
Robotic-assisted repair of post-esophagectomy hiatal hernia: case report and review of technique
Introduction
Hiatal herniation following esophagectomy is an uncommon but potentially life-threatening complication (1). These hernias occur months to years after esophagectomy and are found in up to 15% of patients on routine follow-up imaging (1,2). While post-esophagectomy hiatal hernias can be diagnosed by plain-film x-ray, retrosternal air can be difficult to distinguish from normal postoperative findings following esophagectomy. Therefore, chest and upper abdominal CT scanning is the preferred diagnostic imaging study (3).
Post-esophagectomy hiatal hernias presents in two distinct fashions: para-conduit hernias and redundant hernias (Figure 1) (4). The two may present similarly with symptoms of obstruction but are distinct anatomically. Para-conduit herniation occurs when an intrabdominal conduit herniates alongside the gastric conduit. Often such para-conduit hernias will involve small and/or large bowel, however, any intra-abdominal contents can be involved. In contrast, a redundant conduit occurs when a dilated gastric conduit herniates into the mediastinum causing dysmotility and obstruction (4). Occasionally, a patient can have a simultaneous para-conduit hernia and redundant conduit, which can both contribute to mechanical obstruction (4).
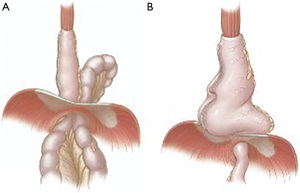
The etiology of para-conduit hiatal hernias is believed to be secondary to recurrent gastric distention and decompression which eventually widens the hiatus and allows for herniation of intra-abdominal contents alongside the gastric conduit (5,6). Division of the crus during the initial esophagectomy may also increase the risk of para-conduit herniation (5,7,8). Another suspected but poorly studied hypothesis is that incomplete division of the gastrocolic ligament may effectively drag the colon up into the mediastinum with the conduit.
Redundant conduit herniation may result from excess conduit left above the diaphragm during initial esophagectomy, mechanical obstruction from delayed emptying, twisting of the conduit, and/or dysmotility within the conduit (4). Incomplete pull-up of the gastric conduit or failure to excise sufficient length of the distal conduit tip during the index operation creates excess length and redundancy in the mediastinum. Avoiding these technical errors during esophagectomy can reduce the incidence of redundant conduit complications.
Interestingly, post-esophagectomy hiatal hernias are more likely to occur after minimally invasive esophagectomy (MIE) as compared to an open approach (4.5% versus 2.6%) (7,9). This is believed to be secondary to reduced peritoneal adhesion formation at the hiatus following MIE (2,5,6). Amongst open operations, transhiatal esophagectomy is associated with the highest incidence of para-conduit hernias (2). Other risk factors predictive for post-esophagectomy hiatal herniation is the presence of a hiatal hernia prior to esophagectomy and increased body mass index (6). Preventative measures such as prophylactic cruroplasty at the time of esophagectomy or securing the conduit to the diaphragm (gastropexy) have not been shown to reduce incidence of post-esophagectomy hiatal herniation (1,5).
Patients with post-esophagectomy hiatal hernias can present with chest pain, abdominal pain, dysphagia, constipation, nausea, decreased exercise tolerance, and/or shortness of breath (4). Patients may also present acutely with bowel or conduit obstruction, perforation, and necrosis. Over ninety percent of the time, colon is the abdominal organ that herniates alongside a conduit (4). Small bowel and omental para-conduit herniation are also commonly seen. The mean time to presentation of post-esophagectomy hiatal hernias is 2.5 years after esophagectomy (4). However, it is important to note this complication can present in the immediate post-operative period or in a delayed fashion many years post-esophagectomy (4).
Controversy still exists around timing and methodology of repair. Most authors perform repair in symptomatic patients fit for surgery (7). Elective repair for asymptomatic hernias should also be considered as urgent para-conduit hernia repair is associated with mortality rates as high as 25% (7). Preemptive repair may avoid serious complications such as visceral perforation and fecopneumothorax (10). Open and laparoscopic abdominal repair are most described for managing post-esophagectomy hiatal hernias. However, the robot-assisted laparoscopic approach has several key advantages and is an excellent minimally invasive alternative. We present the following article in accordance to the CARE reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-49/rc).
Case presentation
The accompanying video describes robotic repair of a redundant conduit and para-conduit herniation (see Video 1). All procedures were in accordance with the ethical standards of our institution’s research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. All operations were performed at a high-volume robotic foregut center which is a certified mentor site and center of excellence for robotic thoracic and foregut surgery. We utilized the da Vinci XI robotic surgical system (Intuitive Surgical, Sunnyvale, CA, USA) with a four-arm approach.
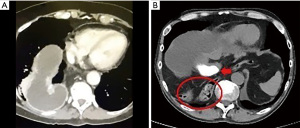
The first case is of a 72-year-old woman who presented with new onset dysphagia two years after her minimally invasive Ivor Lewis esophagectomy. An endoscopy showed a dilated conduit and difficulty traversing the conduit. A CT scan demonstrated a redundant conduit (Figure 2A). The second case is that of a 40-year-old man who presented 2 months after robotic-assisted minimally invasive Ivor Lewis esophagectomy with recurrent aspiration pneumonia. He was found on imaging to have a para-conduit herniation of the colon (Figure 2B).
Discussion
Preoperative planning is critical. We recommend CT scan of the chest and abdomen with intravenous contrast. We also obtain three-dimensional reconstructions to fully understand the anatomy of the hernia and the position of the gastric conduit blood supply. We typically use an optical trocar for entry, but access to the abdomen can be done with any technique favored by the surgeon. Whichever method is chosen, care must be exercised during this reoperative peritoneal entry. The accompanying video depicts port placement. If the index operation was a robot-assisted MIE, we will typically utilize the same ports. We always surveil the abdomen to exclude metastasis.
The tenets of repair are circumferential dissection of the hiatus, preservation of the right gastroepiploic artery, reduction of all intraabdominal contents, and coverage of the hiatal defect with a tension free repair. We preferentially attempt primary repair, if possible, but occasionally mesh is required to ensure a tension-free repair. Although rare, permanent mesh has been associated with erosion into the conduit and gastroepiploic artery. As expected, absorbable mesh has high recurrence rates and should not be used as a bridge. For these reasons, if mesh is required, we prefer not to place mesh directly on the gastric conduit; rather, we perform a diaphragmatic relaxing incision lateral to the crus and cover the incision with non-absorbable mesh reinforcement.
When reducing a para-conduit hernia, it is important to gently reduce the herniated contents, as dilated and unprepped colon is most often involved. All mediastinal attachments are taken down which is facilitated by the robotic platform that allows for dissection high into the chest with superior visualization. As noted earlier, it is common for the gastrocolic ligament in the initial operation to be inadequately divided, and this can serve as a lead point for para-conduit herniation. We therefore ensure complete division of the gastrocolic ligament at the time of post-esophagectomy hiatal hernia repair.
The primary working instruments for this operation are the cadiere forceps and the long bipolar grasper with bipolar energy. Bipolar energy has minimal thermal spread, which is critical when operating near the gastroepiploic artery. We also utilize intraoperative infrared imaging with the administration of intravenous indocyanine green (ICG) dye to delineate the blood supply to the conduit. This is particularly helpful with dense adhesions which may harbor blood supply to the conduit. Notably infrared imaging is available for non-robotic video towers; however, this feature is typically not a standard feature and may employ add-on capital expenditures. The robotic stapler can also be used for thick adhesions near the conduit in the rare circumstance of needing to resect a portion of the dilated, redundant gastric conduit (effectively performing a wedge gastroplasty).
Once intrabdominal contents and/or the redundant conduit are reduced, we aim for circumferential gastropexy of the conduit to the crura with silk suture. One can also consider a pylorus draining procedure particularly if it was not performed (or incompletely performed) at the index operation. Delayed gastric emptying can contribute to redundant conduit or para-conduit hernia formation by dilating the conduit and hiatus over time.
Patients are typically discharged home 1–2 days following hiatal hernia repair. We obtain a barium esophagram prior to starting oral diet to ensure the absence of leak or obstruction. Patients are usually discharged on a liquid diet, which is advanced as an outpatient. These patients often have a history of malignancy and so are screened at least yearly with CT imaging.
As therapies evolve and patients live longer following esophagectomy, we are more likely to encounter delayed post-esophagectomy complications such as redundant gastric conduits and para-conduit herniations. If symptomatic, patients should undergo repair. The robot-assisted approach is a safe and effective minimally-invasive option for this complex problem.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-49/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-49/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-49/coif). ELS is a consultant for Intuitive Surgical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were in accordance with the ethical standards of our institution’s research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gooszen JAH, Slaman AE, van Dieren S, et al. Incidence and Treatment of Symptomatic Diaphragmatic Hernia After Esophagectomy for Cancer. Ann Thorac Surg 2018;106:199-206. [Crossref] [PubMed]
- Ganeshan DM, Correa AM, Bhosale P, et al. Diaphragmatic hernia after esophagectomy in 440 patients with long-term follow-up. Ann Thorac Surg 2013;96:1138-45. [Crossref] [PubMed]
- Kaplan JA, Schecter S, Lin MY, et al. Morbidity and Mortality Associated With Elective or Emergency Paraesophageal Hernia Repair. JAMA Surg 2015;150:1094-6. [Crossref] [PubMed]
- Kent MS, Luketich JD, Tsai W, et al. Revisional surgery after esophagectomy: an analysis of 43 patients. Ann Thorac Surg 2008;86:975-83; discussion 967-74. [Crossref] [PubMed]
- Price TN, Allen MS, Nichols FC 3rd, et al. Hiatal hernia after esophagectomy: analysis of 2,182 esophagectomies from a single institution. Ann Thorac Surg 2011;92:2041-5. [Crossref] [PubMed]
- Vallböhmer D, Hölscher AH, Herbold T, et al. Diaphragmatic hernia after conventional or laparoscopic-assisted transthoracic esophagectomy. Ann Thorac Surg 2007;84:1847-52. [Crossref] [PubMed]
- Oor JE, Wiezer MJ, Hazebroek EJ. Hiatal Hernia After Open versus Minimally Invasive Esophagectomy: A Systematic Review and Meta-analysis. Ann Surg Oncol 2016;23:2690-8. [Crossref] [PubMed]
- van Sandick JW, Knegjens JL, van Lanschot JJ, et al. Diaphragmatic herniation following oesophagectomy. Br J Surg 1999;86:109-12. [Crossref] [PubMed]
- Benjamin G, Ashfaq A, Chang YH, et al. Diaphragmatic hernia post-minimally invasive esophagectomy: a discussion and review of literature. Hernia 2015;19:635-43. [Crossref] [PubMed]
- Kim KW, Lee JI, Kim JS, et al. Fecopneumothoax: a rare case of delayed colon diaphragmatic herniation following esophagectomy. Indian J Surg 2015;77:117-9. [Crossref] [PubMed]
Cite this article as: Watkins AA, Stock CT, Servais EL. Robotic-assisted repair of post-esophagectomy hiatal hernia: case report and review of technique. Ann Esophagus 2022;5:33.


