
The sequel of age and frailty on the pathophysiology and treatment of surgical esophageal diseases
Introduction
The natural aging process can lead to changes in the gastrointestinal tract, including the esophagus. Alterations in the contractility and relaxation of the esophageal musculature can be found in older individuals, even if asymptomatic (1).
In addition, with increasing age, there is a higher occurrence of frailty in the elderly. Frailty is characterized as a decline in functioning in various physiological systems, accompanied by an increased vulnerability to stressors (2). In 2001, Fried et al. (2) described the clinical presentation of frailty as a physical phenotype, a definable biological syndrome. This phenotype of frailty considers three or more positive symptoms or signs in five criteria: weakness, slow walking speed, low physical activity, exhaustion, and unintentional weight loss (2). This process does not only occur as an extreme process of aging, but can also be related to systemic diseases, including malnutrition, inflammatory diseases, and other chronic conditions (3).
There are different scales and indices that can be used to assess frailty, including handgrip strength, gait speed, anthropometric assessment, presence of comorbidities, and functionality. However, a multidimensional approach that evaluates various parameters may be more effective in identifying frail older adults, allowing for early interventions, and reducing the risks of adverse health events (4,5).
It is important to highlight that frailty is a predictive factor of poor prognosis in older adults, increasing rates of mortality, hospitalization, falls, and admission to long-term care institutions (2,6).
This article seeks to comprehensively review the existing literature on the influence and impact of frailty on esophageal disorders, with the goal of enhancing our understanding of the interplay between these two factors in the health of older adults. Specifically, we will conduct a detailed examination of the age and frailty-related changes observed in patients with esophageal diseases, while placing a particular emphasis on their relevance to the field of esophageal surgery.
Motility changes associated with frailty
Healthy aging, also known as senescence, is responsible for several changes throughout the body. As we can see in Figure 1, various studies have sought to correlate esophageal motility changes with the aging process (7,8).
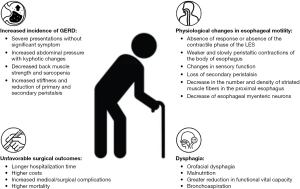
Esophageal manometry studies have shown alterations in the contraction and relaxation of the esophagus in healthy elderly individuals. Djinbachian et al. (9) evaluated manometric parameters considered normal by the Chicago 4.0 classification and observed a positive correlation between age and the integral of the relaxation pressure (IRP) and the integral of the distal contractile integral (DCI), and a negative correlation, to a lesser degree, with distal latency, in symptomatic patients. Jung et al. (10) evaluated asymptomatic healthy individuals to assess the effect of age and sex on manometric profiles and found a positive correlation between age and the IRP and DCI. Possible reasons for altered relaxation of lower esophageal sphincter (LES) are increase in the stiffness of the smooth muscle, reduction of primary and secondary peristalsis and impaired coordination of the upper esophageal sphincter (UES) and pharynx (11). Distal contractile amplitude and duration increased significantly with age, with also may justify the increase of DCI (12).
In older individuals, even if asymptomatic, an inadequate response of the LES can be found, characterized by a partial or complete absence of relaxion phase initiated by deglutition, an absence of the contractile phase after relaxation of LES or both of them. In addition, in the body of the esophagus, peristaltic contractions are weaker and progress more slowly, leading to a higher incidence of disordered contractions (13,14).
The aging process is responsible for several physiological changes in the gastrointestinal system. Among these changes, the sensory function of the esophagus may deteriorate over time, which contributes to the atypical presentation of many esophageal disorders in this age group. In 1964, the concept of presbyesophagus was proposed to explain the changes in peristaltic pressures and esophageal contractility secondary to the natural aging process (7). However, with the advent of esophageal manometry, modern manometric techniques, and classification systems, this concept has been questioned. The majority of elderly patients with abnormal peristalsis are now classified into one of the described motility disorders (8). In spite of motor disorders are more frequent in the old, it does not mean that motility inexorably degrades with time (15). Therefore, many cases of esophageal dysmotility previously labeled as presbyesophagus may be related to damage caused by untreated long-term gastroesophageal reflux disease (GERD), for example (8).
Changes manifest in the physiology of the esophagus and in the symptoms presented in older patients (8). Among the possible causes, the loss of peristaltic function in these patients may be explained by the decrease in the number of esophageal myenteric neurons (16).
The decrease in esophageal innervation is possibly causative of the loss of secondary peristalsis (17). This peristalsis depends on the sensory and motor function of the esophagus, both of which can be affected by aging. Although the thickness of the wall does not change with age, a study by Leese et al. (18) suggests a decrease in the number and density of striated muscle fibers in the proximal esophagus, which was not observed in the smooth muscle portion. Hollis and Castell (19) evaluated the response of esophageal pressures to edrophonium chloride, a cholinergic agonist that stimulates the contraction of the smooth muscle fibers in the body of the esophagus, and noted that in older individuals, pressures do not increase as promptly as in younger individuals, indicating muscle weakening without alteration in neurological function.
Effect of frailty on esophageal diseases
GERD
The changes of sensory function of the esophagus with age contribute to a more severe presentation of GERD without significant symptoms in this population (8). The increase in stiffness and reduction of primary and secondary peristalsis found in the human esophagus with deterioration of esophageal function over the years may contribute to a higher prevalence of GERD in older adults (20).
Frailty may be one of the risk factors for the increased incidence of GERD in the elderly population. In a prospective cohort, Imagama et al. (21) demonstrated that kyphotic changes, vertebral column inclination, decreased back muscle strength, and sarcopenia are significant risk factors for the development of GERD. The study suggests that an increase in intra-abdominal pressure caused by kyphosis can induce a decrease in the LES pressure and hiatal hernia, leading to gastroesophageal reflux. Although not significant, the prevalence of frailty was higher in individuals with GERD when compared to those without the pathology both at the beginning of the study (10.5% vs. 5.7%) and 5 years later, among those who did not have GERD and developed it during those years (10.5% vs. 6.4%) (21).
In a retrospective cross-sectional study involving consecutive outpatients aged 65 years or older, Asaoka et al. (22) examined medical records from a geriatric center. The study assessed various aspects, including patient profiles, osteoporosis evaluation, sarcopenia evaluation, frailty assessment, nutritional status, findings from upper gastrointestinal endoscopy, and questionnaire responses regarding abdominal symptoms. The subjects were then categorized into frailty and non-frailty groups, and the researchers investigated the risk factors for frailty. The results revealed that frailty was significantly associated with a higher prevalence of symptoms related to GERD and constipation. Constipation is a significant risk factor for malnutrition, that is a significant cause of frailty (23), In a logistic regression model for the prevalence of prefrailty, Matsushita et al. showed that chronic constipation was a significant and independent determinant, and they suggested that autonomic failure is associated with prefrailty among older individuals (24). Other factors such as age and sarcopenia were also associated with frailty and contribute to GERD occurrence.
Dysphagia
The process of swallowing is complex and involves different structures, organs, and systems. Difficulty in swallowing, defined as dysphagia, can be broadly categorized into two regions. Oropharyngeal dysphagia involves the oral cavity, striated muscles of the tongue and pharynx and is primarily associated with central neurological disorders and esophageal or conductive dysphagia, is intrinsically related to the smooth muscles of the esophagus and its motility.
A fragility in older individuals is related to a worsening of nutritional status, which has an impact on patients’ lives and increases the risk of morbidity, such as aspiration pneumonia. An institutionalized Japanese elderly population study showed that the occurrence of dysphagia was independently associated with worse nutritional status and frailty (25).
Frail patients have a higher risk of dysphagia, which is associated with adverse outcomes in hospitalizations or surgery, such as longer hospital stays, increased costs, and more complications (26). The main components of frailty that lead to increased dysphagia are sarcopenia, functional impairment, and drugs that affect swallowing (27).
Sarcopenia is the loss of muscle mass that leads to a loss of function (28). There are indirect ways to assess this muscle loss, such as grip strength and walking speed (29). Regarding swallowing function, most studies have evaluated the consequences of a reduction in generalized skeletal muscle in the proximal portion of the esophagus, also composed of striated muscle. It is observed that older patients with sarcopenia have weaker pharyngeal contractility and dysfunction of the UES (30).
Dysphagia tends to be more severe in the elderly and can be caused by health conditions unrelated to swallowing. The increase in inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in response to physiological and environmental stressors associated with a reduction in immune system activity in diseases that course with chronic inflammation has a role in the development of age-related diseases, including frailty (31,32). Diabetes, cardiovascular diseases, obesity, alterations in gut microbiota, and depression are some examples of comorbidities associated with this pathophysiological process (32-34).
Therefore, despite age being a factor for higher prevalence, the presence of dysphagia can result from other factors or comorbidities. Regardless of age, malnutrition and frailty are positively correlated with the severity of dysphagia (35).
Frailty-related dysphagia is also associated with a more pronounced decline in functional vital capacity. In a comparative study between individuals experiencing dysphagia caused by frailty and those with dysphagia resulting from brain injury, multivariate logistic regression analysis demonstrated a significant correlation between forced vital capacity (FVC) and severity scores assessed through videolaryngofluoroscopy in frailty-induced dysphagia (P<0.05). However, no such significance was observed in dysphagia induced by brain injury (P≥0.05) (36). This discrepancy between the two groups can be attributed to the presence of systemic sarcopenia in the first group.
Different forms of assessment can be used to evaluate frailty and estimate the risk of dysphagia (29,37). Despite the differences between assessment models, they all focus on function and physical activity. Changes in oral and swallowing functions are evident in older individuals, which can affect functional domains such as nutrition and sarcopenia in frail elderly, but their evaluation is not included in many frailty assessment models and tools (38). Hathaway et al. (39) showed that while age and hospitalization were associated with lower grip strength as a form of frailty assessment, hospitalized patients also had an association with dysphagia and bronchoaspiration.
Dysphagia is a multifactorial condition that is not only related to swallowing disorders but also to other components of frailty, such as physical, cognitive, and psychological aspects (40). In addition to ensuring adequate nutritional intake, it is important to perform appropriate oral rehabilitation to treat problems related to dentition and xerostomia, as well as to perform resistance training for the tongue and oropharyngeal muscles (41). Early detection of dysphagia and treatment based on nutritional rehabilitation and resistance physical exercises can modify poor prognostic factors for clinical and surgical outcomes associated with frailty (42).
Effect of frailty on the outcomes of esophageal surgery
The occurrence of unfavorable surgical outcomes in older patients is multifactorial and depends on factors such as comorbidities, functional status, and cognition (43-45). Among the factors of poor prognosis, dysphagia appears to be an independent risk factor for the occurrence of unfavorable outcomes in surgical patients. Longer hospitalization time, higher costs, and increased medical/surgical complications were observed among frail and non-frail patients. In turn, the prevalence of dysphagia is higher in populations with frailty (46).
Esophagectomy
The elderly patient undergoing esophagectomy for cancer usually has a coexistence of other diseases that can adversely affect their postoperative course. These comorbidities seem to play a more important role than age alone. Madhavan et al. (47) found that a population over 70 years of age would be more prone to greater perioperative morbidity and mortality in esophagectomy for cancer. Pultrum et al. (48) found in their study that, after multivariate analysis, age alone (above 70 years) was not a prognostic indicator for survival, but the presence of comorbidities was a significant factor.
Considering that frailty affects postoperative outcomes (49), Lee et al. (50) evaluated the effects of frailty in a retrospective cohort of patients undergoing surgical resection for esophageal cancer. Populations between 18 and 65 years and over 65 years were observed. In both groups, frail patients had higher mortality and postoperative respiratory failure, showing that frailty is an important factor independent of age.
Sarcopenia as a component of frailty evaluation also has an important role as a predictor of favorable outcome. A lower muscle mass in patients undergoing esophagectomy for cancer is an independent predictor of mortality and disease-free survival time (45).
Heller’s myotomy
Laparoscopic Heller’s myotomy (LHM), per-oral endoscopic myotomy (POEM), and pneumatic dilatation (PD) are well-established methods for treating achalasia. In older patients, less invasive procedures are preferred to reduce the risk of complications. However, in specialized centers, treatment of the oldest-old patients should be based solely on their physiological and mental health, not their age (51). In a multicenter study, Zotti et al. (51) reviewed outcomes and changes in routine clinical therapeutic options in achalasia patients over 80 years of age and found that LHM can be safely performed in patients with achalasia aged 80 years or older and should be considered as the first choice for treatment since it is associated with a low rate of complications and a satisfactory improvement in symptoms.
Laparoscopic antireflux surgery
The studies for the evaluation and definition of better surgical techniques often exclude older populations, leading to uncertainty regarding the best therapeutic strategy for this population. Retrospective studies with octogenarian populations for laparoscopic anti-reflux surgery show, on the other hand, that despite higher rates of morbidity and mortality compared to individuals under 80 years of age, elective repair of paraesophageal hiatal hernia can be performed in octogenarians, especially in patients without comorbidity (52), with symptomatic improvement and minimal morbidity and mortality (52,53). It is evident that age per se is not a complicating factor, and it is mainly linked to the presence of comorbidities and the patient’s clinical condition. However, studies with this specific population are more necessary for better evaluation and therapeutic definition.
Management of frailty and esophageal diseases
A comprehensive care plan for frailty should address various aspects, including polypharmacy (whether rational or nonrational), management of sarcopenia, treatable causes of weight loss, and the underlying causes of exhaustion, such as depression, anemia, hypotension, hypothyroidism, and B12 deficiency (54).
To prevent malnutrition and complications related to dysphagia, such as aspiration pneumonia, effective interventions have been studied (55). Sire conducted a review of different approaches to managing sarcopenic dysphagia, malnutrition, and oral frailty in the elderly (55).
Oral rehabilitation interventions include functional training, compensatory maneuvers, postural adjustments, swallowing maneuvers, and dietary modifications (55). In the case of sarcopenic dysphagic patients, adopting an upright seated position with head/neck flexed is recommended as it optimizes swallowing performance (56,57). Postural adjustments have also been shown to significantly improve self-perceived difficulties in swallowing maneuvers (57,58). Specifically, maintaining an upright 90° seated position for at least 30 minutes after eating reduces the risk of food inhalation (58,59).
Tongue-pressure resistance training, a strengthening exercise, has been found to improve hyoid bone movements, tongue pressure, and the width of the UES (60). Additionally, modifying the consistency of solid and/or liquid foods can enhance the safety and effectiveness of oral feeding and intake for dysphagic patients (61).
Given the increased risk of complications and higher mortality associated with frailty, a frailty screen should be included in the perioperative evaluation of elderly patients undergoing elective major surgery (62). A detailed assessment of frail patients is necessary to identify the underlying causes of their frailty. Implementing multimodal prehabilitation programs may improve the perioperative prognosis for frail patients (63). Diagnosing frailty enables the determination of patients’ eligibility for surgeries, as well as prehabilitation and rehabilitation programs, which can help reduce postoperative complications, hospital length-of-stay, and improve outcomes (64).
Conclusions
The natural aging process is responsible for physiological changes in esophageal motility, but these changes alone cannot be blamed for the pathological alterations found in the elderly population. On the other hand, frailty is an isolated risk factor for the occurrence of severe gastroesophageal reflux, unfavorable surgical outcomes, and dysphagia.
Different instruments can be used to assess frailty and sarcopenia, which hinders result comparisons and the establishment of protocols for studies. The physiopathology involved in this process is complex and further studies are necessary for better understanding. The exact mechanisms underlying the impact of frailty on esophageal disorders remain uncertain and appear to be multifactorial. However, the impact on clinical features is significant. The management of this disorder encompasses various strategies, including improving nutritional status, addressing sarcopenia, and implementing oral rehabilitation. Whenever possible, these interventions should be instituted before considering invasive procedures.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-23-9/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-23-9/coif). F.A.M.H. serves as an unpaid editorial board member of Annals of Esophagus from September 2022 to August 2024. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cock C, Omari T. Systematic Review of Pharyngeal and Esophageal Manometry in Healthy or Dysphagic Older Persons (>60 years). Geriatrics (Basel) 2018;3:67. [Crossref] [PubMed]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. [Crossref] [PubMed]
- Hiesmayr M, Tarantino S, Moick S, et al. Hospital Malnutrition, a Call for Political Action: A Public Health and NutritionDay Perspective. J Clin Med 2019;8:2048. [Crossref] [PubMed]
- Faller JW, Pereira DDN, de Souza S, et al. Instruments for the detection of frailty syndrome in older adults: A systematic review. PLoS One 2019;14:e0216166. [Crossref] [PubMed]
- Furtado GE, Letieri R, Hogervorst E, et al. Physical Frailty and cognitive performance in older populations, part I: systematic review with meta-analysis. Cien Saude Colet 2019;24:203-18. [Crossref] [PubMed]
- Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752-62. [Crossref] [PubMed]
- Soergel KH, Zborlaske FF, Amberg JR. Presbyesophagus, esophageal motility in nonagenarians. J Clin Invest 1964;43:1472-9. [Crossref] [PubMed]
- DeVault KR. Presbyesophagus: Do modern data support the concept? Curr GERD Rep 2007;1:84-90. [Crossref]
- Djinbachian R, Marchand E, Yan W, et al. Effects of Age on Esophageal Motility: A High-Resolution Manometry Study. J Clin Med Res 2021;13:413-9. [Crossref] [PubMed]
- Jung KW, Jung HY, Myung SJ, et al. The effect of age on the key parameters in the Chicago classification: a study using high-resolution esophageal manometry in asymptomatic normal individuals. Neurogastroenterol Motil 2015;27:246-57. [Crossref] [PubMed]
- Achem SR, Devault KR. Dysphagia in aging. J Clin Gastroenterol 2005;39:357-71. [Crossref] [PubMed]
- Richter JE, Wu WC, Johns DN, et al. Esophageal manometry in 95 healthy adult volunteers. Variability of pressures with age and frequency of "abnormal" contractions. Dig Dis Sci 1987;32:583-92. [Crossref] [PubMed]
- Grande L, Lacima G, Ros E, et al. Deterioration of esophageal motility with age: a manometric study of 79 healthy subjects. Am J Gastroenterol 1999;94:1795-801. [Crossref] [PubMed]
- Khan TA, Shragge BW, Crispin JS, et al. Esophageal motility in the elderly. Am J Dig Dis 1977;22:1049-54. [Crossref] [PubMed]
- Vaiano T, Herbella FAM. Esophageal aging: are presbyesophagus and Berstein test back? Ann Esophagus 2018;1:9. [Crossref]
- Eckardt VF, LeCompte PM. Esophageal ganglia and smooth muscle in the elderly. Am J Dig Dis 1978;23:443-8. [Crossref] [PubMed]
- Ren J, Shaker R, Kusano M, et al. Effect of aging on the secondary esophageal peristalsis: presbyesophagus revisited. Am J Physiol 1995;268:G772-9. [PubMed]
- Leese G, Hopwood D. Muscle fibre typing in the human pharyngeal constrictors and oesophagus: the effect of ageing. Acta Anat (Basel) 1986;127:77-80. [Crossref] [PubMed]
- Hollis JB, Castell DO. Esophageal function in elderly man. A new look at "presbyesophagus". Ann Intern Med 1974;80:371-4. [Crossref] [PubMed]
- Gregersen H, Pedersen J, Drewes AM. Deterioration of muscle function in the human esophagus with age. Dig Dis Sci 2008;53:3065-70. [Crossref] [PubMed]
- Imagama S, Ando K, Kobayashi K, et al. Increase in lumbar kyphosis and spinal inclination, declining back muscle strength, and sarcopenia are risk factors for onset of GERD: a 5-year prospective longitudinal cohort study. Eur Spine J 2019;28:2619-28. [Crossref] [PubMed]
- Asaoka D, Takeda T, Inami Y, et al. The Association between Frailty and Abdominal Symptoms: A Hospital-based Cross-sectional Study. Intern Med 2020;59:1677-85. [Crossref] [PubMed]
- Fávaro-Moreira NC, Krausch-Hofmann S, Matthys C, et al. Risk Factors for Malnutrition in Older Adults: A Systematic Review of the Literature Based on Longitudinal Data. Adv Nutr 2016;7:507-22. [Crossref] [PubMed]
- Matsushita E, Okada K, Ito Y, et al. Characteristics of physical prefrailty among Japanese healthy older adults. Geriatr Gerontol Int 2017;17:1568-74. [Crossref] [PubMed]
- Nishida T, Yamabe K, Honda S. The Influence of Dysphagia on Nutritional and Frailty Status among Community-Dwelling Older Adults. Nutrients 2021;13:512. [Crossref] [PubMed]
- Cohen SM, Lekan D, Risoli T Jr, et al. Association Between Dysphagia and Inpatient Outcomes Across Frailty Level Among Patients ≥ 50 Years of Age. Dysphagia 2020;35:787-97. [Crossref] [PubMed]
- Stoschus B, Allescher HD. Drug-induced dysphagia. Dysphagia 1993;8:154-9. [Crossref] [PubMed]
- Morley JE, von Haehling S, Anker SD, et al. From sarcopenia to frailty: a road less traveled. J Cachexia Sarcopenia Muscle 2014;5:5-8. [Crossref] [PubMed]
- Wakasugi Y, Tohara H, Machida N, et al. Can grip strength and/or walking speed be simple indicators of the deterioration in tongue pressure and jaw opening force in older individuals? Gerodontology 2017;34:455-9. [Crossref] [PubMed]
- Kunieda K, Fujishima I, Wakabayashi H, et al. Relationship Between Tongue Pressure and Pharyngeal Function Assessed Using High-Resolution Manometry in Older Dysphagia Patients with Sarcopenia: A Pilot Study. Dysphagia 2021;36:33-40. [Crossref] [PubMed]
- Webster JM, Kempen LJAP, Hardy RS, et al. Inflammation and Skeletal Muscle Wasting During Cachexia. Front Physiol 2020;11:597675. [Crossref] [PubMed]
- Soysal P, Arik F, Smith L, et al. Inflammation, Frailty and Cardiovascular Disease. In: Veronese N (ed). Frailty and Cardiovascular Diseases. Advances in Experimental Medicine and Biology. USA: Springer International Publishing AG; 2020.
- Mayerl H, Stolz E, Freidl W. Frailty and depression: Reciprocal influences or common causes? Soc Sci Med 2020;263:113273. [Crossref] [PubMed]
- Assar ME, Laosa O, Rodríguez Mañas L. Diabetes and frailty. Curr Opin Clin Nutr Metab Care 2019;22:52-7. [Crossref] [PubMed]
- Ahn DH, Yang HE, Kang HJ, et al. Changes in etiology and severity of dysphagia with aging. Eur Geriatr Med 2020;11:139-45. [Crossref] [PubMed]
- Lee BJ, Lee SC, Choi HY, et al. Correlation between Forced Vital Capacity and the Severity of Frailty-Induced Dysphagia. J Clin Med 2022;11:1962. [Crossref] [PubMed]
- Nkonde C, Bell B, Tait A, et al. The prevalence of oral frailty and its association with dysphagia, frailty and formal care needs. Age Ageing 2023;52: [Crossref]
- Robison R, Focht Garand KL, Affoo R, et al. New horizons in understanding oral health and swallowing function within the context of frailty. Age Ageing 2023;52:afac276. [Crossref] [PubMed]
- Hathaway B, Vaezi A, Egloff AM, et al. Frailty measurements and dysphagia in the outpatient setting. Ann Otol Rhinol Laryngol 2014;123:629-35. [Crossref] [PubMed]
- Nishida T, Yamabe K, Honda S. Dysphagia is associated with oral, physical, cognitive and psychological frailty in Japanese community-dwelling elderly persons. Gerodontology 2020;37:185-90. [Crossref] [PubMed]
- Morley JE. Editorial: Oral Frailty. J Nutr Health Aging 2020;24:683-4. [Crossref] [PubMed]
- Wakabayashi H. Role of Nutrition and Rehabilitation in the Prevention and Management of Sarcopenia and Frailty. In: Kato A, Kanda E, Kanno Y (eds). Recent Advances of Sarcopenia and Frailty in CKD. Springer, Singapore; 2020.
- Whittle J, Wischmeyer PE, Grocott MPW, et al. Surgical Prehabilitation: Nutrition and Exercise. Anesthesiol Clin 2018;36:567-80. [Crossref] [PubMed]
- Chou W, Lam S, Kumar B. 'Clinical frailty is a risk factor of adverse outcomes in patients with esophageal cancer undergoing esophagectomy: analysis of 2011-2017 US hospitals'. Dis Esophagus 2022;35:doac013. [Crossref] [PubMed]
- Sheetz KH, Zhao L, Holcombe SA, et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus 2013;26:716-22. [Crossref] [PubMed]
- Cohen SM, Porter Starr KN, Risoli T Jr, et al. Association between Dysphagia and Surgical Outcomes across the Continuum of Frailty. J Nutr Gerontol Geriatr 2021;40:59-79. [Crossref] [PubMed]
- Madhavan A, Kamarajah SK, Navidi M, et al. The impact of age on patients undergoing transthoracic esophagectomy for cancer. Dis Esophagus 2021;34:doaa056. [Crossref] [PubMed]
- Pultrum BB, Bosch DJ, Nijsten MW, et al. Extended esophagectomy in elderly patients with esophageal cancer: minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol 2010;17:1572-80. [Crossref] [PubMed]
- Garland M, Hsu FC, Shen P, et al. Optimal Modified Frailty Index Cutoff in Older Gastrointestinal Cancer Patients. Am Surg 2017;83:860-5. [Crossref] [PubMed]
- Lee DU, Hastie DJ, Fan GH, et al. Clinical frailty is a risk factor of adverse outcomes in patients with esophageal cancer undergoing esophagectomy: analysis of 2011-2017 US hospitals. Dis Esophagus 2022;35:doac002. [Crossref] [PubMed]
- Zotti OR, Herbella FAM, Armijo PR, et al. Achalasia Treatment in Patients over 80 Years of Age: A Multicenter Survey. J Laparoendosc Adv Surg Tech A 2020;30:358-362. [Crossref] [PubMed]
- Straatman J, Groen LCB, van der Wielen N, et al. Treatment of paraesophageal hiatal hernia in octogenarians: a systematic review and retrospective cohort study. Dis Esophagus 2018; [Crossref] [PubMed]
- Cowgill SM, Arnaoutakis D, Villadolid D, et al. Results after laparoscopic fundoplication: does age matter? Am Surg 2006;72:778-83; discussion 783-4. [Crossref] [PubMed]
- Dent E. Comprehensive care plan for frailty: addressing polypharmacy, management of sarcopenia, treatable causes of weight loss, and causes of exhaustion. J Frailty Aging 2019;8:63-5.
- Sire L. Interventions for prevention of malnutrition and dysphagia-related complications in the elderly. Aging Clin Exp Res 2022;34:1225-34.
- Aoyama K, Kunieda K, Shigematsu T, et al. Bridge Swallowing Exercise for Gastroesophageal Reflux Disease Symptoms: A Pilot Study. Prog Rehabil Med 2022;7:20220054. [Crossref] [PubMed]
- Alghadir AH, Anwer S, Brismée JM. Effect of head and neck positions on oropharyngeal swallowing: a systematic review. J Oral Rehabil 2017;44:877-86.
- Ness-Jensen E, Hveem K, El-Serag H, et al. Lifestyle Intervention in Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol 2016;14:175-82.e1-3.
- Baijens LW, Clavé P, Cras P, et al. European Society for Swallowing Disorders - European Union Geriatric Medicine Society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin Interv Aging 2016;11:1403-28. [Crossref] [PubMed]
- Namiki C, Hara K, Tohara H, et al. Tongue-pressure resistance training improves tongue and suprahyoid muscle functions simultaneously. Clin Interv Aging 2019;14:601-8. [Crossref] [PubMed]
- Sura L, Madhavan A, Carnaby G, et al. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging 2012;7:287-98. [PubMed]
- Alvarez RE, Woods AK. Frailty assessment in the perioperative setting: a clinical review. Aging Med (Milton) 2018;1:50-6.
- Alvarez-Nebreda ML, Bentov N, Urman RD, et al. Recommendations for Preoperative Management of Frailty from the Society for Perioperative Assessment and Quality Improvement (SPAQI). J Clin Anesth 2018;47:33-42. [Crossref] [PubMed]
- Cappe A, Parreira T, Oosterhoff P, et al. Frailty in older adults undergoing cardiac surgery: an overview of current evidence and future directions. J Thorac Dis 2023;15:420-30.
Cite this article as: Andrade ML, Herbella FAM. The sequel of age and frailty on the pathophysiology and treatment of surgical esophageal diseases. Ann Esophagus 2024;7:5.


