. Role of radiofrequency ablation in Barrett’s esophagus. Ann Esophagus 2018;1:5.