
Dedicated services for Barrett’s esophagus—a survey and service assessment of provision in United Kingdom hospitals
Introduction
Specialist services in medicine are increasingly recognised as gold standard care, in gastroenterology one such example for Barrett’s esophagus (BE), has not been formally explored in the UK. BE is a condition of glandular metaplasia developing within the esophagus which is a risk factor for esophageal adenocarcinoma (EAC) (1). The rate of progression to EAC is in the region of 0.3% per annum (2,3). BE is estimated to affect 2% of the general population with a rising prevalence in more socioeconomically developed countries. EAC, once invasive, carries a poor prognosis with a 10-year survival of 12% (4). Esophageal cancer has been highlighted as one of the cancers of unmet need by Cancer Research UK and they estimate 59% of esophageal cancers are preventable (4). In order to detect dysplasia and early cancers, international and national guidelines advise regular surveillance endoscopy procedures performed every 2–5 years, during which visible abnormalities are targeted for biopsy and quadrantic biopsies every 1–2 cm (Seattle protocol biopsies) are performed to try to detect early changes (5-7). Retrospective studies have shown there are improved outcomes for patients on surveillance (8) and adherence to Seattle protocol leads to greater benefit for the detection of dysplasia (9). However, there is evidence to suggest that guidelines and Seattle protocol are poorly adhered to, with clinicians routinely under-sampling BE segments and this is more common for longer segments despite their increased risk of dysplastic changes (10). Missed cancer rates can be high with UK data showing up to 12.7% were missed at an index endoscopy (11), and in a meta-analysis, 25.3% of BE associated EAC cases were reported to have been missed at diagnosis (12). A survey of clinicians taken during the AspECT trial, a large randomised controlled trial comparing the role of esomeprazole at different doses with or without aspirin, showed 90% of respondents would under-biopsy, with 92% of respondents being concerned about the level of evidence for surveillance, however, this survey pre-dated the British Society of Gastroenterology (BSG) guidelines (13).
Factors associated with better adherence have been reviewed in a 2020 systematic review, which looked at 56 studies (14). Pooled adherence to guidelines ranged from 18–89% overall, with adherence to guideline surveillance intervals for non-dysplastic BE of 55% and for low-grade dysplasia (LGD) 50%. For the Seattle protocol biopsies adherence was 49%. Factors associated with better adherence included university hospital endoscopy units, use of a dedicated list or service, shorter segment of BE and endoscopists who were employed as salaried clinicians opposed to those who were dependent on productivity.
In a recent research priority setting exercise looking at clinician and patient priorities for BE research (15), the 4th out of the top ten priorities was: “Should surveillance and new patient clinics for Barrett’s esophagus be done by a dedicated service? How would this compare with existing standards of practice in the UK and what effect would this have on patients (e.g., precancer diagnosis rates, patient education, quality of life and satisfaction)?”
Historically, BE endoscopy surveillance has been performed on routine endoscopy lists, occurring amongst varied other procedures such as colonoscopy. Dedicated endoscopy services for BE surveillance can involve dedicated lists, performed by endoscopists with an interest in BE skilled in surveillance technique and knowledge of the guidelines. Dedicated clinics for follow-up care involve specific clinics or specific clinicians named as leads for BE who see BE patients in their named clinics (Table 1).
Table 1
| Type of Barrett’s esophagus service | Dedicated service | Standard care |
|---|---|---|
| Endoscopy surveillance | Specific endoscopy lists | Barrett’s esophagus cases occur on any endoscopy list performed by a clinician trained in diagnostic upper GI endoscopy |
| Performed by endoscopists with a special interest in Barrett’s esophagus surveillance or upper GI disease | No specific team or endoscopist allocated to cases | |
| Clinic services | Clinic appointments allocated to specific clinicians or nurses who have a special interest in Barrett’s esophagus and knowledge of the disease and management | New patients diagnosed with Barrett’s esophagus seen in any appointment with a gastroenterology consultant or trainee |
GI, gastrointestinal.
There are no randomised controlled trials looking at the use of dedicated services for BE surveillance endoscopy; two cohort studies have been performed comparing a dedicated service with standard care. In an observational cohort study by Ooi et al. they defined a dedicated service as endoscopy performed by endoscopists trained in Seattle protocol, Prague classification and lesion recognition, on dedicated slots or endoscopy lists. The cohort who had their endoscopy performed by these endoscopists was compared with a retrospective cohort who had their surveillance endoscopy on a general endoscopy list. The dedicated service resulted in increased dysplasia detection from 8% to 18% (P<0.001) (16).
A prospective single centre cohort study by Britton et al. showed significantly better adherence to the Seattle protocol (72% vs. 42% vs. 50%, P<0.0001, dedicated vs. prospective non dedicated vs. retrospective data) on dedicated lists, though did not meet statistical significance for dysplasia detection (4.3% vs. 2.6%, P=0.41) (17). They defined a dedicated service as conducted by a single endoscopist with a specialist interest in BE, whereas the non-dedicated arm had their surveillance endoscopy performed on any standard endoscopy session performed by an endoscopist qualified in diagnostic upper GI endoscopy.
For dedicated clinics, few studies have been performed although one study using a cohort that predated the current BSG guidelines showed some clinical benefits of a dedicated BE clinic including changes to medication (17%) and cessation of surveillance (11%) (18). In this study they did not clearly define the logistics of the BE clinic but stated that during consultations a review of the diagnosis was made, clear information was provided to patients, symptoms reviewed and shared decision making regarding future surveillance was made.
These studies suggest there is a role for dedicated BE endoscopy and follow-up clinic care, this service review aims to see what provision already exists in the UK of these types of services.
Aim
- To determine the use of dedicated BE surveillance endoscopy pathways and dedicated BE clinic services in UK hospitals;
- To determine whether this practice is used primarily in tertiary referral centres or if it is more widely available in the non-tertiary general hospital setting;
- To explore what is the local provision of advanced endoscopic therapy such as endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD) and radiofrequency ablation (RFA) and if this has any association with the use of dedicated endoscopy lists for surveillance, and BE specific clinics.
The results are presented in accordance with the SURGE reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-12/rc).
Methods
Study design
A 9-question survey was devised (see Appendix 1 for the full questionnaire). The survey was devised to combine some multiple-choice questions, covering key clinical aspects of a BE service, and open-ended items to allow for more detailed responses where needed. This was a novel tool designed to gather specific factual clinical service information and hence has not been through pretesting or validation testing. There was no scoring system attributed, services were either reported present or not and descriptive data about services was obtained.
Data collection, sample and survey administration
All UK endoscopy units known to the Joint Advisory Group (JAG) which provide endoscopy services for adult patients were contacted via telephone or email by one clinical research fellow and 3 final year medical students (E.R., Y.K., Y.L., J.K.). If they responded, the survey was provided via email or a Survey Monkey online platform link. If there was no response on initial contact further attempts were made, up to 2 further times. The sample population was obtained from the JAG database of known UK endoscopy units on 24th March 2020. Paediatric units and private hospitals were excluded, all other adult units were included hence representative of UK adult endoscopy care. The rationale for the sample size was to contact all adult trusts to gain insight in the practice of as many types of endoscopy units, from a wide geographical and clinical variance. Other information was obtained regarding JAG accreditation and acute status of the unit from JAG team. No incentive was provided for completion of the survey.
Ethics
No ethics application was required as the project falls within quality improvement/service evaluation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All data are anonymised.
Statistical analysis
Categorical variables are described as proportions and analyses were performed using the Chi-square test to compare different groups. Two-tailed P value <0.05 was considered statistically significant. Endoscopy departments who did not respond to the survey were excluded from the main analysis due to missing data. As the proportion of missing values per each questionnaire item was low (averaged 3%), missing values were treated as 0/no. Response rates were calculated as a percentage. Non-responder characteristics were compared with responder characteristics. The statistical software used was RStudio Version 4.0.3 (packages: dplyr ver. 1.0.6, ggplot2 ver. 3.3.3, ComplexHeatmap ver. 2.7.11, UpSetR ver. 1.4.0).
Results
In total, 265 departments (~96%) were contacted with a response rate of 61.9% (164/265). Response rates varied across the devolved nations: England 70.7%, Northern Ireland 50%, Scotland 35.9%, Wales 42.1%. Of departments which responded, 92.1% (151/164) performed BE surveillance in their unit and 56.3% (85/151) reported having a dedicated BE endoscopy service.
Units performing BE surveillance
Table 2 outlines the department characteristics of the units which perform BE surveillance, stratified by whether they have a dedicated BE endoscopy service or not, alongside non-responder characteristics. A greater percentage of English departments that responded had a dedicated BE endoscopy service followed by Scotland, Wales then Northern Ireland. Similar proportions of the units (with or without a dedicated endoscopy service) were JAG accredited; however, there was a greater proportion of acute/large units within the dedicated service group.
Table 2
| Department characteristics | No dedicated BE service (% of total) | Dedicated BE service (% of total) |
Total | Non-responder units |
|---|---|---|---|---|
| Country | ||||
| England | 51 (39.8) | 77 (60.2) | 128 | 54 |
| Scotland | 4 (50.0) | 4 (50.0) | 8 | 25 |
| Wales | 5 (71.4) | 2 (28.6) | 7 | 11 |
| Northern Ireland | 6 (75.0) | 2 (25.0) | 8 | 8 |
| Sector | ||||
| Acute/large | 62 (43.4) | 81 (56.6) | 143 | 82 |
| Non-acute/small | 4 (50.0) | 4 (50.0) | 8 | 19 |
| JAG status | ||||
| Accredited | 31 (42.5) | 42 (57.5) | 73 | 30 |
| Assessed: improvements required | 11 (42.3) | 15 (57.7) | 26 | 13 |
| Not awarded | 8 (33.3) | 16 (66.7) | 24 | 10 |
| Not assessed/undergoing assessment | 16 (57.1) | 12 (42.9) | 28 | 48 |
Values are number (proportion). BE, Barrett’s esophagus; JAG, Joint Advisory Group.
Descriptive results and main findings
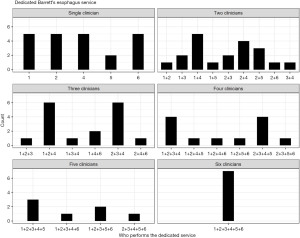
When asked who was running the endoscopy service, most departments reported a mixture of gastroenterologists, surgeons, nurses and fellows, with 25.9% (22/85) reporting a single clinician. The majority of units had more than one clinician providing their dedicated endoscopy service, with wide variation in the combinations involved, outlined in Figure 1.
Association with the use of advanced imaging techniques and chromoendoscopy
Regarding specific interventions and techniques, having a dedicated BE endoscopy service was associated with the local availability of high-resolution white light (92.9% vs. 71.2%, P=0.001) and acetic acid use (83.5% vs. 48.5%, P<0.001) (Table 3). There was no significant difference for virtual chromoendoscopy techniques (P=0.28) or dye chromoendoscopy (e.g., indigo carmine) (P=0.25) (Table 3). Combinations of the provided services are summarised in Figure 2.
Table 3
| Service provided | No dedicated BE service (n=66) | Dedicated BE service (n=85) | P value |
|---|---|---|---|
| High resolution white light | 47 (71.2) | 79 (92.9) | 0.001 |
| Ascetic acid | 32 (48.5) | 71 (83.5) | <0.001 |
| Chromoendoscopy | 31 (47.0) | 49 (57.6) | 0.25 |
| NBI | 60 (90.9) | 82 (96.5) | 0.28 |
| RFA | 13 (19.7) | 37 (43.5) | 0.004 |
| EUS | 20 (30.3) | 54 (63.5) | <0.001 |
| EMR | 25 (37.9) | 51 (60.0) | 0.01 |
| ESD | 3 (4.5) | 29 (34.1) | <0.001 |
Values are number (proportion). BE, Barrett’s esophagus; NBI, narrow-band imaging; RFA, radiofrequency ablation; EUS, endoscopic ultrasound; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection.

Local availability of advanced endoscopic therapy
Having a dedicated endoscopy BE service was associated with local availability of RFA (43.5% vs. 19.7%, P=0.004), endoscopic ultrasound (63.5% vs. 30.3%, P<0.001), EMR (60.0% vs. 37.9%, P=0.01) and ESD (34.1% vs. 4.5%, P<0.001) (Table 3). However, many Trusts without local availability of these services also reported a dedicated BE endoscopy service suggesting practice in the non-tertiary setting [dedicated service with no local EMR 34/85 (40.0%), dedicated service with no local ESD 56/85 (65.9%)]. There was no significant difference in provision of a dedicated BE endoscopy service when accounting for JAG accreditation status (P=0.89), and there were no significant associations found between the size of the units (acute/large verses non-acute/small) and local availability of the services we asked about (Table S1).
When asked about having a named lead clinician for BE services this was found in 94 units performing BE surveillance. Units which reported having a dedicated BE endoscopy service were significantly more likely to have a named lead for BE within their unit (85.9% vs. 31.8%, P<0.001) though this was not universally reported across units.
There were significant associations with having a named lead clinician, namely the availability of acetic acid spray (47.4% no named lead vs. 80.9% named lead, P<0.001), and with the local availability of advanced modalities of RFA (P=0.001), EUS (P<0.001), EMR (P=0.002) and ESD (P=0.02) (Table S2). The differences in services provided by the departments stratified by the named lead type is presented in Table S3.
Follow-up care
Only 56 out of 151 departments performing BE surveillance offered a dedicated BE clinic. Trusts which reported a dedicated endoscopy service were significantly more likely to have a dedicated BE clinic (52.9% vs. 16.7%, P<0.001). When asked to define what they meant by a dedicated clinic, 36/56 (64.3%) stated this was a specific clinic list which was filled with BE cases, with the rest reporting BE patients would be seen by specific clinicians in their clinic. There was no significant difference in the provision of specific clinic/follow-up care when accounting for the units JAG accreditation status (57.5% vs. 55.1%, P=0.89) or size (56.6% vs. 50.0%, P=0.77).
Discussion
This is the largest study to the authors knowledge exploring the role of dedicated services for BE patients in the UK, achieving a high response rate for the survey-based study. All UK countries were represented with a breadth of types of endoscopy units responding—not limited to tertiary centres. This study shows a clear association between dedicated services and quality endoscopy standards such as the local availability of high-resolution white light endoscopy and lead clinician allocation. The need in BE equates to the concern that despite surveillance endoscopy and knowledge about the aetiology of BE associated EAC, 10-year survival rates are still dismal and there are significant rates of missed dysplasia during endoscopy (19). Prior evidence for the role of dedicated endoscopy includes one study showing improved dysplasia detection (16), other cohort data showing improved adherence to surveillance biopsies and standard BSG endoscopy reporting guidelines (17). There is strong evidence that increased esophageal inspection time improves dysplasia detection (20), in the same way that colonoscopy withdrawal time has been shown to improve adenoma detection. The most recent update to the American College of Gastroenterology guidelines recommends the use of chromoendoscopy, either virtual or with dye spray such as acetic acid alongside high resolution white light endoscopy and Seattle protocol biopsies, however, they do not comment on training the workforce in these modalities (6). For bowel cancer screening colonoscopy in the UK a formal training pathway is required which involves a combination of taught academic courses, mentorship and skills training (21), a similar training pathway could be developed for a dedicated BE surveillance endoscopy workforce. Currently in the UK BE surveillance may be performed by any endoscopist who is certified through the JAG to perform diagnostic upper GI endoscopy (22). Skills in performing it are developed through the training pathway but depend on the interest and skills of the trainers involved. There are lesion recognition training programs available such as the BORN (Barrett’s esophagus-related neoplasia) project (23), which uses interactive videos of endoscopic procedures on BE cases where the user can delineate lesions and target biopsies on frames of the video and compare with expert opinions. As demonstrated by our data, though lots of dedicated endoscopy services are available, much of the bulk of BE work is undertaken by the general endoscopy workforce. Work could be done to formalise training in BE lesion recognition during endoscopy accreditation which may be helpful to reduce missed dysplasia. Alongside this departments need to prioritise the time required to complete quality endoscopic surveillance; ring-fencing dedicated BE endoscopy lists may be one way to achieve this. The European Society of Gastrointestinal Endoscopy (ESGE) guidelines for BE advocate for BE surveillance to be performed within specifically allocated time slots, and that BE segments >10 cm should be referred to expert centres for surveillance endoscopy (24). Our study suggests dedicated endoscopy services correspond with other best practice in terms of endoscopy, namely the availability of high-resolution white light endoscopes, and studies are now needed to prove if this translates to improved clinical and patient-reported outcomes.
Dedicated clinic services have been utilised in other aspects of gastroenterology care, with specific clinics available for inflammatory bowel disease (IBD) including helplines and specialist hepatology clinics that aim to help hard to reach populations (25-27). The BSG guideline advises all new diagnoses of BE should have an outpatient appointment to discuss the diagnosis, our study shows there are dedicated clinics in practice in the UK though the evidence base is limited. Indeed, only one study has looked at the role of a dedicated clinic for BE and showed medication was changed in 17% of cases and in 11% of cases, surveillance was stopped (18). Inappropriate use of surveillance, e.g., for shorter segments, lack of intestinal metaplasia and for those in whom it is inappropriate due to comorbid state, is an ongoing concern and has the potential for cost savings if appropriately stopped. At present, risk stratification models to determine which patients are more at risk of EAC in the BE population have been described looking at demographics, length of BE, smoking history, family history and obesity (28). These have not been formally incorporated into guidelines, meaning judgements must be made and decision making shared between clinicians and patients. Hence, we see there could be a potential benefit to concentrating clinic and follow-up services to those with an interest in BE via dedicated slots to make the most appropriate decisions and aid patient education. Qualitative and quantitative studies have shown patients lack disease-specific knowledge and have high rates of cancer worry—an area which could be addressed in dedicated clinics (29-31).
Limitations
In this study, nearly all units in the UK were contacted, but there was a response rate of 61%; however, compared with other survey-based studies this is a high response rate. This means however that there are data lacking from the non-responders, and many of these were in England and Scotland, this could be addressed by including questions regarding BE services in JAG census data to gain a more rounded understanding of current practices. Also dedicated services have not been defined in guidelines or in the literature so what a dedicated service represents in one unit may differ in another. Further work is required to create best practice guidelines for how BE dedicated services should be established when more evidence is available. Another limitation of this design of study is it is not possible to attribute a causal relationship between the presence of a dedicated service and the local availability of certain services or endoscopy practice, randomised controlled trials are needed to show a causal link between improvements in clinical and patient outcomes and a dedicated service for this patient group.
Conclusions
In conclusion, this is the largest survey of UK endoscopy services about BE care provision at the time of publication. This has potential wider generalisability to other health care systems given the clinical services explored, e.g., BE surveillance endoscopy, RFA, high-resolution white-light endoscopy (HRWLE) and acetic acid, are available in other economically developed countries. If further work shows improved outcomes from a dedicated endoscopy or follow-up service, the model of dedicated care for BE could potentially be replicated. This study shows that the practice of using dedicated services for BE is quite widespread, but not the standard in UK hospitals. Where present, it is associated with but not limited to hospitals with local availability of advanced techniques and technologies. Further work is required to define the key components of a dedicated service for BE, determine both clinical and patient reported outcomes, as well as cost-benefit analysis to confirm if this practice should be utilised more widely.
Acknowledgments
We are grateful to the staff at the Joint Advisory Group for endoscopy at the Royal College of Physicians for the assistance with the list of hospitals and to all the responding endoscopy units for the time taken to respond for their contribution to this work. We are grateful for the assistance of Dr. Lisa McNeill in distributing the survey in Northern Ireland. This work was presented as a poster at the British Society of Gastroenterology conference 2021.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-12/rc
Data Sharing Statement: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-12/dss
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-12/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-22-12/coif). E.R. has received research funding from Medtronic Ltd. for other projects and honoraria from Janssen and Takeda. Y.A. has received research funding from Medtronic Ltd. and Cancer Research UK for other projects. S.H. is a board member of Phagenesis Ltd., Chief Scientific Officer and Director of the company. S.H. owns foundation shares in this company. S.H. has received speaker honoraria from the Chinese Dysphagia Research Forum 2021 and NIHR funding for research into pharyngeal stimulation in dysphagic stroke. S.H. received support to attend the ESSD meeting in Brussels, 2020 of Euros 450.00 for flights and 1 night accommodation. S.H. has a patent pending for Anisys Go and Lyft a device for measuring sphincter function and providing biofeedback. S.H. is paid 2 days per week to the University of Manchester for his role as interim Vice-chair of NICE. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. No ethics application was required as the project falls within quality improvement/service evaluation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All data are anonymised.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nowicki-Osuch K, Zhuang L, Jammula S, et al. Molecular phenotyping reveals the identity of Barrett's esophagus and its malignant transition. Science 2021;373:760-7. [Crossref] [PubMed]
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375-83. [Crossref] [PubMed]
- Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst 2011;103:1049-57. [Crossref] [PubMed]
- Cancer Research UK. Cancer research UK Oesophageal cancer statistics [Internet]. CRUK. 2020 [cited 2020 Oct 1]. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer
- Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7-42. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline. Am J Gastroenterol 2022;117:559-87. [Crossref] [PubMed]
- Bennett C, Moayyedi P, Corley DA, et al. BOB CAT: A Large-Scale Review and Delphi Consensus for Management of Barrett's Esophagus With No Dysplasia, Indefinite for, or Low-Grade Dysplasia. Am J Gastroenterol 2015;110:662-83. Erratum in: Am J Gastroenterol 2015 Jun;110(6):943. doi: 10.1038/ajg.2015.151. [Crossref] [PubMed]
- Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett's esophagus-associated esophageal adenocarcinomas. Gastroenterology 2013;145:312-9.e1. [Crossref] [PubMed]
- Peters FP, Curvers WL, Rosmolen WD, et al. Surveillance history of endoscopically treated patients with early Barrett's neoplasia: nonadherence to the Seattle biopsy protocol leads to sampling error. Dis Esophagus 2008;21:475-9. [Crossref] [PubMed]
- Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett's esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 2009;7:736-42. [Crossref] [PubMed]
- van Putten M, Johnston BT, Murray LJ, et al. 'Missed' oesophageal adenocarcinoma and high-grade dysplasia in Barrett's oesophagus patients: A large population-based study. United European Gastroenterol J 2018;6:519-28. [Crossref] [PubMed]
- Visrodia K, Singh S, Krishnamoorthi R, et al. Magnitude of Missed Esophageal Adenocarcinoma After Barrett's Esophagus Diagnosis: A Systematic Review and Meta-analysis. Gastroenterology 2016;150:599-607.e7; quiz e14-5. [Crossref] [PubMed]
- Das D, Ishaq S, Harrison R, et al. Management of Barrett's esophagus in the UK: overtreated and underbiopsied but improved by the introduction of a national randomized trial. Am J Gastroenterol 2008;103:1079-89. [Crossref] [PubMed]
- Roumans CAM, van der Bogt RD, Steyerberg EW, et al. Adherence to recommendations of Barrett's esophagus surveillance guidelines: a systematic review and meta-analysis. Endoscopy 2020;52:17-28. [Crossref] [PubMed]
- Britton J, Gadeke L, Lovat L, et al. Research priority setting in Barrett's oesophagus and gastro-oesophageal reflux disease. Lancet Gastroenterol Hepatol 2017;2:824-31. [Crossref] [PubMed]
- Ooi J, Wilson P, Walker G, et al. Dedicated Barrett's surveillance sessions managed by trained endoscopists improve dysplasia detection rate. Endoscopy 2017;49:524-8. [Crossref] [PubMed]
- Britton J, Chatten K, Riley T, et al. Dedicated service improves the accuracy of Barrett's oesophagus surveillance: a prospective comparative cohort study. Frontline Gastroenterol 2019;10:128-34. [Crossref] [PubMed]
- Anagnostopoulos GK, Pick B, Cunliffe R, et al. Barrett's esophagus specialist clinic: what difference can it make? Dis Esophagus 2006;19:84-7. [Crossref] [PubMed]
- Visrodia K, Iyer PG, Schleck CD, et al. Yield of Repeat Endoscopy in Barrett's Esophagus with No Dysplasia and Low-Grade Dysplasia: A Population-Based Study. Dig Dis Sci 2016;61:158-67. [Crossref] [PubMed]
- Gupta N, Gaddam S, Wani SB, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett's esophagus. Gastrointest Endosc 2012;76:531-8. [Crossref] [PubMed]
- UK Health Security Agency. Guidance on education and training requirements for specialist screening practitioners [Internet]. UK Government Bowel cancer screening programme guidance. 2021 [cited 2022 Jul 11]. Available online: https://www.gov.uk/government/publications/bowel-cancer-screening-specialist-screening-practitioner/guidance-setting-out-the-skills-competencies-and-education-and-training-required-of-specialist-screening-practitioners-ssps#education-and-training-requirements
- JAG Joint advisory group. JETS Certification Pathways Trainee Certification Process [Internet]. Available online: https://www.thejag.org.uk/Downloads/JAG/JAG certification/JAG certification criteria and application process.pdf
- Bergman JJGHM, de Groof AJ, Pech O, et al. An Interactive Web-Based Educational Tool Improves Detection and Delineation of Barrett's Esophagus-Related Neoplasia. Gastroenterology 2019;156:1299-1308.e3. [Crossref] [PubMed]
- Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017;49:191-8. [Crossref] [PubMed]
- Leach P, De Silva M, Mountifield R, et al. The effect of an inflammatory bowel disease nurse position on service delivery. J Crohns Colitis 2014;8:370-4. [Crossref] [PubMed]
- Fofaria RK, Barber S, Adeleke Y, et al. Stratification of inflammatory bowel disease outpatients by disease activity and risk of complications to guide out-of-hospital monitoring: a patient-centred quality improvement project. BMJ Open Qual 2019;8:e000546. [Crossref] [PubMed]
- O'Sullivan M, Jones AM, Gage H, et al. ITTREAT (Integrated Community Test - Stage - TREAT) Hepatitis C service for people who use drugs: Real-world outcomes. Liver Int 2020;40:1021-31. [Crossref] [PubMed]
- Parasa S, Vennalaganti S, Gaddam S, et al. Development and Validation of a Model to Determine Risk of Progression of Barrett's Esophagus to Neoplasia. Gastroenterology 2018;154:1282-1289.e2. [Crossref] [PubMed]
- Britton J, Taxiarchi P, Martin G, et al. Comparative quantitative survey of patient experience in Barrett's oesophagus and other gastrointestinal disorders. BMJ Open Gastroenterol 2020;7:e000357. [Crossref] [PubMed]
- Britton J, Hamdy S, McLaughlin J, et al. Barrett's oesophagus: A qualitative study of patient burden, care delivery experience and follow-up needs. Health Expect 2019;22:21-33. [Crossref] [PubMed]
- van der Ende-van Loon MCM, Nieuwkerk PT, van Stiphout SHC, et al. Barrett Esophagus: Quality of life and factors associated with illness perception. United European Gastroenterol J 2022;10:721-9. [Crossref] [PubMed]
Cite this article as: Ratcliffe E, Liew Y, Kuan J, Kim Y, Kopczynska M, Britton J, McLaughlin J, Hamdy S, Ang Y. Dedicated services for Barrett’s esophagus—a survey and service assessment of provision in United Kingdom hospitals. Ann Esophagus 2023;6:39.


