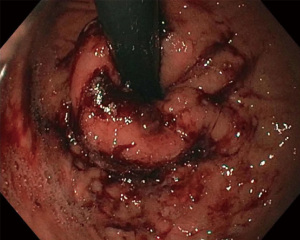
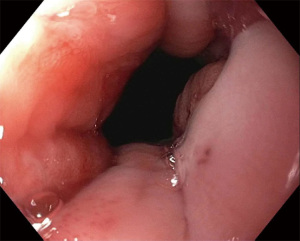
A narrative review of minimally invasive procedures for gastroesophageal reflux disease: endoscopic antireflux procedures (TIF and Stretta)
Introduction
Gastroesophageal reflux disease (GERD) is defined as reflux of gastric contents into the esophagus or oropharynx, and can range from asymptomatic mild disease to severe esophagitis and metaplasia (Barrett’s esophagus). Its prevalence ranges from 2.5% to 25% in Western countries, and is associated with obesity and diet (1,2). Initial management with mild symptoms involves lifestyle modifications, including weight loss, elevating the head of the bed at night and avoiding meals less than two hours prior to bedtime, and avoidance of dietary triggers (caffeine, foods high in fat content, peppermint, carbonated beverages) (3). For severe symptoms or erosive esophagitis, proton pump inhibitors (PPI) are recommended in addition to lifestyle modification (4).
When patients have symptoms despite maximal medical therapy, patients do not wish to take lifelong medication, or there are complications of GERD present (stricture, severe esophagitis, etc.) intervention is indicated (5). Several endoscopic and surgical techniques exist. Surgical fundoplication to augment the pressure of the lower esophageal sphincter (LES) is the gold standard intervention. Laparoscopic fundoplication is associated with durable long term symptom relief, but is also associated with morbidity such as dysphagia, bloating, inability to belch, and flatulence, especially in complete fundoplication versus partial (6). In addition, surgical fundoplication is associated with an up to 15% failure rate in some studies, resulting in recurrent symptoms (7). Patients and physicians are aware of these potential undesired outcomes and therefore are sometimes fearful of surgical intervention.
Over the past two decades, endoscopic therapies to improve GERD have emerged, which work by augmenting the barrier between the esophagus and the stomach (8). Many procedures have been developed over this time including injectables and suturing devices, but have not withstood the test of time. Currently 2 procedures prevail in the United States for the endoscopic management of GERD. The two techniques that will be discussed in this review are the transoral incisionless fundoplication (TIF), and the radiofrequency energy delivery to the LES (Stretta) procedures. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-15/rc).
TIF
Introduction
TIF is a completely endoscopic procedure that creates a two to four centimeter long partial fundoplication that is 270°. Indications for TIF include patients suffering from GERD/heartburn despite maximal medical therapy, patients with small (<2 cm) or no hiatal hernia, LA grade B or lower esophagitis, and patients that do not desire long-term medical treatment or surgical treatment for GERD. Contraindications for TIF include hiatal hernia larger than 2 cm, Hill grade 3 or higher, prior antireflux surgery or other esophageal surgery and pathology, and BMI over 35 (9). Barrett’s esophagus, or high grade esophagitis, is also a contraindication, as maximal reflux control (i.e., laparoscopic fundoplication) is recommended to reduce progression of dysplasia (10). In addition those with such severe disease often also have an associated hiatal hernia. Thus, laparoscopic fundoplication is preferred in patients with severe disease or metaplasia/dysplasia, as well as those with large hiatal hernias.
Evaluation
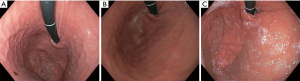
Patients are worked up for TIF similarly to standard surgical fundoplication. Patients should undergo a minimum of 4 studies for a complete esophageal and esophagogastric junction evaluation. All should have an endoscopy, esophagram contrast study, esophageal manometry and pH-imetry for complete evaluation. The most critical of these studies is the endoscopic evaluation. The physician performing the TIF should do an endoscopic evaluation prior to the TIF to ensure the diaphragmatic opening is not too large (hill 3 or greater). The axial or vertical excursion of the stomach should be evaluated on initial approach to the GEJ as well as after the stomach is distended to not miss a sliding hiatal hernia. Additionally, appropriate attention should be given to the retroflexed distention of the fundus to appropriately evaluate the Hill grade. We suggest at least 60 seconds. The presence of a hill 3 valve indicates a large crural opening. In general reflux is attributed to dysfunction of the LES and the poor approximation of the crural fibers. If the crural opening is too large, then the TIF will be minimally successful as the hiatal hernia component of the reflux would not be addressed. Those patients should have a hiatal hernia repair in addition to the fundoplication (Figure 1). In addition, upright refluxers seem to do better than supine refluxers after the TIF. This should be assessed on the pH evaluation (11). The TIF reduces the distensibility of the LES and creates a true valve reducing TLESR’s and reflux.
Technique
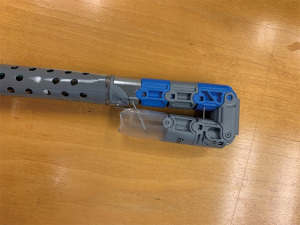
The TIF procedure is performed under general anesthesia in the outpatient setting or endoscopy lab. An endoscopic suturing device called the EsophyX (EndoGastric Solutions, Redmond, WA, USA) is used in conjunction with a gastroscope (Figure 2). A baseline endoscopy is performed prior to device insertion. The gastroscope is inserted into the device and secured in place, The device and gastroscope are inserted together into the esophagus and then the stomach, using high flow CO2 for insufflation.
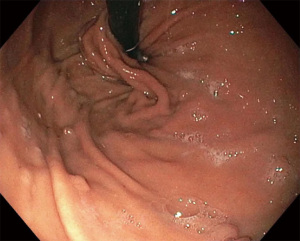
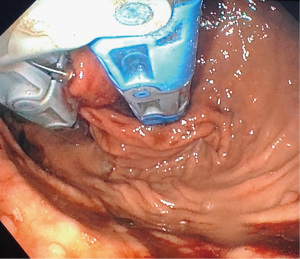
There are usually two operators present: one to use the EsophyX device and another to manipulate the endoscope (10). A standard endoscope can be used. First, an endoscopy is performed and the z line is measured along with ensuring there is not a prohibitive hiatal hernia or food in the stomach (food will block the suction ports of the device and prohibit it from working properly). The scope and the device are then inserted together. Of note, the distention of the esophagus with the device helps avoid narrowing of the GE junction at the time of the procedure. With the endoscope placed through the device and retroflexed, the device is further inserted into the stomach under vision, and the device is retroflexed to the gastroesophageal junction and turned to the 11 o’clock position (Figure 3). A helical retractor is then deployed from the device and engaged at the Z line to invaginate it into the stomach while the stomach is desufflated, creating the channel for the fundoplication. The device then closes over this tissue, the stomach is reinsufflated, and H-type polypropylene fasteners are deployed full thickness (one side in the stomach and the other side in the esophagus), creating a serosa to serosa plication (Figure 4). Two to four fasteners are placed in the 11 o’clock position, by rotating back and forth to ensure adequate coverage at each position (Figure 5). The device is then opened, and the helical retractor is released and retracted, and the device is rotated to the opposite side and deployed again. This is repeated a third time centrally, in about the 6 o’clock position. The end result is a 2 to 4 centimeter long, >240° fundoplication (9-12). An endoscopy is then repeated to inspect the fundoplication, the stomach is desufflated, and the procedure is completed (Figure 6). In general 10 of the T-fasteners are placed, however more can be used if needed. The procedure is reproducible with a short learning curve for skilled endoscopists and generally takes under 1 hour to perform. The goal is to create a 3 cm serosa to serosa full thickness approximation that is approximately 270 degrees such that there is effacement of the GE junction at the base and the esophagus is almost pushed further below the diaphragm using the device during the procedure.
Outcomes
Compared to maximum strength medical therapy with PPI, TIF is associated with significant improvement in typical symptoms of GERD (12). In a prospective randomized trial of 63 patients randomized to TIF or maximum standard dose PPI, at 6 months TIF was associated with elimination of troublesome GERD symptoms in 97% of patients, compared to 50% of patients randomized to PPI (13). Another randomized prospective trial of 60 patients showed similar results at 6 months with significant improvement in GERD related quality of life in TIF patients compared to PPI alone, along with decreased acid exposure in the distal esophagus and higher rates of normalization of pH. However, at 12 months, although GERD related quality of life remained significantly higher in TIF patients, the objective parameters of distal esophageal acid exposure, pH normalization, and resumption of PPI usage had no significant differences (14). In a study looking at long term follow up in 41 patients post TIF, 36/41(87.8%) had either reduced the frequency of PPI intake by half, or stopped taking PPI entirely at 36 months, with significant improvement in GERD related quality of life. By 6 years, follow up was limited to 14 patients, and although not significant, 12/14 (85.7%) had stopped or halved the frequency of PPI usage. Associations significantly associated with improvement in PPI use and GERD related quality of life included hiatal hernia <2 cm, lack of ineffective esophageal motility, and >240° fundoplication with the use of additional fasteners (15,16). Meta-analyses have shown mixed results with regards to long term durability, but are confounded by the fact that a newer generation of the EsophyX device has since come into use (17). Major adverse events include perforation, bleeding, infection, pneumothorax, and mediastinal abscess, and occur at a rate of 1.5% to 5% (10-15). A very nice study studying distensibility of the LES noted a significant difference in transient relaxations of the LES but minimal gas bloat in these patients (12).
The transoral fundoplication is an excellent procedure to have in the armamentarium for those managing GERD. Particularly in patients with minimal hiatal hernia, it is extremely effective and fairly durable. We have several patients We follow, and even at 4 to 5 years they are happy with the results and remain of maintenance therapy for GERD. The TIF is also being considered in more complex situations. We have utllized the procedure after a Nissen fundoplication where the stomach has slipped slightly through the plication and the plication may have loosened. The TIF seems to pull the stomach back down below the plication and secure the stomach within the plication in a non-slip fashion. Anecdotally this relieves the cough that can be associated with that anatomic variation. Another place where TIF is being considered is for reflux after a peroral endoscopic myotomy. This is still very controversial and requires further evaluation.
The other situation worth discussing is performing a TIF instead of a surgical fundoplication at the time of surgical hiatal hernia repair. Some surgeons have opted to even combine this procedure with a surgical hiatal hernia repair. The patients perceive the plication portion of the surgery to have the unwanted outcomes, and the TIF is perceived and shown to have less bloating and dysphagia. There is a strong group of proponents for this approach. Although the TIF appears very similar to a Toupet fundoplication, the argument is that the TIF has far more points of fixation and therefore is likely to be more durable. Several feasibility studies have demonstrated good outcomes with this approach (16). In the author’s opinion, this is an option but does require some further investigation in a comparative trial. In summary the TIF procedure is safe and effective and has a reasonable learning curve for most endoscopists.
Stretta procedure
Introduction
The Stretta procedure (Mederi Therapeutics, Greenwich, CT, USA) received FDA approval in 2000, and is the most commonly performed endoscopic antireflux procedure worldwide (Figure 7) (18,19). The procedure introduces radiofrequency energy delivery into the muscularis propria of the LES and gastric cardia. The exact mechanisms by which this improves reflux symptoms is not completely clear, but early studies showed that the induced-inflammation results in collagen deposition that bulks the sphincter, thereby decreasing compliance, and additionally lowering incidences of transient LES relaxation (20). These transient inappropriate relaxations of the LES result from low gastric yield pressure which is an additional cause of GERD. The fibers causing the TLESR’s are far fewer than those preserving normal relaxation. Therefore ablation of these fibers does not affect the normal function of the LES. In addition, it is hypothesized that neurolysis leads to decreased sensitivity to esophageal acid exposure, reducing symptoms of GERD. This has not been demonstrated in any studies. In addition, neurolysis does not explain the objective decrease in acid exposure seen in several studies (20,21). This neurolysis is less likely to be the effect of the procedure.

Indications for the Stretta procedure include patients who have frequent symptoms as well as objective evidence of GERD despite maximal medical therapy but with some response to medication, a 2-cm or smaller hiatal hernia, normal esophageal motility, desire to avoid long term medical therapy, and poor candidates for surgery or those that wish to avoid surgery (22). Contraindications include a hiatal hernia >2 cm and high grade esophagitis or dysplasia. An absolute contraindication is the presence of Barrett’s as this obscures the LES landmark that is the basis of this procedure. Although anecdotal, the author notes less success in those with an LES pressure under 15 mmHg as the augmentation of LES pressure appears to be less the mechanism of action than the reduction in TLESR’s. Many patients who are ideal candidates for the Stretta procedure are those who rarely get referred to the interventionist, as these patients have relatively normal appearing anatomy on endoscopy. However the pH studies demonstrate pathologic GERD with a correlation in symptoms. Unfortunately, if the anatomy appears fairly normal and the patient has nonerosive esophagitis, many do not take the next step to order pH studies in these patients despite ongoing symptoms that do not respond to medications. The visual observation of the anatomy should not preclude further objective studies in those with severe symptoms of reflux or who cannot stop maximal medical therapy.
Technique
An advantage of the Stretta procedure is that it can be done in an outpatient setting, under conscious sedation or general anesthesia. If conscious sedation is chosen, care should be taken to pay close attention to patient discomfort. Discomfort is most apparent during distribution of radiofrequency energy, and the patient should be medicated accordingly. It is imperative for the endoscopist and anesthesia professional to communicate about giving the patient adequate analgesia and not overpower the patient with sedation. Some operators may choose to perform the procedure under general anesthesia for this reason.
Endoscopy is first performed to establish a baseline and to see the extent of GERD, and to measure the anatomic landmarks. The entire procedure is based on the endoscopic measurement of the location of the LES. A guidewire is then passed through the endoscope, and the Stretta device is passed over the guidewire to be 1 cm proximal to the Z line. The remainder of the procedure is performed blind without endoscopic guidance. Early on endoscopic guidance and fluoroscopic guidance were considered, however the procedure is very reproducible and safe when performed with good technique with low potential for complication.
Suction and irrigation are primed, and the device’s balloon is inflated to oppose the walls of the basket to the mucosal surface of the esophagus. A pressure valve is hooked to the inflation syringe to avoid over inflation of the balloon. The four electrodes located at cardinal directions, each 5.5 mm in length, are then deployed into the muscularis propria. 5W of radiofrequency energy is then delivered over 1 minute, with computer-controlled temperature monitoring to a target of 85 ℃ in the muscularis layer. Continuous cold water (not saline as this will conduct the energy) irrigation is applied to the mucosa to avoid thermal injury, with a target temperature <50 ℃. If temperatures reach above this threshold in any area or the impedance feedback gets too high, that electrode will cease to deliver energy.
After the energy delivery is completed, the electrodes are retracted and the balloon deflated, and the catheter is withdrawn a few centimeters to suction any esophageal fluid that has built up. The device is then rotated 45° and readvanced to the same level, the balloon reinflated, and the electrodes deployed again, delivering two sets of radiofrequency energy at each level. This is repeated at 5 mm increments for a total of 2 cm of length, with 8 total treatment sets in the esophagus. After finishing the four esophageal levels, the device is then advanced past the Z line into the stomach, and the balloon fully inflated to 25 cc. The device is then pulled back with minimal pressure until resistance is met (no greater than 2 cm above the measured Z line), in order to deploy the device at the hiatus and the gastric cardia. The treatment is performed three times at this level, rotating 30° to either side. This is repeated with the balloon inflated to 22 cc (5 mm proximal to this level), and again performed three times at this level. There is some variation to technique at this level, with some operators performing serial treatments down to 2 cm into the cardia if the pullbacks come back too far (greater than 2 cm above the z line) (22). The idea is to avoid deploying the delivery needles in the mediastinum where they can potentially go across the wall of the esophagus. It is ideal if the LES is under the protection of the diaphragmatic fibers and further in the abdominal cavity. When this is completed, the procedure is finished, and the device can be removed.
A completion endoscopy is performed to examine the effects of the procedure, and remove any residual fluid to prevent aspiration (Figure 8). Often minimal change is seen on the esophageal and gastric mucosa. Post procedure, PPIs are continued for three weeks and tapered off as symptoms improve (23). Carafate is used for the first week. Symptoms are expected to improve over 6 months as this is how normal healing occurs. So patients should be educated that they may not experience immediate relief, but that the effects of the procedure are expected to evolve over time. The collagen deposition is part of the healing process and part of the mechanism of action of this procedure.
Outcomes
With regard to efficacy, as this technique has been present for 20 years, there is a spectrum of data largely finding it to be effective. Several analyses have demonstrated its effectiveness both in the short term and the long term. A systematic review and meta-analysis of 28 controlled and cohort studies of 2,468 patients showed that Stretta significantly improved health-related quality of life, incidence of erosive esophagitis, and esophageal acid exposure, as well as elimination of PPI use in 51% of patients, with a mean follow-up of 25.4 months (24). Another systematic review and meta-analysis with 1,441 patients from 18 studies showed significant improvement subjectively in heartburn related quality of life, quality of life in reflux and dyspepsia score, and objectively in decreased DeMeester score compared to pre-op (25). Long term 10 year follow up has also been studied. In a prospective analysis of 217 patients with GERD before and after the Stretta procedure, at 10 years 72% of patients had normalization of the GERD-health-related quality of life score, 64% had a 50% or greater reduction in PPI use, and 54% of patients reported a 60% or greater improvement in quality of life. In addition, out of 33 patients that had Barrett’s esophagus pre procedure, 85% had regression confirmed on biopsy (26). These patients had noncircumferential short segment Barrett’s and this procedure should be used sparingly in those with Barrett’s. The presence of Barrett’s obscures the squamocolumnar junction. So this may result in the procedure not being performed properly if this landmark cannot be properly identified.
Adverse events of the Stretta procedure as determined by a meta-analysis comprising 2,468 patients occurred at a rate of 0.93% (24). The most common adverse events were erosions and mucosal lacerations, with serious adverse events such as esophageal perforation and aspiration pneumonia occurring very rarely. This is one of the safest endoscopic interventions for GERD. In addition, this procedure does not inhibit or limit any future procedures. Not only has this procedure been used to treat primary GERD, but it has also been a great adjunct for those with recurrent reflux after a plication or after bariatric procedures (27). This is a valuable procedure to have in one’s armamentarium. The biggest challenge with this procedure remains reimbursement. Despite 20 years of data, many insurers still view this procedure as experimental.
This procedure has also been utilized in challenging patients such as after a Nissen and after bariatric procedures. There has been reasonable effectiveness when chosen carefully for the appropriate patients (28). The nice thing about the procedure is that there is little downside and will not interfere with any future procedure in this area. Therefore, those who treat reflux should keep this procedure in their armamentarium.
Discussion
Despite the widespread incidence of GERD, the use of endoscopic therapies is underutilized. This is multifactorial. The first challenge is getting patients referred to the interventional endoscopist. Although there is objective evidence showing that the TIF and Stretta procedures can improve quality of life, decrease overall medication use, and reduce acid exposure in the esophagus, referring physicians continue to prescribe medications for management. Patients are often not given the option for another intervention. Many patients in our practice say, “My physician never told me there were other ways to treat my reflux. I found out about you because of a friend you treated.” Therefore those of us who offer these procedures need to continue to educate the medical and patient communities about alternatives to pharmacotherapy. With proper patient selection, particularly in patients with non-erosive reflux disease, the TIF or Stretta procedures can be advantageous, as patients with non-erosive reflux disease are less likely to respond to PPI therapy compared to patients with erosive esophagitis (16,29). General indications for these endoscopic procedures include symptoms as well as objective evidence of GERD despite medical therapy, a 2 cm or smaller hiatal hernia, desire to avoid long term medical therapy, and poor candidates for surgery or those that wish to avoid surgery. General contraindications include high grade esophagitis, metaplasia or dysplasia, hiatal hernia >2 cm, and any esophageal anatomic abnormality preventing proper device insertion, such as esophageal stricture narrowing. Laparoscopic fundoplication is preferred in patients with severe disease or metaplasia/dysplasia, as well as those with large hiatal hernias, as hiatal hernia and Hill grade 3 or above are associated with recurrence and need for revisional interventions (30). Thus, prior to endoscopic intervention, an endoscopy should be performed. In addition, we recommend that all patients with continued symptoms despite lifestyle modifications and medical therapy have objective pH testing, even in the setting of a normal appearing upper endoscopy, as they may still have pathologic GERD and benefit from intervention.
The second big barrier is the lack of coverage by insurance companies. Although these technologies have been around for almost two decades, insurers still deem these interventions as experimental. The medical community should recognize these procedures will not be as effective as surgery, but are a great middle ground between medications and surgery. Perhaps with broader education and outreach demonstrating the effectiveness of these procedures, referrals will increase and patients will have access to appropriate treatments. The growing amount of data surrounding the effectiveness of these procedures as well as patients wanting to reduce PPI therapy will hopefully increase the widespread use of these interventions. Only a small percentage of patients who are candidates for antireflux procedures are referred for evaluation.
Conclusions
Endoscopic antireflux procedures are important treatment modalities as an “in between” medical therapy alone and surgical antireflux treatment. In the correct patient population (presence of GERD/heartburn, hiatal hernia 2 cm or less, normal esophageal motility, and LA grade B esophagitis or lower) who are poor surgical candidates or who desire a decrease in medication requirement but prefer a non-surgical treatment, endoscopic antireflux procedures such as the TIF and Stretta procedures demonstrate safety and efficacy. These procedures can also be very effective for those whom medications are not effective, but their anatomy is relatively normal and therefore does not warrant surgical intervention (30). Literature supports that ideal patient candidates will likely have subjective and objective improvements, and although they are not meant to eliminate the need for PPI use entirely, the endoscopic antireflux procedures can provide a significant improvement in quality of life and amount of PPI usage over time (9). As the procedures are performed more commonly, long-term outcomes will be further studied, and the TIF and Stretta procedures will become even more prevalent.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Timothy M. Farrell and Geoffrey Kohn) for the series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-15/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-15/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-15/coif). The series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Savarino E, de Bortoli N, De Cassan C, et al. The natural history of gastro-esophageal reflux disease: a comprehensive review. Dis Esophagus 2017;30:1-9. [PubMed]
- Savarino E, Marabotto E, Bodini G, et al. Epidemiology and natural history of gastroesophageal reflux disease. Minerva Gastroenterol Dietol 2017;63:175-83. [PubMed]
- Mehta RS, Song M, Staller K, et al. Association Between Beverage Intake and Incidence of Gastroesophageal Reflux Symptoms. Clin Gastroenterol Hepatol 2020;18:2226-2233.e4. [Crossref] [PubMed]
- Sigterman KE, van Pinxteren B, Bonis PA, et al. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2013;2013:CD002095. [Crossref] [PubMed]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-28; quiz 329. Erratum in: Am J Gastroenterol 2013;108:1672. [Crossref] [PubMed]
- Ludemann R, Watson DI, Jamieson GG, et al. Five-year follow-up of a randomized clinical trial of laparoscopic total versus anterior 180 degrees fundoplication. Br J Surg 2005;92:240-3. [Crossref] [PubMed]
- Fuchs KH, Babic B, Breithaupt W, et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc 2014;28:1753-73. [Crossref] [PubMed]
- Fass R. An Overview of Transoral Incisionless Fundoplication and Magnetic Sphincter Augmentation for GERD. Gastroenterol Hepatol (N Y) 2017;13:50-2. [PubMed]
- Nabi Z, Reddy DN. Endoscopic Management of Gastroesophageal Reflux Disease: Revisited. Clin Endosc 2016;49:408-16. [Crossref] [PubMed]
- Fernando HC. Endoscopic fundoplication: patient selection and technique. J Vis Surg 2017;3:121. [Crossref] [PubMed]
- Rinsma NF, Smeets FG, Bruls DW, et al. Effect of transoral incisionless fundoplication on reflux mechanisms. Surg Endosc 2014;28:941-9. [Crossref] [PubMed]
- Testoni PA, Mazzoleni G, Testoni SG. Transoral incisionless fundoplication for gastro-esophageal reflux disease: Techniques and outcomes. World J Gastrointest Pharmacol Ther 2016;7:179-89. [Crossref] [PubMed]
- Trad KS, Barnes WE, Simoni G, et al. Transoral incisionless fundoplication effective in eliminating GERD symptoms in partial responders to proton pump inhibitor therapy at 6 months: the TEMPO Randomized Clinical Trial. Surg Innov 2015;22:26-40. [Crossref] [PubMed]
- Witteman BP, Conchillo JM, Rinsma NF, et al. Randomized controlled trial of transoral incisionless fundoplication vs. proton pump inhibitors for treatment of gastroesophageal reflux disease. Am J Gastroenterol 2015;110:531-42. [Crossref] [PubMed]
- Testoni PA, Testoni S, Mazzoleni G, et al. Long-term efficacy of transoral incisionless fundoplication with Esophyx (Tif 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: a prospective single-center study. Surg Endosc 2015;29:2770-80. [Crossref] [PubMed]
- Chang KJ, Bell R. Transoral Incisionless Fundoplication. Gastrointest Endosc Clin N Am 2020;30:267-89. [Crossref] [PubMed]
- Huang X, Chen S, Zhao H, et al. Efficacy of transoral incisionless fundoplication (TIF) for the treatment of GERD: a systematic review with meta-analysis. Surg Endosc 2017;31:1032-44. [Crossref] [PubMed]
- Stretta Doc: Stop Heartburn Reflux and GERD with Stretta, 2020. Available online: https://strettadoc.com/wp-content/uploads/2017/12/DeviceGraphic1.png
- Ravich WJ. Endoluminal reflux therapy: what do "FDA clearance" and "FDA approval" mean? Gastrointest Endosc 2008;68:845-8. [Crossref] [PubMed]
- Hopkins J, Switzer NJ, Karmali S. Update on novel endoscopic therapies to treat gastroesophageal reflux disease: A review. World J Gastrointest Endosc 2015;7:1039-44. [Crossref] [PubMed]
- Triadafilopoulos G. Radiofrequency treatment for gastroesophageal reflux disease. UpToDate. Available online: https://www.uptodate.com/contents/radiofrequency-treatment-for-gastroesophageal-reflux-disease
- Sandhu DS, Fass R. Stretta therapy in the management of gastroesophageal reflux disease (GERD). Ann Esophagus 2019;2:1. [Crossref]
- Richards WO, Houston HL, Torquati A, et al. Paradigm shift in the management of gastroesophageal reflux disease. Ann Surg 2003;237:638-47; discussion 648-9. [Crossref] [PubMed]
- Fass R, Cahn F, Scotti DJ, et al. Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg Endosc 2017;31:4865-82. [Crossref] [PubMed]
- Perry KA, Banerjee A, Melvin WS. Radiofrequency energy delivery to the lower esophageal sphincter reduces esophageal acid exposure and improves GERD symptoms: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech 2012;22:283-8. [Crossref] [PubMed]
- Noar M, Squires P, Noar E, et al. Long-term maintenance effect of radiofrequency energy delivery for refractory GERD: a decade later. Surg Endosc 2014;28:2323-33. [Crossref] [PubMed]
- Mattar SG, Qureshi F, Taylor D, et al. Treatment of refractory gastroesophageal reflux disease with radiofrequency energy (Stretta) in patients after Roux-en-Y gastric bypass. Surg Endosc 2006;20:850-4. [Crossref] [PubMed]
- Guerron D, Portenier D. A case series on gastroesophageal reflux disease and the bariatric patients: Stretta therapy as a non-surgical option. Bariatric Times 2016;13:18-20.
- Long JD, Orlando RC. Nonerosive reflux disease: a pathophysiologic perspective. Curr Gastroenterol Rep 2008;10:200-7. [Crossref] [PubMed]
- Franciosa M, Triadafilopoulos G, Mashimo H. Stretta Radiofrequency Treatment for GERD: A Safe and Effective Modality. Gastroenterol Res Pract 2013;2013:783815. [Crossref] [PubMed]
Cite this article as: Fastiggi MT, Khaitan L. A narrative review of minimally invasive procedures for gastroesophageal reflux disease: endoscopic antireflux procedures (TIF and Stretta). Ann Esophagus 2022;5:43.