Per-oral endoscopic myotomy (POEM) for achalasia: techniques and outcomes
Achalasia is a rare condition with an annual incidence of 1 to 2 cases per 100,000 people (1-4). It is an esophageal motility disorder, characterized by incomplete lower esophageal sphincter (LES) relaxation leading to dysphagia. Achalasia is caused by inflammation and degeneration of the inhibitory, nitric-oxide-producing ganglion cells in the myenteric plexus of the LES and distal esophageal smooth muscle (5,6). This loss of inhibitory neurons causes increased basal sphincter pressure and loss of sphincter relaxation in conjunction with aperistalsis of the esophagus (7). The etiology of achalasia is predominately idiopathic; however, achalasia is occasionally a genetically predisposition, associated with autoimmune disorders, or secondary to infection or inflammation, such as with Chagas disease (8). Most patients are diagnosed between 30 and 60 years of age with similar incidence between men and women (4,8,9).
Clinical presentation and diagnosis
Achalasia presents with progressive dysphagia to solids and liquids in 82–100% of patients, regurgitation in 76–91%, heartburn in 27–42%, chest pain in 25–64%, weight loss in 35–91%, and nocturnal cough and aspiration in 8% of patients (2,10-12). Nutritional deficiencies and malnutrition are also common; 75% of patients with achalasia had low pre-albumin levels in one study (13). The gold standard of diagnosis is high-resolution esophageal manometry (HREM).
All patients with achalasia display impaired esophagogastric junction (EGJ) relaxation; however, patterns of pressurization and relaxation seen on manometry vary, which led to the characterization of 3 distinct achalasia subtypes and the development and refinement of the Chicago Classification (2,9,14). Type I, also known as classic achalasia, occurs in 20–40% of patients and is characterized by the absence of panesophageal pressurization and complete aperistalsis with a distal contractile integral (DCI) <100 mmHg/s/cm. Type II achalasia, which occurs in 50–70% of patients making it the most common subtype, also displays complete aperistalsis. However, manometry will show uniform pressures across the entire esophagus >30 mmHg in >20% of swallows. Lastly, type III achalasia, or the “spastic” subtype, is characterized by 2 or more premature lumen-obliterating spastic contractions in >20% of swallows with or without periods of compartmentalized esophageal pressurization and DCI >450 mmHg/s/cm (2,15-17). This is the least frequent subtype and is observed in only 5% of patients with achalasia. Of the less frequent symptoms, patients with type II achalasia are most likely to have weight loss, and patients with type III achalasia and female patients are most likely to report chest pain and least likely to have weight loss (2,18). Histologically, patients with type I or type II achalasia have aganglionosis and neuronal loss, whereas patients with type III achalasia have preserved ganglion cells (2).
A barium esophagogram is frequently obtained in the workup of patients with achalasia and shows dilation of the esophagus, a narrow “bird-beak” appearance of the EGJ, delayed emptying, and aperistalsis (19). However, the esophagogram may appear normal in up to 1/3 of patients (12). Esophagogastroduodenoscopy (EGD) is also performed to rule out pseudoachalasia from a malignancy. In patients with achalasia, the LES typically appears normal to thickened and does not open spontaneously with passage of the endoscope but requires some gentle forward pressure with a classic “pop” when traversing the non-relaxed LES (2,12,20).
Medical management
Medical management of achalasia may temporarily improve symptoms in up to 75% of patients and includes calcium channel blockers and nitrates (2). Non-surgical interventions for achalasia include botulinum injection and pneumatic dilation. Localized administration of botulinum toxin to the LES improves symptoms in up to 90% of patients; however, up to 50% have return of symptoms within 1 year (2). Pneumatic dilation improves symptoms in 90% of patients at 6 months and 44% at 6 years, but dilation may be more effective in some patients than in others. Clinical series have reported a 37% response at 3 years with a single balloon dilation vs. 86% with graded dilations and that balloon dilation is more frequently effective in patients older than 45 years and with type II achalasia (2,21,22).
Surgical management
Surgical treatments of achalasia include laparoscopic Heller myotomy (LHM) or robotic-assisted Heller myotomy (RHM) with fundoplication, per-oral endoscopic myotomy (POEM), and esophagectomy as a last resort if other treatments fail or if the patient has a tortuous megaesophagus (2). LHM or RHM is most favorable in patients with type II achalasia due to a 93% effectiveness in treating type II achalasia as compared with type I (81% effectiveness) or type III (71–86% effectiveness) (2,23). In addition, combining partial fundoplication (either Dor or Toupet) with LHM has reduced the incidence of postoperative gastroesophageal reflux disease (GERD) from >40% to <15% (24,25). The clinical efficacy of LMH is reported as 87% to 92% (8,26). Ali and colleagues compared LHM, RHM, and POEM between 2013 and 2017 and found that LHM had a significantly higher complication rate (17.5%) as compared with RHM (0%) or POEM (1.1%) (27).
History of POEM
POEM was introduced in 1980 by Ortega and colleagues who first performed an endoscopic transmucosal myotomy with an electrosurgical knife in dogs (20,28-30). Despite having outcomes comparable with Heller myotomy, there was a concern that full thickness esophageal incision would increase the risk of esophageal perforation and subsequent mediastinal perforation, and as a result, the technique failed to gain momentum initially. Over the course of the next 30 years, however, some important modifications were made that increased the safety of the procedure including a submucosal tunneling technique.
During submucosal tunneling, a mucosal incision is made proximal to the myotomy and a submucosal tunnel is created to provide access so that only the circular muscle fibers are divided (29,31,32). In 2010, Inoue and colleagues utilized this technique in a 17-patient cohort with achalasia and demonstrated improvement of dysphagia similar to that seen with Heller myotomy (33). Several other studies have shown similar safety and efficacy of POEM as LHM with no difference in postsurgical quality of life and similarly reduced Eckardt scores (34-36). Additionally, two European and US multicenter trials have reported a response rate of over 90% with similar efficacy of POEM as compared with LHM for treatment of all three types of achalasia (35,37).
In our practice, POEM remains the preferred approach for the management of achalasia, especially for type I and type III. We do customize the management of type II achalasia as per patient’s symptoms and preference. Most of them still get POEM but we do counsel and offer RHM with modified Dor fundoplication in patients with symptomatic GERD and increase acid exposure of distal esophagus on Bravo test.
Technique of POEM
POEM involves four main steps that vary in specifics between surgeons and institutions. Endotracheal intubation is typically performed, and the patient is placed in the supine position. General anesthesia, instead of sedation, decreases the risk of aspiration, bleeding, perforation, and insufflation-related complications (38-40). Carbon dioxide insufflation is preferred as it resolves more quickly than room air, is noncombustible and inexpensive, and the abdomen remains accessible during the procedure to assess distension or pneumoperitoneum (8,39). Peak airway pressures are also monitored as elevated pressures can indicate pneumoperitoneum requiring needle decompression (39,41).
At the start of the procedure, an EGD is performed to evaluate the esophageal anatomy. The EGJ is noted, and the esophagus is cleared of the food debris. If the surgeon is unable to clear the esophagus of food, then the procedure should be postponed to avoid risking mediastinal contamination. The distance to the LES is measured from the incisors. The site of the myotomy, anterior vs. posterior, remains debatable. Earlier descriptions detailed anterior myotomy. Most experts, including experts at our institution, prefer posterior myotomy in the supine position, especially if the patient had Heller myotomy previously.
Step 1: mucosotomy
The gastroscope is aligned to make sure the 12-o-clock and 6-o-clock positions are identified, and the scope is oriented in the lumen of esophagus. The clear plastic cap is installed on the tip of the scope, and the holes are oriented such that they are placed next to the suction channel.
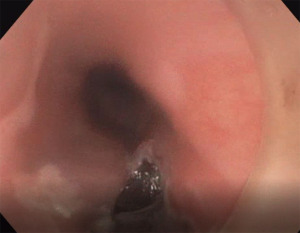
The site of mucosotomy is selected 12 to 15 cm from the incisors. The mucosotomy is placed more proximally in patients with type III achalasia so a longer myotomy can be performed. A 25-G Carr-Locke injection needle (US Endoscopy, Mentor, OH, USA) is used to inject 3 mL of a mixture of normal saline and indigo carmine dye into the submucosal space to raise a bleb at the selected site posteriorly at 6-o-clock position. Subsequently, a HybridKnife I-type (Erbe, Tuebingen, Germany) is used to make a linear mucosotomy (Figure 1). The tip of the gastroscope with the plastic cap is pushed into the submucosal tunnel.
Step 2: creation of the submucosal tunnel
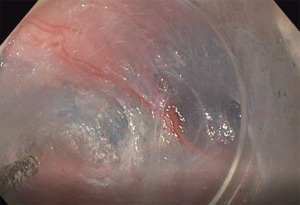
A diluted solution is injected to raise the areolar submucosal fibers off the muscular layer. The HybridKnife is used in cutting mode to cut the fibers and expose the muscular layer (Figure 2). It is crucial to make the tunnel uniformly wide. The tunnel is extended caudally to 2–3 cm distal to the EGJ. The space gets tighter past the EGJ, and a partial myotomy may be required to pass the gastroscope distal to EGJ.
Step 3: myotomy
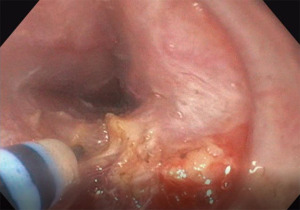
The myotomy is started 3 cm caudal to the mucosotomy site. We usually perform the myotomy at the 5-o-clock position and attempt to cut only the circular muscle layer, sparing the longitudinal layer (Figure 3). However, a full thickness myotomy has not been shown to have negative consequences and yields similar outcomes. The myotomy is extended 2–3 cm distal to the EGJ, and the clasp and sling gastric fibers are also transected. Coagulation is used for larger vessels.
After conclusion of the myotomy, 5 mL of the diluted solution is injected, which allows visualization of a greyish discoloration of the mucosa from the lumen of stomach on retroflexion and confirms that the myotomy extends beyond the EGJ.
Achieving a complete myotomy is the goal of POEM. We use the following tricks to identify the EGJ and ensure complete myotomy:
- Endoscopically measuring the distance of the EGJ from the incisors and making sure we cover this distance and pass the endoscope beyond the EGJ in the submucosal tunnel;
- Injecting dye in the submucosal space and confirming endoscopically that we are beyond the EGJ;
- The EGJ is usually evident as a very tight space during the endoscopic procedure, which opens up once we transverse the EGJ. Attention to the resistance present when performing submucosal tunneling and the myotomy is, therefore, helpful in identifying the EGJ;
- When we encounter the “spermal vessels” in the gastric cardia, it helps us to confirm that the myotomy was carried out beyond the EGJ.
Step 4: closure of the mucosotomy
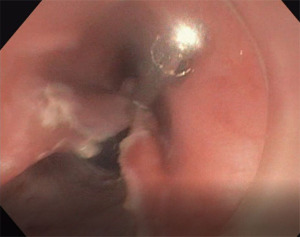
The mucosotomy site is closed using 4 or 5 Resolution Clips (Boston Scientific, Marlborough, MA, USA) (Figure 4).
We follow these patients in the postoperative period with barium swallow studies and a quality-of-life questionnaire. We document their Eckerdt score 2 weeks, 6 months, 1 year, 2 years, 5 years and 10 years after the procedure to quantitate the resolution and reappearance of achalasia symptoms. We may perform redo POEM if needed. If a patient has an Eckerdt score >3, however, we recommend RHM.
Variations in technique
The main variations in the POEM technique between surgeons and institutions are anterior vs. posterior myotomy, length and depth of myotomy, and closure methods. Anterior and posterior approaches have been shown to have similar efficacy, with the anterior approach initially showing approximately 85% efficacy in some studies (37). Khashab and colleagues randomized 150 patients to receive POEM with either an anterior or posterior approach and ultimately showed that posterior myotomy was not inferior (42). Technical success was not statistically different between the approaches (97.3% anterior; 100% posterior; P=0.23). Abnormal esophageal acid exposure (49% vs. 42%), rate of adverse events (11% vs. 9%) clinical success based on Eckardt questionnaire score <3 (90% vs. 89%), and GERD requiring proton pump inhibitor use (19% vs. 20%) were also not significantly different 1 year after POEM. Additionally, post-procedural pain and length of hospital stay (2 days) were similar with each approach. Two patients in each group had inadvertent mucosotomies, and closure was found to be easier in patients assigned to the posterior approach, with fewer clips needed. A higher success rate was also observed with the posterior approach in patients with prior achalasia treatment history, such as a LHM or a previous anterior POEM attempt (42,43).
Two smaller, single-center randomized trials also compared anterior approaches with posterior approaches. Ramchandani and colleagues found similar procedure times, postoperative Eckardt scores, and rates of esophagitis. However, they found more inadvertent mucosotomies in the patients who underwent myotomy with the anterior approach (20% vs. 3.3%) and increased abnormal DeMeester scores in the patients who underwent myotomy with the posterior approach (37% vs. 16%) (44). Tan and colleagues observed similar clinical success at mean follow up of 15.5 months and no difference in postoperative manometry, adverse events, and abnormal esophageal acid exposure between the two approaches (45). Overall, the anterior approach theoretically preserves the oblique muscle fibers of the LES decreasing the risk of reflux as compared with the posterior approach, which has decreased bleeding risk due to avoidance of the anterior submucosal space that contains arterial branches from the left gastric artery (42). Tanaka and colleagues described a unique alteration of the posterior approach in which they expose the two penetrating vessels at the boundary of the circular and oblique muscles. Preserving the oblique muscle was shown to decrease the rate of GERD as detected by acid exposure time during pH monitoring (31.3% vs. 58.1%) (46).
Outcomes of POEM
Overall, the complication rate after POEM is low; complications in 3.2% of patients were reported by Inoue and colleagues and on the high end, complications were observed in 7.5% of patients in an international, multicenter study by Haito-Chavez and colleagues (47). Reported complications after POEM include mucosal perforation, bleeding, pneumothorax, pneumoperitoneum, pneumomediastinum, subcutaneous emphysema, pleural effusion, pneumonia, GERD, and esophagitis. The majority of these complications can be managed conservatively (8,47,48). Complications secondary to insufflation are seen in 7.6% to 55.5% of patients (39,49,50). Longer procedure time and full thickness myotomy increase the likelihood of these complications associated with barotrauma (8,38). Shiwaku and colleagues analyzed 1,346 patients who underwent POEM from 2008 to 2015 and also noted low rate of adverse events (3.7%). All the adverse events that they observed resolved with conservative treatment, and POEM relieved achalasia with a response rate of 94.7% at 1 year postoperatively (51).
GERD is the most common complication after POEM, but there are conflicting data regarding the incidence of increased acid exposure, GERD symptoms, and esophagitis after POEM (8). GERD after POEM was reported in 20% to 46% of patients in an analysis by the POEM White Paper Committee in 2014 (8,52). A meta-analysis by Repici and colleagues predicted a GERD rate after POEM of 19.0% in a pooled rates analysis and higher rates of abnormal pH studies after POEM (53). A meta-analysis by Schlottman and colleagues found that POEM was associated with a higher likelihood of abnormal esophageal acid exposure [odds ratio (OR) =4.3], erosive esophagitis (OR =9.31) and GERD symptoms (OR =1.69) (36). Inoue and colleagues analyzed 500 consecutive achalasia patients treated with POEM and noted GERD in 21.3% of patients postoperatively (48). Sanaka and colleagues compared patients undergoing POEM with patients undergoing LHM with Dor fundoplication (43). Esophageal acid exposure, confirmed by pH study 2 months postoperatively, was higher after POEM as compared with LHM (abnormal DeMeester score 54.8% vs. 17.4%; P=0.005). However, there was no significant difference in GERD symptoms (28%), basal LES pressure, or LES relaxation pressure between the techniques, which may be secondary to preserving anatomical barriers, such as suspensory ligaments and the angle of His, when performing POEM (43). The discordant findings between abnormal acid exposure and GERD symptoms might be also secondary to denervation of the esophagus after achalasia. There were no differences noted based on achalasia subtype.
Shiwaku and colleagues and others have reported lower rates of GERD and esophagitis after POEM and poor correlation between GERD symptoms and pH study findings (37,51,54-58). Kumbhari and colleagues found an abnormal DeMeester score in 57.8% of patients and erosive esophagitis in 23.2%. Interestingly, 60% of patients had no GERD symptoms after POEM despite abnormal DeMeester scores (59,60).
Due to higher rates of esophageal acid exposure after POEM, experts recommend counseling patients preoperatively on potential long-term effects of POEM despite variability in the reported rates of GERD symptoms. All patients with an abnormal pH study should be placed on long-term proton pump inhibitor therapy (43,61).
In randomized, multicenter, clinical trials, POEM resulted in better mid-term outcomes than pneumatic dilation and similar outcomes as LHM (62,63). At the end of a 2-year follow-up period, 63 patients randomized to undergo POEM had a higher incidence of treatment success (92%) than 63 patients randomized to undergo pneumatic dilation (54%). Treatment success in the study was defined as Eckardt score ≤3 and the absence of severe complications or the need for retreatment. More patients who underwent POEM had GERD 2 years after the procedure as compared with those who underwent dilation, however (62). When outcomes after POEM were compared with outcomes after LHM in a randomized trial with 112 patients who underwent POEM and 109 patients who underwent LHM, clinical success was seen in 83% of patients after POEM and 82% of patients after LHM, proving non-inferiority of POEM as compared with LHM. Again, more GERD was observed after POEM; 44% of patients had reflux esophagitis 2 years after POEM vs. 29% with reflux esophagitis after LHM (63).
Additionally, several systematic reviews have compared POEM with LHM and found similar or slightly better outcomes with POEM, and that POEM is significantly beneficial in patients with type III achalasia due to ability to perform a longer myotomy (36,64,65). The International Per Oral Endoscopic Myotomy Survey (IPOEMS) highlighted experience from 16 centers from their adoption of the technique through early 2012. They observed decreased Eckardt scores after POEM in >90% of patients 9 months after surgery (66). Additionally, Inoue’s first 500 procedures showed >90% efficacy with Eckardt score <3 and decreased LES pressure 3 years after myotomy, which was confirmed by Crespin and colleagues and by other studies (8,40,48,67). A large meta-analysis in 2016 by Akintoye and colleagues reported up to 98% efficacy of POEM 12 months after surgery (49).
In conclusion, there are several treatment options available for achalasia including myotomy, and POEM is a novel technique using endoscopic approach for myotomy. POEM has become the preferred approach for treating type III achalasia. Postoperative GERD remains the main shortcoming of POEM, because the myotomy is not complimented with anti-reflux partial fundoplication, which is routinely performed during RHM or LHM. Therefore, the decision to pursue POEM as a treatment option is ultimately reliant on the surgeon’s preferences and experience and the patient’s physiology, co-morbidities, and achalasia subtype.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Abbas E. Abbas and Roman V. Petrov) for the series “New Technologies in Esophageal Surgery and Endoscopy” published in Annals of Esophagus. The article has undergone external peer review.
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-47/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-47/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadowski DC, Ackah F, Jiang B, et al. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil 2010;22:e256-61. [Crossref] [PubMed]
- Patel DA, Lappas BM, Vaezi MF. An Overview of Achalasia and Its Subtypes. Gastroenterol Hepatol (N Y) 2017;13:411-21. [PubMed]
- Harvey PR, Thomas T, Chandan JS, et al. Incidence, morbidity and mortality of patients with achalasia in England: findings from a study of nationwide hospital and primary care data. Gut 2019;68:790-5. [Crossref] [PubMed]
- Wadhwa V, Thota PN, Parikh MP, et al. Changing Trends in Age, Gender, Racial Distribution and Inpatient Burden of Achalasia. Gastroenterology Res 2017;10:70-7. [Crossref] [PubMed]
- Holloway RH, Dodds WJ, Helm JF, et al. Integrity of cholinergic innervation to the lower esophageal sphincter in achalasia. Gastroenterology 1986;90:924-9. [Crossref] [PubMed]
- Sodikoff JB, Lo AA, Shetuni BB, et al. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil 2016;28:139-45. [Crossref] [PubMed]
- Pandolfino JE, Gawron AJ. Achalasia: a systematic review. JAMA 2015;313:1841-52. [Crossref] [PubMed]
- Cappell MS, Stavropoulos SN, Friedel D. Updated Systematic Review of Achalasia, with a Focus on POEM Therapy. Dig Dis Sci 2020;65:38-65. [Crossref] [PubMed]
- Gennaro N, Portale G, Gallo C, et al. Esophageal achalasia in the Veneto region: epidemiology and treatment. Epidemiology and treatment of achalasia. J Gastrointest Surg 2011;15:423-8. [Crossref] [PubMed]
- Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol 1998;27:21-35. [Crossref] [PubMed]
- Fisichella PM, Raz D, Palazzo F, et al. Clinical, radiological, and manometric profile in 145 patients with untreated achalasia. World J Surg 2008;32:1974-9. [Crossref] [PubMed]
- Howard PJ, Maher L, Pryde A, et al. Five year prospective study of the incidence, clinical features, and diagnosis of achalasia in Edinburgh. Gut 1992;33:1011-5. [Crossref] [PubMed]
- Zifodya JS, Kim HP, Silver HJ, et al. Tu1199 nutritional status of patients with untreated achalasia. Gastroenterology 2015;148:S-819-20. [Crossref]
- Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0©. Neurogastroenterol Motil 2021;33:e14058. [Crossref] [PubMed]
- Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 2008;135:1526-33. [Crossref] [PubMed]
- Torresan F, Ioannou A, Azzaroli F, et al. Treatment of achalasia in the era of high-resolution manometry. Ann Gastroenterol 2015;28:301-8. [PubMed]
- Vaezi MF, Pandolfino JE, Yadlapati RH, et al. ACG Clinical Guidelines: Diagnosis and Management of Achalasia. Am J Gastroenterol 2020;115:1393-411. [Crossref] [PubMed]
- Patel DA, Naik R, Slaughter JC, et al. Weight loss in achalasia is determined by its phenotype. Dis Esophagus 2018; [Crossref] [PubMed]
- Rohof WO, Lei A, Boeckxstaens GE. Esophageal stasis on a timed barium esophagogram predicts recurrent symptoms in patients with long-standing achalasia. Am J Gastroenterol 2013;108:49-55. [Crossref] [PubMed]
- Schaheen LW, Sanchez MV, Luketich JD. Peroral Endoscopic Myotomy for Achalasia. Thorac Surg Clin 2018;28:499-506. [Crossref] [PubMed]
- Vela MF, Richter JE, Khandwala F, et al. The long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol 2006;4:580-7. [Crossref] [PubMed]
- Farhoomand K, Connor JT, Richter JE, et al. Predictors of outcome of pneumatic dilation in achalasia. Clin Gastroenterol Hepatol 2004;2:389-94. [Crossref] [PubMed]
- Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 2013;144:718-25; quiz e13-4. [Crossref] [PubMed]
- Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 2009;249:45-57. [Crossref] [PubMed]
- Richards WO, Torquati A, Holzman MD, et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg 2004;240:405-12; discussion 412-5. [Crossref] [PubMed]
- Schlottmann F, Andolfi C, Kavitt RT, et al. Multidisciplinary Approach to Esophageal Achalasia: A Single Center Experience. J Laparoendosc Adv Surg Tech A 2017;27:358-62. [Crossref] [PubMed]
- Ali AB, Khan NA, Nguyen DT, et al. Robotic and per-oral endoscopic myotomy have fewer technical complications compared to laparoscopic Heller myotomy. Surg Endosc 2020;34:3191-6. [Crossref] [PubMed]
- Gulati S, Emmanuel A, Inoue H, et al. Peroral endoscopic myotomy: a literature review and the first UK case series. Clin Med (Lond) 2017;17:22-8. [Crossref] [PubMed]
- Inoue H, Tianle KM, Ikeda H, et al. Peroral endoscopic myotomy for esophageal achalasia: technique, indication, and outcomes. Thorac Surg Clin 2011;21:519-25. [Crossref] [PubMed]
- Ortega JA, Madureri V, Perez L. Endoscopic myotomy in the treatment of achalasia. Gastrointest Endosc 1980;26:8-10. [Crossref] [PubMed]
- Pasricha PJ, Hawari R, Ahmed I, et al. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy 2007;39:761-4. [Crossref] [PubMed]
- Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc 2007;65:688-94. [Crossref] [PubMed]
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- Awaiz A, Yunus RM, Khan S, et al. Systematic Review and Meta-Analysis of Perioperative Outcomes of Peroral Endoscopic Myotomy (POEM) and Laparoscopic Heller Myotomy (LHM) for Achalasia. Surg Laparosc Endosc Percutan Tech 2017;27:123-31. [Crossref] [PubMed]
- Peng L, Tian S, Du C, et al. Outcome of Peroral Endoscopic Myotomy (POEM) for Treating Achalasia Compared With Laparoscopic Heller Myotomy (LHM). Surg Laparosc Endosc Percutan Tech 2017;27:60-4. [Crossref] [PubMed]
- Schlottmann F, Luckett DJ, Fine J, et al. Laparoscopic Heller Myotomy Versus Peroral Endoscopic Myotomy (POEM) for Achalasia: A Systematic Review and Meta-analysis. Ann Surg 2018;267:451-60. [Crossref] [PubMed]
- Von Renteln D, Fuchs KH, Fockens P, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology 2013;145:309-11.e1-3.
- Wang X, Tan Y, Zhang J, et al. Risk factors for gas-related complications of peroral endoscopic myotomy in achalasia. Neth J Med 2015;73:76-81. [Crossref] [PubMed]
- Bang YS, Park C. Anesthetic Consideration for Peroral Endoscopic Myotomy. Clin Endosc 2019;52:549-55. [Crossref] [PubMed]
- Bechara R, Onimaru M, Ikeda H, et al. Per-oral endoscopic myotomy, 1000 cases later: pearls, pitfalls, and practical considerations. Gastrointest Endosc 2016;84:330-8. [Crossref] [PubMed]
- Löser B, Recio Ariza O, Saugel B, et al. Anesthesia for Patients Undergoing Peroral Endoscopic Myotomy Procedures: A Review of the Literature. Anesth Analg 2020;130:1331-40. [Crossref] [PubMed]
- Khashab MA, Sanaei O, Rivory J, et al. Peroral endoscopic myotomy: anterior versus posterior approach: a randomized single-blinded clinical trial. Gastrointest Endosc 2020;91:288-297.e7. [Crossref] [PubMed]
- Sanaka MR, Thota PN, Parikh MP, et al. Peroral endoscopic myotomy leads to higher rates of abnormal esophageal acid exposure than laparoscopic Heller myotomy in achalasia. Surg Endosc 2019;33:2284-92. [Crossref] [PubMed]
- Ramchandani M, Nabi Z, Reddy DN, et al. Outcomes of anterior myotomy versus posterior myotomy during POEM: a randomized pilot study. Endosc Int Open 2018;6:E190-8. [Crossref] [PubMed]
- Tan Y, Lv L, Wang X, et al. Efficacy of anterior versus posterior per-oral endoscopic myotomy for treating achalasia: a randomized, prospective study. Gastrointest Endosc 2018;88:46-54. [Crossref] [PubMed]
- Tanaka S, Toyonaga T, Kawara F, et al. Novel per-oral endoscopic myotomy method preserving oblique muscle using two penetrating vessels as anatomic landmarks reduces postoperative gastroesophageal reflux. J Gastroenterol Hepatol 2019;34:2158-63. [Crossref] [PubMed]
- Haito-Chavez Y, Inoue H, Beard KW, et al. Comprehensive Analysis of Adverse Events Associated With Per Oral Endoscopic Myotomy in 1826 Patients: An International Multicenter Study. Am J Gastroenterol 2017;112:1267-76. [Crossref] [PubMed]
- Inoue H, Sato H, Ikeda H, et al. Per-Oral Endoscopic Myotomy: A Series of 500 Patients. J Am Coll Surg 2015;221:256-64. [Crossref] [PubMed]
- Akintoye E, Kumar N, Obaitan I, et al. Peroral endoscopic myotomy: a meta-analysis. Endoscopy 2016;48:1059-68. [Crossref] [PubMed]
- Ren Z, Zhong Y, Zhou P, et al. Perioperative management and treatment for complications during and after peroral endoscopic myotomy (POEM) for esophageal achalasia (EA) (data from 119 cases). Surg Endosc 2012;26:3267-72. [Crossref] [PubMed]
- Shiwaku H, Inoue H, Yamashita K, et al. Peroral endoscopic myotomy for esophageal achalasia: outcomes of the first over 100 patients with short-term follow-up. Surg Endosc 2016;30:4817-26. [Crossref] [PubMed]
- NOSCAR POEM White Paper Committee; Stavropoulos SN, Desilets DJ, et al. Per-oral endoscopic myotomy white paper summary. Gastrointest Endosc 2014;80:1-15.
- Repici A, Fuccio L, Maselli R, et al. GERD after per-oral endoscopic myotomy as compared with Heller's myotomy with fundoplication: a systematic review with meta-analysis. Gastrointest Endosc 2018;87:934-43.e18. [Crossref] [PubMed]
- Minami H, Inoue H, Haji A, et al. Per-oral endoscopic myotomy: emerging indications and evolving techniques. Dig Endosc 2015;27:175-81. [Crossref] [PubMed]
- Familiari P, Gigante G, Marchese M, et al. Peroral Endoscopic Myotomy for Esophageal Achalasia: Outcomes of the First 100 Patients With Short-term Follow-up. Ann Surg 2016;263:82-7. [Crossref] [PubMed]
- Swanstrom LL, Kurian A, Dunst CM, et al. Long-term outcomes of an endoscopic myotomy for achalasia: the POEM procedure. Ann Surg 2012;256:659-67. [Crossref] [PubMed]
- Familiari P, Greco S, Gigante G, et al. Gastroesophageal reflux disease after peroral endoscopic myotomy: Analysis of clinical, procedural and functional factors, associated with gastroesophageal reflux disease and esophagitis. Dig Endosc 2016;28:33-41. [Crossref] [PubMed]
- Jones EL, Meara MP, Schwartz JS, et al. Gastroesophageal reflux symptoms do not correlate with objective pH testing after peroral endoscopic myotomy. Surg Endosc 2016;30:947-52. [Crossref] [PubMed]
- Kumbhari V, Familiari P, Bjerregaard NC, et al. Gastroesophageal reflux after peroral endoscopic myotomy: a multicenter case-control study. Endoscopy 2017;49:634-42. [Crossref] [PubMed]
- Kumbhari V, Tieu AH, Onimaru M, et al. Peroral endoscopic myotomy (POEM) vs laparoscopic Heller myotomy (LHM) for the treatment of Type III achalasia in 75 patients: a multicenter comparative study. Endosc Int Open 2015;3:E195-201. [Crossref] [PubMed]
- Kahrilas PJ, Katzka D, Richter JE. Clinical Practice Update: The Use of Per-Oral Endoscopic Myotomy in Achalasia: Expert Review and Best Practice Advice From the AGA Institute. Gastroenterology 2017;153:1205-11. [Crossref] [PubMed]
- Ponds FA, Fockens P, Lei A, et al. Effect of Peroral Endoscopic Myotomy vs Pneumatic Dilation on Symptom Severity and Treatment Outcomes Among Treatment-Naive Patients With Achalasia: A Randomized Clinical Trial. JAMA 2019;322:134-44. [Crossref] [PubMed]
- Werner YB, Hakanson B, Martinek J, et al. Endoscopic or Surgical Myotomy in Patients with Idiopathic Achalasia. N Engl J Med 2019;381:2219-29. [Crossref] [PubMed]
- Andolfi C, Fisichella PM. Meta-analysis of clinical outcome after treatment for achalasia based on manometric subtypes. Br J Surg 2019;106:332-41. [Crossref] [PubMed]
- Zhang Y, Wang H, Chen X, et al. Per-Oral Endoscopic Myotomy Versus Laparoscopic Heller Myotomy for Achalasia: A Meta-Analysis of Nonrandomized Comparative Studies. Medicine (Baltimore) 2016;95:e2736. [Crossref] [PubMed]
- Stavropoulos SN, Modayil RJ, Friedel D, et al. The International Per Oral Endoscopic Myotomy Survey (IPOEMS): a snapshot of the global POEM experience. Surg Endosc 2013;27:3322-38. [Crossref] [PubMed]
- Crespin OM, Liu LWC, Parmar A, et al. Safety and efficacy of POEM for treatment of achalasia: a systematic review of the literature. Surg Endosc 2017;31:2187-201. [Crossref] [PubMed]
Cite this article as: Musgrove K, Spear C, Abbas FA, Abbas G. Per-oral endoscopic myotomy (POEM) for achalasia: techniques and outcomes. Ann Esophagus 2023;6:20.