
Endoscopic management of complications—Ovesco/stent for management of anastomotic leaks: a narrative review
Introduction
Background
Despite surgical improvements, esophagic resections have a high morbidity and mortality. Leakage at the esophagic surgery is one of the most common and feared complications (1), which may occur in 5–20% of patients according to published studies. Related complications include mediastinitis, pulmonary infections or severe sepsis and is responsible for 40% of postoperative deaths (2).
The traditional surgical management of postoperative leaks and perforations has high morbidity as well as mortality leading to need for prolonged hospital stay. Anastomotic esophagectomy complications may lead to prolonged length of intensive care unit, elevated costs to the patient and health care system, increased mortality, and reduced quality of life, given the potential for post-leak stricture and prolonged dependence on tube feeds (3).
Anastomotic complications have multivariable causes. A retrospective study by Gonzalez et al. showed that the majority risk for anastomotic leaks or fistulas was pre-operative chemo-radiotherapy (71.4%). Most diagnoses were performed by endoscopic evaluation for clinical deterioration (65.7%). Another interesting result was that most defects were smaller than 0.2 cm, a case where endoscopic treatment with over-the-scope clips (OTSC) can be used with higher success rates (4).
Moreover, the therapeutic approach can be oriented by topography. Hourneaux de Moura et al. suggest that for cervical esophagus wall defects, endoscopic treatment with through-the-scope (TTS) clip is feasible. However, identification of the defect may be difficult and most of self-expanded metal stent (SEMS) placement may not be useful. Cervical perforations have a therapeutic clinical success with non-operative treatment with antibiotics and nil per orally. Thoracic esophagus leak healing is related to the size of the wall defect: small lesions can be healed with non-interventional therapies, while larger defects can be treated with SEMS deployment if the defect is not larger than 70% of the lumen circumference. Surgery is the only choice for large esophagus wall defects or when patients do not improve with alternative options (5).
Esophagic reoperations have high mortality rates, as high as 40% (2). It is mandatory to consider other therapeutic methods and endoscopic alternatives in the treatment of gastrointestinal fistulas (6,7). With advancement of endoscopic techniques and devices, many options have been attempted for treatment of gastrointestinal perforations. These include endoscopic clips, clips and endoloops, SEMSs, fibrin sealants, and others. OTSC promote a higher improvement approach for these patients (8).
Traditionally, endoscopic healing is performed with TTS clips and/or deployment of covered stents; however, TTS clips are limited to mucosa and submucosa applications. Stents have considerable adverse event rates, including migration, pain, and needs for long-term therapy (9). We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-1/rc).
Objectives
In this narrative review, we discuss the advancements in the endoscopic management of post esophagectomy anastomotic defects focused on preserving the gastric tube, using the full-thickness clips closure system and combined therapies.
Methods
Research selection
A review of the literature was performed using CENTRAL, MEDLINE, LILACS databases from 2010 through May 2021. The search strategy included the key terms: “anastomotic leak”; “esophagus”; “endoscop*”; “surgical instruments”; “minimally invasive therapy”; “clip”; “padlock”; “over-the-scope-clip”; “stent”. This research is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of Search (specified to date, month and year) | 29th, December, 2021 |
| Databases and other sources searched | CENTRAL, MEDLINE, LILACS |
| Search terms used (including MeSH and free text search terms and filters) | “anastomotic leak”; “esophagus”; “endoscop*”; “surgical instruments”; “minimally invasive therapy”; “clip”; “padlock”; “over-the-scope-clip”; “stent”. |
| Timeframe | Research and selection process: 12/29/2021–01/20/22. Collection data and results analysis: 01/21/22–02/21/22. Paper development: 02/22/22–03/03/22. Start submission process: 03/10/22. |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | All studies; only English papers. No limits of publication date. |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Selection process was conducted independently and, if necessary, consensus was obtained with both authors. |
| Any additional considerations, if applicable | NA |
Selection criteria
Inclusion criteria was English language, focus on esophagus endoscopic therapy, and comparing or describing OTSC applications. The exclusion criteria were articles not pertinent to the focused purpose of the study.
Discussion
Narrative
Endoscopic conventional treatment options
Many studies examined the successful management of post esophagectomy anastomotic leak with endoscopic application of covered SEMS. Others therapeutic uses for stents include spontaneous or iatrogenic perforation and fistulae. It is important to note that associated mediastinal or pleural drainage are required due to contamination, as well as nutritional therapy support with enteral or parenteral nutrition. Smaller defect size and early intervention are predictors of therapy clinical success (10).
Until now, no randomized prospective trials have been published regarding the application of OTSC for this problem. However, there are many retrospective multicenter series that confirmed clinical success ranging from 63% to 89%. Clinical success is defined as closure of the gastrointestinal wall defect with non-operative approach (11).
The SEMS failure therapy rate is around 15%, sometimes requiring another surgery that results in high morbidity. Stents related complications are tissue ingrowth and overgrowth, mucosal damage, aspiration, reflux, and bleeding (11).
Nowadays, other endoscopic therapies appear to be superior than SEMS for gastrointestinal leaks and fistulas, such as endoscopic vacuum therapy (EVT) that has substantially enlarged the proportion of patients who can be managed without further surgery (12).
A recent systematic review and meta-analysis involving 5 papers and 274 patients showed that EVT promotes 21% improvement in complete fistula closure compared with the metallic stent group (P=0.0003). EVT demonstrated a 12% reduction in mortality compared to stenting (P=0.006) and a shorter (−14.22 days) duration of therapy (P<0.00001). There was a 24% reduction in adverse events (P=0.0001) (13).
Endoscopic clips: TTS clips versus OTSC
The rationale of treating gastrointestinal leaks using endoscopic clips started in 1975, with endoscopic therapeutic clips use for hemostasis (14). TTS clip use for anastomotic damage was first related in the middle of the 90’s (15). A narrative review with 12 studies analyzing clinical success treatment of esophageal perforation with endoscopic clips found a rate of 56–100% (11).
Since then, several varieties of TTS clips are now available, including rotatable devices and clips that can be reopened. However, commercially available clips have many limitations, such as suboptimal use for damaged or previously manipulated tissues. There is small success in healing complex gastrointestinal wall defects. Because of this, a lot of clips are frequently used to treat esophagogastric leaks with suboptimal clinical success rate. This situation demands innovative clip technology that results in higher curative rates and stimulates non-operative approach (16,17).
OTSC system was introduced (Ovesco Endoscopy GmbH, Tübingen, Germany) which simulates a surgical full-thickness closure, promoting an endoscopic alternative. The OTSC system is a pre-loaded clip in an application cap, connected in the endoscope like a band ligation device. The delivery system is quite similar to band ligation, too (17). OTSC is made for long-term implantation, significant full-thickness apposing with a high compression force until healing was complete (18).
OTSC devices
OTSC®
The OTSC® system basically resembles devices used for rubber band ligation of esophageal varices. A firing cap is attached on the end of the scope. They have two diameters device with variable chamber sizes (11 to 14 mm) compatible with many endoscope types (12).
According to the type of lesion, different clips are available. The OTSC® have three different models: spiked teeth (for all tissue use), blunt teeth (acute and soft tissue), and longer pointed teeth (for gastric use) (11).
The Ovesco system has twin grasper with independent jaws that approximate the gastrointestinal tract wall before deployment (16).
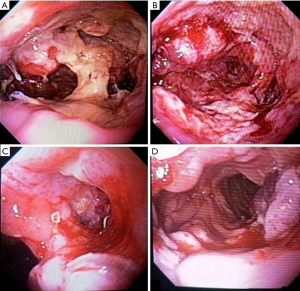
As compared to TTS clips, the Ovesco clip grasps more tissue and promotes more compressive linear force. OTSC® have also been used in non-variceal gastrointestinal bleeding approach when conventional therapy was not effective. The OTSC is safe and MRI compatible, because it is made up of nitinol alloy, and has a bear claw shape (Figure 1A) (4).

The OTSC system deployment is composed for many levels (19): (I) the transparent cap is mounted on the distal end of the scope; (II) the proximal ends have a band ligation like delivery system, controlled by an auxiliary person; (III) choose the deployment site and apply suction with or without grasper help to approximate tissue; (IV) fire the proximal delivery system and apply the OTSC. Remember to choose the optimal model before firing: blunt teeth (lower traumatic force and acute wall defects), spiked teeth (fibrotic tissue) or sharp teeth (to bowel wall) (19).
Padlock® Clip Closure System
The Padlock® clip (Aponos Medical Co., Kingston, NH, USA) is an OTSCs that proposes another technique for full-thickness apposing and has a different design (20).
The Padlock® clip is made by a nitinol ring with hexagonal form and needles pointing towards each other (Figure 1B). It provides full-thickness apposing with radial force by the six daggers, limiting the depth of penetration. The free spaces between the daggers optimize blood flow and the healing process.
The Padlock clip is preloaded in a cap attached on the tip of the scope (Figure 1B). The Padlock clip device is available in two sizes: for upper GI endoscopes (diameter: 9.5–11 mm); and for lower GI endoscopes (diameter: 11.5–14 mm).
The tissue chamber diameter is fixed, but the depth of the tissue chamber depends on the model. The tissue chamber depth is 1 cm in the standard model; however, for lower GI endoscopy size, the depth varies (8–20 mm), depending on the diameter of the scope. The clip is applicated by a delivery system, very similar to conventional TTS delivery system (20).
Although both are OTSC, OTSC and Padlock have many significant differences. The star shape and the convergent daggers promote a firm apprehension, which is not seen in the Ovesco (Figure 1C). The Padlock clip system does not require the operating channel of the endoscope, which is occupied when using the Ovesco clip (20) (Figure 2). Differences between the OTSC are listed in Table 2.
Table 2
| OTSC® | Padlock® Clip Closure System |
|---|---|
| Bear claw-shaped | Hexagonal shaped |
| Ligating band like fire | Through the scope clip like fire |
| Three different teeth with linear force | 6 linear needles with circumferential force |
| Occupied endoscopic work channel | Free endoscopic work channel |
| Fixed sizes | Variable chamber depth (8–20 mm) |
OTSC, over-the-scope clip.
General applications
A single center between 2018 and 2019 performed GI bleeding treatment with Padlock clip in 4 patients and for EFTR in 3 patients with success. Patient follow-up occurred at 1 and 3 weeks postoperatively. Padlock was delivered after apposing the tissue defect into the cap, without assistance of any forceps. Because of this, the free work channel in the scope permitted blood and fluids aspiration while adjusting to the perfect position (20).
A retrospective single center study with 101 OTSC applications showed that 92.8% of patients were successfully treated with OTSC and clinical success was reached in 89.3% of patients. OTSC deployment has clinical success in 85.4% patients for treatment of upper GI bleeding. It is important to note that, besides technical success of OTSC application for perforation treatment was 100%, only half of the cases reached clinical success. In fact, this situation was not related only to OTSC therapy, but involved the multidisciplinary approach results. The use for prevention perforation or bleeding after endoscopic submucosal dissection (ESD) was effective (100%). The overall application-related complications were low, just 2% (21).
Contraindications
An important limit of the OTSC therapy is defects larger than 2.5 cm. However, one may apply more than one clip, according to anatomy (16).
For Padlock Clip the own guide use device information mentions that your application is feasible for EFTR and hemostasis for lesions until 1.5 cm and for ulcers and fistulas treatment until 2 cm.
Adverse events
A few complications of OTSC placement have been reported and, in general, are minor and self-resolving problems like deployment failure, proximal tissue ulceration, and laceration (21).
OTSC for esophageal anastomotic leaks
OTSC application was indicated for small esophagic wall defects (<1–2 cm), if it has good blood flow and if the lesion was accessible by endoscopy, using the OTSC like isolated or associated endoscopic therapy (12).
The first OTSC application for esophageal anastomotic leaks was described by Pohl et al. in 2010 (17). One case with successful primary OTSC application with curative results and a second case with failed therapy with OTSC has been reported. For this case, the authors hypothesized that ablation of epithelialized mucosal edges prior to application of the OTSC would have promoted lasting occlusion.
Since then, many case series were published. Mennigen et al. (12) reported 14 patients that used the OTSC application for primary gastrointestinal wall defect therapy. Median follow-up was 5.5 months. The immediate clinical success rate was 100%, confirmed by endoscopy, including two cases of bronchial and pleural fistula which had failed SEMS. However, the long-term follow-up clinical success rate was 79%, without related adverse events.
The rationale that OTSC could be a rescue alternative to SEMS was published by Gómez et al. in a post-sleeve acute and proximal gastric fistula therapy that endoscopic treatment with SEMS failed, using an OTSC device deployment. A follow-up barium study showed no extravasation through the site of the fistula and the patient continued to tolerate oral intake, maintained a stable weight, and denied abdominal pain or fever (22).
In one of the largest case series for OTSC in gastrointestinal fistulas, 12 consecutive patients underwent OTSC placement to treat anastomotic or traumatic gastroesophageal wall defects (6–25 mm). All patients had clinical success without complications. Most fistulas was sealed in a few days after a single OTSC use (16).
Apparently, the treatment of anastomotic defects with OTSC could not be for acute situations only. Galizia et al. presented three cases of chronic esophagojejunal anastomotic leaks successfully treated with OTSC (23) with good results. In all cases, clip application was simple, safe, and effective, without early and late complications.
A rare, prospective cases series showed that for post-sleeve fistula treatment with OTSC, 54.5% were healed with primary efficacy, 36.5% with secondary efficacy, and treatment failed in 1 patient (9%), thus resulting in a 91% success rate (19).
The post procedure patient should be made nil per os until a contrast esophagogram can be obtained, usually within 24 hours. After that, if there is no leak evidence, the patient can start clear liquids oral intake and do a low progression to soft diet in a few days. If the leak persists, perform another endoscopic and imaging study to decide which complimentary therapy is better (11).
The use of OTSC is less expensive than a conventional surgical approach: in an Italian hospital the OTSC procedure carries a cost of about US$1,050, whereas a surgical reintervention would cost US$3,800 (16).
In Brazil, just a few months ago we have OTSC available for clinical use. We have a successful Padlock application for an acute esophagojejunal anastomotic leak. This patient was a 58-year-old man who underwent total gastrectomy for gastric cancer with a long Roux-en-Y and an end-to-side stapled esophagojejunostomy. On post-operative day 7, he presented with 50% anastomotic dehiscence and a pleural fistula confirmed by methylene blue oral test and endoscopic examination. We decided for endoscopic treatment with intraluminal EVT for one week and, after that, we applied two Padlock Clips with success and leak and fistula closure (Figure 3). The patient was discharged from the hospital one day later without complications and with oral liquid and soft diet. One month follow-up was good, and the patient has tolerating oral nutrition.
Limitations of this review and need for future research
Until now, the published literature regarding over-the-scopes clips has limited quality of evidence. We summarized a few case series and discussed the rationale, application, techniques, complications, and clinical success. Larger clinical studies are warranted, including a multicenter randomized clinical trial comparing OTSC versus others endoscopic therapy for gastrointestinal wall defects.
Summary
The approach to esophagic wall defects is complex and demands a multidisciplinary decision involving endoscopy, critical care, and surgical teams (17).
OTSC application is suitable for chronic and acute small defects (as isolated or complimentary option) or bronchopleural fistulas that have infection control or previous drainage (12).
The OTSC system could be used in GI fistulae with a non-infected tract or associated abscess and an opening smaller than 2 cm to be efficiently closed. In addition, recent fistulae could be better managed with an OTSC system because high fibrosis seems to be a limitation of its efficacy (19).
OTSC are an interesting endoscopic option for primary treatment of esophagic leak, fistulas, and perforations or could be a complementary therapy, such as EVT and OTSC, reducing hospitalization time and treatment costs, with good results as a minimally invasive endoscopic therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alejandro Nieponice) for the series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-1/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-1/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-1/coif). The series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” was commissioned by the editorial office without any funding or sponsorship. HGG reports being TOPMED speaker and consultant and Avanos Medical Speaker. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raymond D. Complications of esophagectomy. Surg Clin North Am 2012;92:1299-313. [Crossref] [PubMed]
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71-5. [PubMed]
- Famiglietti A, Lazar JF, Henderson H, et al. Management of anastomotic leaks after esophagectomy and gastric pull-up. J Thorac Dis 2020;12:1022-30. [Crossref] [PubMed]
- Gonzalez JM, Servajean C, Aider B, et al. Efficacy of the endoscopic management of postoperative fistulas of leakages after esophageal surgery for cancer: a retrospective series. Surg Endosc 2016;30:4895-903. [Crossref] [PubMed]
- Hourneaux de Moura EG, Toma K, Goh KL, et al. Stents for benign and malignant esophageal strictures. Ann N Y Acad Sci 2013;1300:119-43. [Crossref] [PubMed]
- de Moura DTH, de Moura BFBH, Manfredi MA, et al. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc 2019;11:329-44. [Crossref] [PubMed]
- Mercky P, Gonzalez JM, Aimore Bonin E, et al. Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc 2015;27:18-24. [Crossref] [PubMed]
- Nasa M, Sharma ZD, Choudhary NS, et al. Over-the-scope clip placement for closure of gastrointestinal fistula, postoperative leaks and refractory gastrointestinal bleed. Indian J Gastroenterol 2016;35:361-5. [Crossref] [PubMed]
- Bège T, Emungania O, Vitton V, et al. An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc 2011;73:238-44. [Crossref] [PubMed]
- Voermans RP, Le Moine O, von Renteln D, et al. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol 2012;10:603-8. [Crossref] [PubMed]
- Watkins JR, Farivar AS. Endoluminal Therapies for Esophageal Perforations and Leaks. Thorac Surg Clin 2018;28:541-54. [Crossref] [PubMed]
- Mennigen R, Colombo-Benkmann M, Senninger N, et al. Endoscopic closure of postoperative gastrointestinal leakages and fistulas with the Over-the-Scope Clip (OTSC). J Gastrointest Surg 2013;17:1058-65. [Crossref] [PubMed]
- do Monte ES Junior, de Moura DTH, Ribeiro IB, et al. Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: Systematic review and meta-analysis. Dig Endosc 2021;33:892-902. [PubMed]
- Haider S, Kahaleh M. The use of endoscopic clipping devices in the treatment of iatrogenic duodenal perforation. Gastroenterol Hepatol (N Y) 2010;6:660-1. [PubMed]
- Rodella L, Laterza E, De Manzoni G, et al. Endoscopic clipping of anastomotic leakages in esophagogastric surgery. Endoscopy 1998;30:453-6. [Crossref] [PubMed]
- Manta R, Manno M, Bertani H, et al. Endoscopic treatment of gastrointestinal fistulas using an over-the-scope clip (OTSC) device: case series from a tertiary referral center. Endoscopy 2011;43:545-8. [Crossref] [PubMed]
- Pohl J, Borgulya M, Lorenz D, et al. Endoscopic closure of postoperative esophageal leaks with a novel over-the-scope clip system. Endoscopy 2010;42:757-9. [Crossref] [PubMed]
- Schurr MO, Hartmann C, Ho CN, et al. An over-the-scope clip (OTSC) system for closure of iatrogenic colon perforations: results of an experimental survival study in pigs. Endoscopy 2008;40:584-8. [Crossref] [PubMed]
- Surace M, Mercky P, Demarquay JF, et al. Endoscopic management of GI fistulae with the over-the-scope clip system (with video). Gastrointest Endosc 2011;74:1416-9. [Crossref] [PubMed]
- Goenka MK, Rodge GA, Tiwary IK. Endoscopic Management with a Novel Over-The-Scope Padlock Clip System. Clin Endosc 2019;52:574-80. [Crossref] [PubMed]
- Wedi E, Gonzalez S, Menke D, et al. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol 2016;22:1844-53. [Crossref] [PubMed]
- Gómez V, Lukens FJ, Woodward TA. Closure of an iatrogenic bariatric gastric fistula with an over-the-scope clip. Surg Obes Relat Dis 2013;9:e31-3. [Crossref] [PubMed]
- Galizia G, Napolitano V, Castellano P, et al. The Over-The-Scope-Clip (OTSC) system is effective in the treatment of chronic esophagojejunal anastomotic leakage. J Gastrointest Surg 2012;16:1585-9. [Crossref] [PubMed]
Cite this article as: Guedes HG, de Moura EGH. Endoscopic management of complications—Ovesco/stent for management of anastomotic leaks: a narrative review. Ann Esophagus 2022;5:16.



