Treatment of Barrett’s esophagus: a narrative review
Introduction
Barrett’s esophagus (BE) is a condition defined by the replacement of the normal stratified squamous epithelium that lines the distal portion of the esophagus by metaplastic columnar epithelium (1,2). BE is an acquired condition. Most cases of BE are secondary to the damaging effects of gastroesophageal reflux disease (GERD) (3). The major significance of BE is related to its tendency to progress to esophageal adenocarcinoma (EAC) via the metaplasia-dysplasia-carcinoma sequence (4). BE is the only recognized premalignant lesion of EAC and it carries an 11-fold higher risk of EAC compared to the general population (5,6). Risk of BE progression is dependent on the degree of dysplasia. Management of BE often focuses on treating the underlying GERD and varies based on the presence versus absence of dysplasia. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-63/rc).
Methods
This is a narrative review incorporated from electronic databases (including PubMed, Google Scholar, UpToDate, ScienceDirect) spanning from 1990 to 2020 sampling from multiple centers in both a generalized population as well as age and gender specific. Analysis includes multivariate, meta-analysis, retrospective, and structured questionnaires. The research designs comprise prospective and retrospective cohort studies, case- control, and sham-controlled trials (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of Search | 12/1/2020 to 1/11/2022 |
| Databases and other sources searched | PubMed, Google Scholar, UpToDate, ScienceDirect |
| Search terms used | Barrett’s esophagus, GERD, esophageal cancer, dysplasia, mucosa |
| Timeframe | October 1990 to March 2020 |
| Inclusion and exclusion criteria | Inclusion criteria: single and multi-centered, prospective and retrospective cohort studies, case-control, sham-controlled trials, multivariate, meta-analysis, retrospective, structured questionnaires; exclusion criteria: none |
| Selection process | Authors conducted independently |
Epidemiology
BE is thought to develop as a result of chronic exposure of the distal esophageal mucosa to acid that occurs with GERD. A proportion of 10–20% of patients with GERD present with BE, although selection bias exists secondary to the populations that are generally screened (1,7). While GERD is believed to be the major catalyst to BE development, there are other risks factors predicting BE development and subsequent progression of BE to dysplasia and EAC. As indicated in Table 2, other well-established risk factors for BE include age greater than 50 years, male gender, central obesity, white race, presence of hiatal hernia, and cigarette smoking (8-10). Family history of BE or EAC is a particularly strong risk factor, and one study utilizing a structured questionnaire given to 164 subjects reported an increased risk in first- and second-degree relatives of patients with BE (24% vs. 5%, P<0.005) (11). Interestingly, Helicobacter pylori has been reported to be protective against BE secondary to urease activity by decreasing acidity in the stomach (12,13).
Table 2
| GERD |
| Age greater than 50 years old |
| Central obesity |
| White race |
| Presence of hiatal hernia |
| Cigarette smoking |
| Family history of BE or EAC |
GERD, gastroesophageal reflux disease; BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma.
In 2019, a systematic review and meta-analysis was performed to assess the correlation of the risk of BE in the general population based on the number of risk factors while controlling for potential confounders. Forty-nine studies through October 2018 were analyzed (307,273 individuals, 1,948 with BE). The results of the analysis revealed the prevalence of BE for several populations as: low-risk general population, 0.8%; GERD, 3%; GERD plus presence of any other risk factor, 12.2%; family history, 23.4%; age >50, 6.1%; obesity, 1.9%; and male sex, 6.8%. When controlling the study region, age, and gender in a meta-regression, there was a positive linear relationship between the number of risk factors and the prevalence of BE (14).
In another systematic review and meta-analysis performed in 2018, 20 studies (including 74,943 patients) were analyzed to detect the risk factors associated with the progression of BE with and without LGD to BE with HGD or EAC. They found that the risk factors for the progression of BE included increasing age, male sex, ever smoking, longer BE segment length, and LGD. Alcohol use and obesity was not associated with risk of progression. Therefore, they concluded that patients with these risk factors should undergo more intensive surveillance or endoscopic therapy (15).
Pathophysiology
The process of developing BE is said to occur in two steps. The first step occurs secondary to the reflux of both gastric fluid and duodenal secretions onto the squamous epithelium of the esophagus which transforms it into simple columnar epithelium, that of the cardiac mucosa. This chronic mucosal injury occurs over a span of a few years and involves an inflammatory cascade that stimulates cellular proliferation and genetic alterations that then induces genetic destabilization. The second step spans over 5 to 10 years and involves intestinal metaplasia via the development of goblet cells (Figure 1). Once BE is present, the development of low-grade dysplasia (LGD) to high-grade dysplasia (HGD) and eventually adenocarcinoma occurs through the metaplasia-dysplasia-carcinoma sequence (Figure 2). Alterations of architecture and cytologic processes differentiate LGD from HGD (16). Notwithstanding this increased cancer risk, the annual rate of progression of BE to EAC is 0.1% to 0.5% and the rate of progression of HGD to EAC is 6% (17).
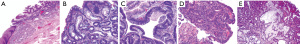
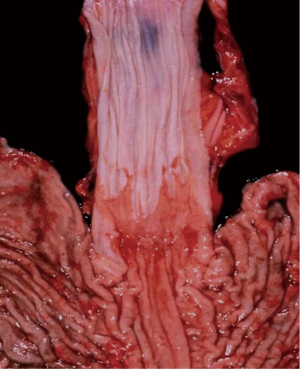
Of the patients with GERD, about 10–15% will develop BE. The normal esophageal squamous mucosa transforms into simple columnar epithelium is provoked chronic injury from recurrent reflux. Studies have shown that the duration of reflux symptoms was an important factor for BE development. The damage that acid causes to the esophageal epithelium creates dilated intercellular spaces that causes an increase in the transepithelial permeability allowing for larger molecules to diffuse across. This exposes basal layer stem cells to reflux fluid that induces a cascade of events leading to cell edema and eventual cell death. Phenotypic transformation of squamous cells into columnar mucosal cells then occurs due to a combination of tissue reparative processes in the setting of an acidic environment. Two pathways exist for the transformation of columnar epithelium to BE, gastric differentiation or intestinal differentiation. Gastric differentiation consists of the formation of parietal cells within glands. Intestinal differentiation consists of the formation of goblet cells within the columnar epithelium that is induced by intestinalizing gene expression. Intestinal differentiation is unfavorable in comparison due to its capability of further progression to epithelial dysplasia and adenocarcinoma. With this information in mind, it is important to note that BE is the strongest predicting factor of EAC even though only a small percentage of patients with BE will develop cancer (18).
Treatment
Barrett’s esophagus without dysplasia
Medical therapy
In patients with non-dysplastic BE, the primary goal is that of preventing dysplastic progression while mitigating GERD symptoms (19). First-line therapy to regulate GERD in BE patients is medical therapy with proton pump inhibitors (PPIs). The mechanism of action of PPIs is to block gastric acid secretion by irreversibly binding to and inhibiting the hydrogen-potassium ATPase pump that resides on the luminal surface of the parietal cell membrane (20). In a multivariate analysis of 236 subjects with non-dysplastic BE, 56 patients developed dysplasia, 14 of which had high-grade dysplasia. This revealed a 75% reduction of dysplasia development among patients who received PPI therapy versus no therapy or histamine 2-receptor antagonist (H2RA) therapy (95% CI: 0.13–0.47, P<0.0001). Moreover, a longer duration of PPI therapy correlated with a decreased incidence of dysplasia (21). In a single-center, prospective interventional controlled study, 50 patients with BE were included over 2 years of receiving PPI therapy. At each visit, the length of BE was analyzed via endoscopic biopsies at intervals of 1 cm. It was shown that those with short-segment Barrett’s esophagus (SSBE) had circumferential extension of 1.5 cm before treatment vs. 0.8 cm after treatment and maximal proximal extension of 2.3 cm before treatment versus 1.1 cm after treatment (22).
Other classes of chemoprevention in BE are aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), and statins, although the data that is currently available is uncertain and therefore is not recommended routinely (23).
In the wide scheme of things, PPIs are largely well tolerated and have shown to aid in the impedance of dysplastic changes of BE via its acid suppression properties as well as anti-inflammatory effects. Although the progression of BE to dysplasia and subsequently EAC is not completely understood and there are conflicting results in its role as chemoprevention, it remains first-line therapy as it provides excellent control of GERD.
Antireflux surgery
In the instance that medical therapy has failed to control GERD symptoms, surgical intervention with antireflux surgery (ARS) is an alternative option. The method of reflux prevention in ARS differs from medical therapy in that it is geared toward mechanical deficits such as an incompetent lower esophageal sphincter (LES), impaired esophageal motility, or hiatal hernia that allows for reflux into the distal esophagus. One study evaluated 186 laparoscopic fundoplications (Nissen 98% and Toupet 2%) and found that 82% of patients stated their preoperative reflux symptoms were gone, concluding it was a safe antireflux therapy (24).
A subgroup of patients exists that would benefit more from a gastric bypass versus a fundoplication for control of reflux symptoms. In obese patients, they generally have inadequate outcomes or relapse of symptoms after undergoing fundoplication secondary to increased intraabdominal pressure causing disruption of the wrap. In a 2017 literature review involving 121 patients with a mean body mass index (BMI) of 37.17 kg/m2, laparoscopic Nissen fundoplication was compared to laparoscopic gastric bypass. There was found to be an increased risk of complications in the gastric bypass group, but it nonetheless is the preferred operation in the morbidly obese population for the added benefits of concomitant weight loss (25).
Conflicting evidence exists in terms of whether or not surgical intervention should be employed in the prevention of BE progression. A 2003 meta-analysis that compared patients with BE undergoing ARS versus medical therapy showed that the incidence rate of cancer in the ARS group was 3.8 cancers/1,000 patient-years versus 5.3 in the medical group (P=0.29). This concluded that the risk of EAC in BE patients is not statistically decreased by ARS, although strong selection bias existed in that the data was resulted from nonrandomized cohort studies (26). In contrast to this, a 2001 study of 97 patients with BE were analyzed at a medium of five years after undergoing antireflux surgery. In 44% of the patients, there was regression from low-grade dysplasia to non-dysplastic BE. In 14% of the patients, there was regression from intestinal metaplasia to cardiac mucosa. None of the patients developed either high-grade dysplasia or EAC (27).
It has been stated and demonstrated in studies that antireflux surgery in combination with endoscopic therapy might prevent progression and possibly regression of dysplastic and metaplastic changes in the esophagus. However, such studies include small numbers of patients and short follow-up therefore was not able to address long-term success. Because this is still difficult to prove and the supporting evidence is inconclusive, further evaluation with larger controlled trials and longer-term follow-up is necessary to better define the success of this approach for preventing low-grade dysplasia’s progression to esophageal cancer (28).
In order to determine whether or not ARS should be employed in effort to alter risk of malignant progression in BE, it would be beneficial for future studies to include a larger population, a longer median follow-up, and involvement of different cohorts. In the interim, ARS can be used to control the symptomatology in reflux patients who have either failed medical therapy or are non-compliant with medication and lifestyle modifications.
Barrett’s esophagus with low-grade dysplasia
There is a dispute in the optimal treatment of LGD because of the inconsistency that exists within its diagnosis. The current guidelines state that the diagnosis of LGD must be confirmed and agreed upon by two pathologists (29). In a multicenter analysis of 210 patients with BE and LGD followed for an average of 6.2 years, there was a 1.6%/year incidence of developing HGD and 0.44%/year incidence of developing EAC (30). All patients with LDG should be on a PPI and undergo surveillance with endoscopy with biopsy within six months of reflux control. With that being said, the risk of progression exists and therefore endoscopic ablation therapy should be considered. A 2017 meta-analysis of 2,746 patients studied the risk of disease progression in those with LGD treated with either radiofrequency ablation (RFA) or surveillance endoscopy only. The study concluded that the incidence rate of disease progression in the surveillance group was significantly higher than the RFA group (0.022 vs. 0.005, P<0.001) (31).
Barrett’s esophagus with high-grade dysplasia
In comparison to LGD, the majority of patients with HGD must undergo endoscopic treatment because of its increased risk of progression. In a 2008 meta-analysis of 236 patients with HGD who underwent surveillance endoscopies, approximately 6% developed EAC (17). An important initial step in these patients is a repeat endoscopy with the use of white light-endoscopy and narrow band imaging to locate potential lesions or nodules within the mucosa. Because there is no risk of lymph node metastasis in HGD, endoscopic techniques should be employed. The thought behind this is that normal squamous epithelium replaces the abnormal tissue once it is either removed or destroyed before it becomes invasive. There are a multitude of treatment modalities that exist and they are separated into two types. The first group are tissue-6 acquiring techniques that include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). The second group are ablative techniques that include RFA, multipolar electrocoagulation (MPE), argon plasma coagulation (APC), cryotherapy (CRY), and photodynamic therapy (PDT) (3).
Endoscopic mucosal resection
EMR is the preferred initial treatment modality for nodular BE because it allows for comprehensive histologic analysis, given the fact that it preserves tissues rather than destroying it. This process begins by introducing either normal saline or epinephrine into the submucosal layer in order to elevate the lesion off of the muscularis propria. There are two methods that can be performed. The cap-assisted method targets the lesion and then retracts into the cap via suction, followed by resection via an electrocautery snare. The ligation-assisted method aspirates tissues via suction, followed by creating a pseudopolyp with a band ligation device and subsequent electrocautery (Figure 3). Although these techniques have similar outcomes, ligation-assisted is preferred because of its ability to resect several lesions with one kit, thereby making it more cost-friendly as well quicker (32).
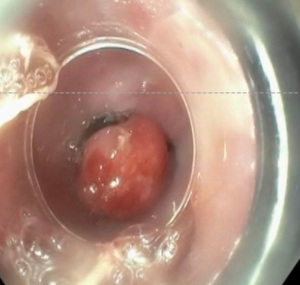
In a 2009 retrospective analysis of 49 patients with histologically confirmed BE with HGD or intramucosal carcinoma (IMC), normal squamous epithelium was seen on surveillance biopsies in 96.9% of patients with a mean remission time of 22.9 months. Also noted in this study was the development of esophageal stenosis, occurring in 37% of patients, all of which were managed by endoscopic dilations or stents. Other possible complications that can occur includes bleeding that occurs immediately after resection and perforation (33).
In 2000, a prospective study was conducted on 64 patients with Barrett’s esophagus (61 patients with early carcinoma, 3 patients with high-grade dysplasia) to investigate the role of endoscopic mucosal resection. They were divided into 2 groups. Group A consisted of 35 patients that met the criteria for low risk (macroscopic types I, IIa, IIb, and IIc, lesion diameter up to 20 mm, mucosal lesion, histological grades G1 and G2 and/or high-grade dysplasia). Group B consisted of 29 patients that met the criteria for high risk. Complete remission was achieved in 97% of the patients in group A and in 59% in group B. In a mean follow-up of 1 year +/− 8 months, recurrent or metachronous carcinomas were found in 14%. Only one major complication occurred, spurting bleeding, that was managed endoscopically (34).
Endoscopic submucosal dissection
ESD is the preferred technique in lesions that are greater than 1.5–2 cm because it allows for a complete en bloc resection of the lesion, rather than in a fragmentary fashion when compared to EMR. ESD also allows for comprehensive histologic analysis of tissue, although is not widely used because of its correlation with substantial adverse effects as well as the technical difficulty that is involved in the procedure. This process begins with introducing saline into the submucosal layer within the area that is marked via coagulation, this lifts the lesions off of the muscularis propria and allows for the dissection knives to perform the submucosal dissection (35).
A 2014 retrospective analysis studied 75 patients with BE who underwent ESD. Results include an en bloc resection rate of 90 % and curative resection of HGD and EAC of 64% and 85% respectively. Potential adverse effects of this procedure include bleeding, perforation, and stricture formation (36).
Radiofrequency ablation
This is the most common ablative technique that is used and is preferred for nonnodular BE. It achieves tissue necrosis by delivering a high-frequency energy to the esophageal mucosa. Either a balloon ablation catheter (for circumferential ablation of BE segments longer than 2 cm) or a focal catheter (for focal ablation of shorter BE segments) is used. Depending on the length of the BE segment, multiple endoscopic treatments with RFA may be necessary for complete dysplasia eradication (37).
A 2009 multi-center sham-controlled trial studied 127 patients with dysplastic BE were randomly assigned to either receive RFA or a sham procedure (the control group). Among those with LGD, 90.5% of those in the RFA group had complete eradication of dysplasia versus 22.7% of those in the control group (P<0.001). Among those with HGD, 81% of those in the RFA group had complete eradication versus 19% of those in the control group (P<0.001). It was also found that the RFA group had less progression of disease (3.6% vs. 16.3%, P=0.03) as well as less cancers (1.2% vs. 9.3%, P=0.045). Overall, RFA is well tolerated and safe. The most common adverse effects include chest pain, dysphagia, strictures requiring dilation, gastrointestinal hemorrhage, and perforation (38).
Multipolar electrocoagulation
This technique involves passing an electrical current through the targeted tissue lesion via an electrical probe at the end of an endoscope (3). A 2011 prospective cohort study involved 166 patients with either nondysplastic BE or evidence of intestine metaplasia with a 10-year follow-up. Findings include recurrent BE in 5% of the patients and HGD or EAC in 0% of the patients, concluding that it is an effective method to ablate BE over the long-term scheme (39).
Argon plasma coagulation
APC conducts and electrical current that results in thermal electrocoagulation via a beam 259 of argon plasma. This is a useful technique mucosal-involving BE because it creates a 2–3 mm depth of necrosis that is evenly distributed (3). A 2003 study was performed and involved 29 patients with HGD who underwent APC with a 7-year follow-up. Results include a response to treatment in 86% of patients and complete regression to neosquamous esophageal mucosa in 75% of patients. They concluded that APC in HGD is an effective treatment, especially in those who are unable to undergo surgical resection (40).
Cryotherapy
CRY is a non-contact method that involves endoscopically spraying liquid nitrogen at −196 °C directly onto the target lesion, thereby freezing and causing tissue destruction. Because no contact is involved, this technique can be employed for irregular lesions. Another added benefit of this technique is the ability to use it as both a first-line treatment for dysplastic BE as well as second-line in previously failed treatment modalities. Adverse effects include pain, strictures requiring dilation, bleeding, and perforation (35).
In 2009, a single-center, nonrandomized cohort study was performed involving 30 patients with HGD or IMC who underwent CRY. 90% of these patients had downgrading of pathology stage after treatment. Elimination of cancer or downgrading of HGD at last follow-up was 68% for HGD and 80% for IMC (41).
Photodynamic therapy
PDT involves administering a systemic photosensitizing agent (e.g., Porfimer sodium) into the patient that is then taken up by neoplastic tissues. The photosensitizing agent is exposed to a portion of the esophagus to light of a specific wavelength, which produces cytotoxicity and leads to dysplasia cell death (35). Adverse effects of this include cutaneous photosensitivity, constipation, vomiting, odynophagia, dysphagia, noncardiac chest pain, dehydration, and stricture formation.
In 2003, a study was performed involving 103 patients with BE who underwent PDT with Porfimer sodium and PPI use. Results include a decrease in length of BE by a mean of 6.92 cm. Of the 65 patients with HGD, (94%) had elimination of HGD. They concluded that Porfimer-photodynamic therapy with supplemental Nd:YAG photoablation as well as concomitant omeprazole reduces the length of BE, eliminates HGD, and may reduce the expected frequency of carcinoma (42).
There is a consecutive case series of 86 patients at a single center that was done to compare effectiveness, safety, and cost of photodynamic therapy and radiofrequency ablation in treatment of Barrett’s dysplasia. Thirty-three patients with high-grade dysplasia had treatment with porfimer sodium photosensitzer. Fifty-three patients with BD (47 with LGD, 6 with HGD) had RFA. The complete histological resolution response of BD was 54.5% with PDT versus 88.7% with RFA. They concluded that RFA had higher rate of complete histological resolution response without any serious adverse events and it was also less costly than PDT for endoscopic treatment of BD (43).
Barrett’s esophagus with T1 carcinoma
Patients with adenocarcinoma at the level of the mucosa (T1a) without lymphovascular invasion are candidates for esophagus-preserving therapies with endoscopic modalities. In a 2009 analysis of 178 patients with T1a EAC, 78% were treated endoscopically and 26% were treated surgically. They concluded that the overall survival in patients with mucosal EAC when treated endoscopically is comparable with that of patients treated surgically. Recurrent carcinoma occurs in a limited proportion of patients treated endoscopically, but all are successfully re-treated without impacting overall survival (44).
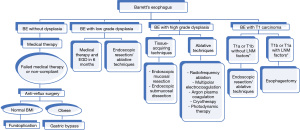
With adenocarcinoma that invades into the submucosa (T1b), nodal metastasis is likely present and therefore esophagectomy with lymph node dissection is recommended. Although a caveat to this exists. In a 2017 analysis within the National Cancer Database, 782 patients with nonmetastatic, Tis, T1a, or T1b EAC who had primary surgical resection and microscopic examination of at least 15 lymph nodes were studied. They found that independent predictors of lymph node metastasis (LNM) included submucosal invasion, lymphovascular invasion (LVI), decreasing differentiation, and tumor size ≥2 cm (P<0.05) (Table 3). For T1a tumors that were ≥2 cm or had poor differentiation, LNM rates were 6.7 and 10.2%, respectively with a 90-day mortality of 3.1%. For T1b tumors that were <2 cm and well differentiated, LNM rate was 4.2% with a 90-day mortality of 6%. The 5-year overall survival in T1a versus T1b was 80.2% versus 64.4% respectively. LNM increased the risk of death for T1a (HR =8.52, 95% CI: 3.13–23.22, P<0.001) and T1b tumors (HR =2.52, 95% CI: 1.59–4.00, P<0.001). Subsequently, they concluded that in T1a EAC with poor differentiation or size ≥2 cm, esophagectomy should be considered, whereas in T1b EAC with low-risk features (well-differentiated, <2 cm, no lymphovascular invasion), endoscopic resection may be sufficient (45). Treatment options for BE is summarized in Figure 4.
Table 3
| Submucosal invasion |
| Lymphovascular invasion |
| Decreasing differentiation |
| Tumor size ≥2 cm |
Discussion
Although there is a strong association between GERD and BE, its development into cancer is a rare dysplastic process that is not completely understood. Nonetheless, surveillance and treatment early on is imperative to preclude this from occurring. Such methods include PPIs, endoscopic mucosal resection, endoscopic submucosal dissection, radiofrequency ablation, multipolar electrocoagulation, argon plasma coagulation, cryotherapy, photodynamic therapy, and esophagectomy. Every patient is unique in not only the pathology that leads to their diagnosis, but how they will respond to the treatment they undergo. Careful evaluation of dysplastic mucosa and management with one of the vast treatment modalities that are available is fundamental in mitigating its potential to become cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Timothy M. Farrell and Geoffrey Kohn) for the series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-63/rc).
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-63/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-63/coif). The series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. DM serves as an unpaid editorial board member of Annals of Esophagus from September 2020 to August 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldblum JR. The significance and etiology of intestinal metaplasia of the esophagogastric junction. Ann Diagn Pathol 2002;6:67-73. [Crossref] [PubMed]
- Quante M, Graham TA, Jansen M. Insights Into the Pathophysiology of Esophageal Adenocarcinoma. Gastroenterology 2018;154:406-20. [Crossref] [PubMed]
- Martinucci I, de Bortoli N, Russo S, et al. Barrett's esophagus in 2016: From pathophysiology to treatment. World J Gastrointest Pharmacol Ther 2016;7:190-206. [Crossref] [PubMed]
- Wiseman EF, Ang YS. Risk factors for neoplastic progression in Barrett's esophagus. World J Gastroenterol 2011;17:3672-83. [Crossref] [PubMed]
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375-83. [Crossref] [PubMed]
- Kambhampati S, Tieu AH, Luber B, et al. Risk Factors for Progression of Barrett's Esophagus to High Grade Dysplasia and Esophageal Adenocarcinoma. Sci Rep 2020;10:4899. [Crossref] [PubMed]
- Cameron AJ, Zinsmeister AR, Ballard DJ, et al. Prevalence of columnar-lined (Barrett's) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology 1990;99:918-22. [Crossref] [PubMed]
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett's esophagus by endoscopy indication. Gastrointest Endosc 2010;71:21-7. [Crossref] [PubMed]
- van Blankenstein M, Looman CW, Johnston BJ, et al. Age and sex distribution of the prevalence of Barrett's esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005;100:568-76. [Crossref] [PubMed]
- Khieu M, Mukherjee S. Barrett Esophagus. Treasure Island (FL): StatPearls Publishing; 2022.
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett's oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002;51:323-8. [Crossref] [PubMed]
- Corley DA, Kubo A, Levin TR, et al. Helicobacter pylori infection and the risk of Barrett's oesophagus: a community-based study. Gut 2008;57:727-33. [Crossref] [PubMed]
- Sharma P, Vakil N. Review article: Helicobacter pylori and reflux disease. Aliment Pharmacol Ther 2003;17:297-305. [Crossref] [PubMed]
- Qumseya BJ, Bukannan A, Gendy S, et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett's esophagus. Gastrointest Endosc 2019;90:707-717.e1. [Crossref] [PubMed]
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors Associated With Progression of Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:1046-1055.e8. [Crossref] [PubMed]
- Oh DS, Demeester SR. Pathophysiology and treatment of Barrett's esophagus. World J Gastroenterol 2010;16:3762-72. [Crossref] [PubMed]
- Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394-8. [Crossref] [PubMed]
- Schlottmann F, Molena D, Patti MG. Gastroesophageal reflux and Barrett's esophagus: a pathway to esophageal adenocarcinoma. Updates Surg 2018;70:339-42. [Crossref] [PubMed]
- Modiano N, Gerson LB. Barrett's esophagus: Incidence, etiology, pathophysiology, prevention and treatment. Ther Clin Risk Manag 2007;3:1035-145. [PubMed]
- Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep 2008;10:528-34. [Crossref] [PubMed]
- El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett's esophagus. Am J Gastroenterol 2004;99:1877-83. [Crossref] [PubMed]
- Gashi Z, Bahtiri E, Gashi A, et al. Proton Pump Inhibitors Diminish Barrett's Esophagus Length: Our Experience. Open Access Maced J Med Sci 2018;6:1041-5. [Crossref] [PubMed]
- Singh T, Sanghi V, Thota PN. Current management of Barrett esophagus and esophageal adenocarcinoma. Cleve Clin J Med 2019;86:724-32. [Crossref] [PubMed]
- Rosenthal R, Peterli R, Guenin MO, et al. Laparoscopic antireflux surgery: long-term outcomes and quality of life. J Laparoendosc Adv Surg Tech A 2006;16:557-61. [Crossref] [PubMed]
- Mendes-Filho AM, Godoy ESN, Alhinho HCAW, et al. Fundoplication conversion in Roux-en-Y gastric bypass for control of obesity and gastroesophageal reflux: systematic review. Arq Bras Cir Dig 2017;30:279-82. [Crossref] [PubMed]
- Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett's esophagus? A meta-analysis. Am J Gastroenterol 2003;98:2390-4. [Crossref] [PubMed]
- Hofstetter WL, Peters JH, DeMeester TR, et al. Long-term outcome of antireflux surgery in patients with Barrett's esophagus. Ann Surg 2001;234:532-8; discussion 538-9. [Crossref] [PubMed]
- dos Santos RS, Bizekis C, Ebright M, et al. Radiofrequency ablation for Barrett's esophagus and low-grade dysplasia in combination with an antireflux procedure: a new paradigm. J Thorac Cardiovasc Surg 2010;139:713-6. [Crossref] [PubMed]
- Naini BV, Souza RF, Odze RD. Barrett's Esophagus: A Comprehensive and Contemporary Review for Pathologists. Am J Surg Pathol 2016;40:e45-66. [Crossref] [PubMed]
- Wani S, Falk GW, Post J, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett's esophagus. Gastroenterology 2011;141:1179-86, 1186.e1.
- Qumseya BJ, Wani S, Gendy S, et al. Disease Progression in Barrett's Low-Grade Dysplasia With Radiofrequency Ablation Compared With Surveillance: Systematic Review and Meta-Analysis. Am J Gastroenterol 2017;112:849-65. [Crossref] [PubMed]
- Blevins CH, Iyer PG. Endoscopic therapy for Barrett's oesophagus. Best Pract Res Clin Gastroenterol 2015;29:167-77. [Crossref] [PubMed]
- Chennat J, Konda VJ, Ross AS, et al. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol 2009;104:2684-92. [Crossref] [PubMed]
- Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology 2000;118:670-7. [Crossref] [PubMed]
- Mansour NM, El-Serag HB, Anandasabapathy S. Barrett's esophagus: best practices for treatment and post-treatment surveillance. Ann Cardiothorac Surg 2017;6:75-87. [Crossref] [PubMed]
- Chevaux JB, Piessevaux H, Jouret-Mourin A, et al. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett's neoplasia. Endoscopy 2015;47:103-12. [PubMed]
- Belghazi K, Bergman J, Pouw RE. Endoscopic Resection and Radiofrequency Ablation for Early Esophageal Neoplasia. Dig Dis 2016;34:469-75. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Allison H, Banchs MA, Bonis PA, et al. Long-term remission of nondysplastic Barrett's esophagus after multipolar electrocoagulation ablation: report of 139 patients with 10 years of follow-up. Gastrointest Endosc 2011;73:651-8. [Crossref] [PubMed]
- Attwood SE, Lewis CJ, Caplin S, et al. Argon beam plasma coagulation as therapy for high-grade dysplasia in Barrett's esophagus. Clin Gastroenterol Hepatol 2003;1:258-63. [Crossref] [PubMed]
- Dumot JA, Vargo JJ 2nd, Falk GW, et al. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc 2009;70:635-44. [Crossref] [PubMed]
- Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett's esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc 2003;58:183-8. [Crossref] [PubMed]
- Ertan A, Zaheer I, Correa AM, et al. Photodynamic therapy vs radiofrequency ablation for Barrett's dysplasia: efficacy, safety and cost-comparison. World J Gastroenterol 2013;19:7106-13. [Crossref] [PubMed]
- Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology 2009;137:815-23. [Crossref] [PubMed]
- Newton AD, Predina JD, Xia L, et al. Surgical Management of Early-Stage Esophageal Adenocarcinoma Based on Lymph Node Metastasis Risk. Ann Surg Oncol 2018;25:318-25. [Crossref] [PubMed]
Cite this article as: Nesheiwat G, Carr R, Molena D, Tang L. Treatment of Barrett’s esophagus: a narrative review. Ann Esophagus 2022;5:44.