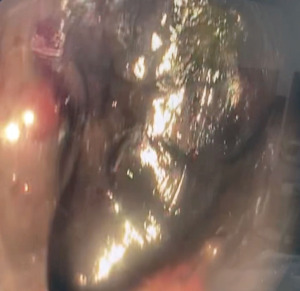A rare case of combined black esophagus and stomach: a case report
Introduction
Black esophagus, also known as acute esophageal necrosis (AEN) is an uncommon pathology, with a predicted incidence of 0.01–0.28% (1). It seems to present most commonly with signs of upper gastrointestinal bleeding, particularly hematemesis (2,3). It has been theorized to result from a combination of hemodynamic compromise, and baseline critical illness (1). There have been several studies highlighting an association with alcohol abuse as well (4,5). Surprisingly, the mortality seems to be relatively low, reported around 32%, with several reports describing resolution of the condition in several patients (5,6). There are extremely limited reports of black stomach, and to our knowledge, we are presenting the first case of combined black esophagus and stomach. We present the following case in accordance with the CARE reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-70/rc).
Case presentation
Our case involves a 75-year-old man with relevant past medical history of reduced ejection fraction heart failure, chronic obstructive pulmonary disease, type A aortic dissection as well as metastatic pancreatic cancer was brought to our hospital following an outpatient cardiac arrest. He had initially contacted emergency medical services for worsening abdominal pain, during their evaluation of the patient he suddenly became unresponsive and was found to be pulseless. He underwent cardiopulmonary resuscitation and had eventual return of system circulation. He was subsequently admitted to the intensive care unit (ICU) for further resuscitation.
Of note several months prior to presentation he was evaluated for possible gastric outlet obstruction by the gastroenterology team. At the time he had presented with gastric distention and thickening at gastrojejunal junction (patient had previously undergone a pancreaticoduodenectomy) on imaging. He was initially decompressed with a nasogastric tube but subsequently had minimal output and was able to tolerate liquid diet. He was being followed by the gastroenterology team for possible stent placement prior to his decompensation.
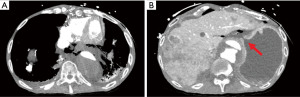
Once further stabilized from the code event he underwent computed tomography angiography (CTA) scan which demonstrated extensive pneumomediastinum, pneumoperitoneum and free fluid (Figure 1A) as well as an apparent perforation at the cardia below the gastroesophageal junction (GEJ) (Figure 1B). At this point our team was consulted for further evaluation. He was noted to be hemodynamically unstable with a pressor requirement. His physical exam was significant for signs of peritonitis. His laboratory results were significant for leukopenia of 3,700, and lactic acidosis of 2.9 mmol/L. Given his hemodynamic instability and medical history the decision was made to proceed with endoscopy to better elucidate the cause of these findings and for possible stent placement. The stent was considered as a temporizing measure given that the patient was not hemodynamically stable enough to tolerate more definitive surgery.
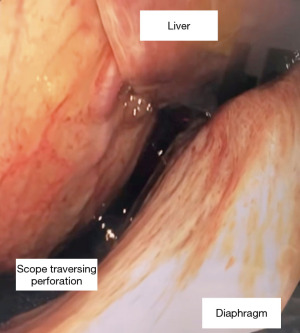
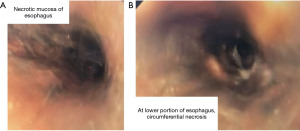
During the endoscopy, at 25 cm from the incisors, patchy black mucosa was encountered (Figure 2A), extending to confluent areas at 30 cm and circumferential necrosis at 40 cm from incisors (Figure 2B). The gastric mucosa of the fundus, body and antrum were necrotic (Figure 3) with the perforation in the fundus with peritoneal viscera and liver visible through the defect (Figure 4). Given the extent of the necrosis, no biopsies were taken out of concern for worsening the perforation. Further interventions were deemed futile and the patient was brought to the ICU for comfort directed care and expired shortly thereafter.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The stomach has a robust blood supply of multiple sources, consisting of the left and right gastric and gastroepiploic arteries, making it incredibly resistant to ischemia. The patient we described had a prior history of type A aortic dissection and repair, and chronic dissection flap extended into the abdominal aorta and iliac arteries. The dissection was stable and appeared unchanged from prior CT, with the celiac and superior mesenteric artery (SMA) arteries patent on imaging. It is possible the visceral circulation was compromised with lower perfusion pressure as a consequence of chronic dissection. This could expose the visceral circulation to hemodynamic insults, especially during the cardiac arrest. It is unclear if the cardiac arrest was secondary to worsening sepsis from primary gastroesophageal ischemia or if primary cardiac arrest resulted in visceral hypoperfusion. However, in either scenario, severe visceral ischemia led to extensive gastroesophageal necrosis.
The reported outcomes of black esophagus are varied in the literature. Some cases described resolution of the necrosis following further resuscitation, with patient not requiring any further surgical intervention (7-9). The management of these so-called “stable” patients is typically supportive, and involves correction of the underlying pathologies, usually involving resuscitative efforts, antibiotics, acid-suppression, and bowel-rest with parenteral nutrition (10). However, the patients in these reports did not have pan-esophageal necrosis, and in most cases only the distal esophagus was involved. A report by Riascos and colleagues demonstrated a case of near pan-esophageal necrosis with perforation (11). The patient underwent a left cervicotomy and right thoracotomy with wide mediastinal drainage for source control initially, followed by return to operating room (OR) for esophagectomy and feeding gastrostomy several days after improved hemodynamics. The patient improved clinically initially, however eventually decompensated and succumbed, likely due to pulmonary embolism. The mortality rate of AEN has been quoted to be as high as 32%, however, this number is likely related to other concurrent illnesses. The mortality specific to AEN is approximately 6% (10). The incidence of perforation secondary to black esophagus is surprisingly only 5% (12), however this is likely an underreported phenomena as patients may be too unstable and expire prior to presentation or procedures that allow for diagnosis.
The most common long-term sequelae of those who survive the acute phase of black esophagus is esophageal stricture, which accounts for 70% of all AEN long-term complications in survivors (13,14).
Black stomach is an even more rare phenomenon, with only a handful of cases described. Two cases of black stomach were reported, secondary to ingestion of caustic substances (15,16), and one case reported in the setting of polycythemia vera (17). The management was supportive until the patients succumbed to their disease. This likely represents a very late stage in their illness and gravity of the disease in this ischemia resistant organ.
There is only one other recently published report of combined necrosis of multiple foregut organs (18). The case presented a combined black esophagus and duodenum with the stomach being spared. This case, to the best of our knowledge, is the first reported case of combined black esophagus and stomach with unfortunately fatal but expected outcome. It is also important to note that given the patients long standing underlying malignancy his death was likely the result of a multitude of factors. Unfortunately, there were no viable operative interventions to correct the problem and all efforts would have been futile. More attention is needed to address early diagnosis of the condition and a meaningful impact on the outcomes. It is important to recognize that both these pathologies are secondary to a primary pathology driving the ischemia. In order to adequately treat patients with this pathology providers should also aim to identify and treat the primary underlying pathology. Finally recognizing the extent of the disease and that mortality associated with this complex may be underreported due to self-selecting nature of patients may help providers offer appropriate treatment options such as comfort care measures.
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA006927.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-70/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-70/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-70/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol 2010;16:3219-25. [Crossref] [PubMed]
- Jacobs BA, Salem GA, Kastens DJ. Black Esophagus: A Rare Cause of Upper Gastrointestinal Bleeding. Clin Gastroenterol Hepatol 2020;18:e57. [Crossref] [PubMed]
- Vohra I, Attar B, Almoghrabi A. Black Esophagus Due to Acute Necrosis. Clin Gastroenterol Hepatol 2021;19:e16. [Crossref] [PubMed]
- Siddiqi A, Chaudhary FS, Naqvi HA, et al. Black esophagus: a syndrome of acute esophageal necrosis associated with active alcohol drinking. BMJ Open Gastroenterol 2020;7:e000466. [Crossref] [PubMed]
- Lamers CR, Mares WGN, Bac DJ. Black esophagus: a case series and literature review of acute esophageal necrosis. Scand J Gastroenterol 2018;53:1421-4. [Crossref] [PubMed]
- Abdullah HM, Ullah W, Abdallah M, et al. Clinical presentations, management, and outcomes of acute esophageal necrosis: a systemic review. Expert Rev Gastroenterol Hepatol 2019;13:507-14. [Crossref] [PubMed]
- Sato T, Banno H, Komori K. Acute esophageal necrosis after endovascular abdominal aneurysm repair. J Vasc Surg Cases Innov Tech 2021;7:597-8. [Crossref] [PubMed]
- Makeen A, Al-Husayni F, Banamah T. Acute Esophageal Necrosis Early after Renal Transplantation. Case Rep Nephrol 2021;2021:5164373. [Crossref] [PubMed]
- Li CJ, Claxton BB, Block P, et al. Acute Esophageal Necrosis Secondary to a Paraesophageal Hernia. Case Rep Gastroenterol 2021;15:594-7. [Crossref] [PubMed]
- Dias E, Santos-Antunes J, Macedo G. Diagnosis and management of acute esophageal necrosis. Ann Gastroenterol 2019;32:529-40. [Crossref] [PubMed]
- Riascos MJ, Watts-Pajaro FA, Uribe-Buritica FL, et al. Sudden Esophageal Necrosis and Mediastinitis Associated with Invasive Candidiasis: A Case Report. Am J Case Rep 2021;22:e928394. [Crossref] [PubMed]
- Richards J. Esophageal necrosis [Internet]. StatPearls [Internet]. U.S. National Library of Medicine; 2021 [cited 2021Nov15]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572075/
- Martins D, Marques R, Costa P, et al. The dark side of the esophagus. Autops Case Rep 2021;11:e2021284. [Crossref] [PubMed]
- Schizas D, Theochari NA, Mylonas KS, et al. Acute esophageal necrosis: A systematic review and pooled analysis. World J Gastrointest Surg 2020;12:104-15. [Crossref] [PubMed]
- Remes-Troche JM A. 'black stomach' due to ingestion of anhydrous calcium chloride. BMJ Case Rep 2013;2013:bcr2012007716. [Crossref] [PubMed]
- Grifson JJ, Perungo T, Amudhan A, et al. Black stomach [Internet]. Journal of Case Reports. [cited 2021Nov15]. Available online: http://www.casereports.in/articles/6/1/Black-Stomach.html
- Azevedo R, Pereira F, Caldeira A. Black stomach: acute gastric wall ischemia due to polycythemia of an unknown origin. Rev Esp Enferm Dig 2019;111:248. [PubMed]
- Saleem S, Weissman S, Ahmad S. The black esophagus and duodenum: a rare case report. Gastroenterol Hepatol Bed Bench 2020;13:264-7. [PubMed]
Cite this article as: Magarinos J, Akcelik A, Schmidt A, Petrov R, Bakhos C. A rare case of combined black esophagus and stomach: a case report. Ann Esophagus 2023;6:35.