
Preoperative anatomic considerations for a cervical or intrathoracic anastomosis: a retrospective cohort study
Introduction
Esophageal cancer surgery includes radical resection of the esophagus and regional lymphadenectomy, preferably using minimally invasive techniques. After both the three-stage McKeown esophagectomy (1), the most frequently performed esophagectomy around the world, as the two-stage transhiatal esophagectomy introduced by Orringer (2), continuity is restored via a cervical esophagogastric anastomosis. Alternatively, after the two-stage esophagectomy described by Ivor Lewis, an intrathoracic anastomosis is created (3). In recent years, this approach is gaining popularity among surgeons due to both the continuous rise of adenocarcinoma of the lower esophagus (4-6), and the increasing evidence that an intrathoracic anastomosis is associated with better functional outcome and less surgery-related complications (7,8).
As the 8the edition of the American Joint Committee on Cancer staging (AJCC) clearly reports assessment of tumor location during endoscopy is crucial (9), as perioperative systemic treatment, radiation fields and type of resection largely depend on it. Nevertheless, current literature only grossly distinguishes proximal, mid or distal esophageal tumors. The distance from the incisors to the upper border of the tumor or Barrett’s segment also play an important role when selecting an intrathoracic or cervical reconstruction (10). Current data reporting the level of the intrathoracic anastomosis are incomplete as anatomical landmarks of the anastomosis lack (11,12) or levels are simply described as cervical or “high”/“low” intrathoracic anastomosis (13,14). As a positive esophageal proximal resection margin is strongly related to poor oncological outcome, knowledge of the postoperative anatomy is a prerequisite for preoperative treatment planning to select the location of the anastomosis (15). Therefore, this study aimed to compare the level of the esophagogastric anastomosis following an intrathoracic or cervical reconstruction and provide its relation to preoperative location of the tumor and estimated proximal resection margin. We present the following article in accordance with the STROBE reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-41/rc).
Methods
This study describes a retrospective anatomical cohort in which all consecutive patients undergoing elective curative esophageal surgery for esophageal cancer between 2010 and 2018 followed by endoscopic evaluation were included. Surgery was performed in the Amsterdam UMC, location VUmc and involved a minimally invasive or open approach according to McKeown, Orringer or Ivor Lewis (1-3). Patient characteristics, surgery, pathology and endoscopy reports were extracted from electronic health records. If available, postoperative computed tomography (CT) images were evaluated.
Anatomical location of the anastomosis
In this study, the intrathoracic anastomosis during a two-stage Ivor Lewis esophagectomy was consistently created at the level of the tracheal carina, at the crossing of the azygos vein. The anastomosis was constructed in a side-to-side fashion using a linear stapled technique with hand-sewn closure of the stapling defect or fully stapled circular technique in an end-to-side or end-to-end fashion. A cervical anastomosis was created following a three-stage McKeown and Orringer esophagectomy. The esophagus was transected at the level of the sternal notch thus preserving as much cervical esophagus as possible. The anastomosis was created using an end-to-side or end-to-end hand-sewn technique.
Clinical level of the anastomosis
The primary aim of this study was to use postoperative endoscopic measurements to depict the upper level of the esophagogastric anastomosis, stratified for a cervical and intrathoracic reconstruction. Endoscopies were requested to dilate esophagogastric strictures, assess anastomotic integrity or insert gastro/jejunal feeding tubes. Endoscopes from Fujifilm or Olympus were used. The mean level of the anastomosis was determined when multiple endoscopies were performed to compensate for inter- and intra-observer variability. As a quality control measure, postoperative CT scans of patients with an intrathoracic anastomosis were assessed to verify that the anastomosis was located at the tracheal carina. Patients with an anastomosis >2 cm above or below the tracheal carina were identified and the endoscopic outcomes were adjusted based on CT measurements.
Preoperative tumor level and resection margins
In all patients, staging endoscopies were used to determine the preoperative tumor level. Proximal resection margins were measured during pathological evaluation. For an estimation of the location of the anastomosis as a function of the length of the patient, linear regression analysis was used.
Statistical analysis and ethical considerations
IBM SPSS statistics (version 23) was used for standard statistical analysis. Continuous variables are expressed as mean ± standard deviation or median and interquartile range (IQR) and frequency percentages are calculated for dichotomous variables. Differences were tested using an unpaired t-tests or Mann-Whitney U test. For an estimation of the location of the anastomosis as a function of the length of the patient, linear regression analysis was used. A two-sided P value of <0.05 was considered statistically significant. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (16). The Medical Ethics Committee of the Amsterdam UMC approved the study protocol (No. 2018.595). All living subjects have been provided the opportunity to opt-out and received a written no objection letter.
Results
Patient characteristics
Between January 2010 and October 2018, 329 patients underwent a curative esophagectomy according to Ivor Lewis, McKeown or Orringer. One or more postoperative endoscopies were performed in 208 patients (63%), to assess or dilate anastomotic strictures (54%), assess anastomotic integrity (32%), place nasogastric/jejunal feeding tube (11%) or for other reasons (3%). This cohort consisted of 158 males and 50 females with a mean age of 65.1±9.3 years, additional characteristics are summarized in Table 1. An Ivor Lewis esophagectomy was performed in 61 patients, resulting in a side-to-side linear stapled (n=42, 69%) or end-to-end/side fully circular stapled (n=19, 31%) intrathoracic anastomosis. In 147 patients an esophagectomy with a cervical anastomosis was performed according to McKeown (n=91, 62%) or Orringer (n=56, 38%). Patient distribution is displayed in a flowchart (Figure 1).
Table 1
| Characteristics | Intrathoracic (n=61) | Cervical (n=147) |
|---|---|---|
| Male gender | 52 (85.2) | 106 (72.1) |
| Age, years | 62.5±8.0 | 66.2±9.6 |
| Length, cm | 178.3±8.2 | 175.4±9.7 |
| ASA score | ||
| I | 8 (13.1) | 11 (7.5) |
| II | 42 (68.9) | 87 (59.2) |
| III | 10 (16.4) | 43 (29.3) |
| IV | 1 (1.6) | 6 (4.1) |
| Neo-adjuvant treatment | ||
| None | 1 (1.6) | 9 (6.1) |
| Chemotherapy | 5 (8.2) | 4 (2.7) |
| Chemoradiotherapy | 55 (90.1) | 134 (91.2) |
| Type of carcinoma | ||
| Adenocarcinoma | 53 (86.9) | 97 (66.0) |
| Squamous cell | 5 (8.2) | 44 (29.9) |
| Other | 3 (4.9) | 6 (4.1) |
| Approach | ||
| Open | 7 (11.5) | 13 (8.8) |
| Minimally invasive | 54 (88.5) | 134 (91.2) |
| Type of procedure | ||
| Ivor Lewis | 61 (100.0) | 0 (0) |
| McKeown | 0 (0) | 91 (61.9) |
| Transhiatal | 0 (0) | 56 (38.1) |
| Configuration | ||
| ETS | 17 (27.9) | 52 (35.4) |
| ETE | 2 (3.3) | 94 (63.9) |
| STS | 42 (68.9) | 1 (0.7) |
| Technique | ||
| Hand-sewn | 0 (0) | 146 (99.3) |
| Linear stapled | 42 (68.9) | 1 (0.7) |
| Circular stapled | 19 (31.2) | 0 (0) |
| Radical surgery | 57 (93.4) | 132 (89.8) |
Data are n (%) or mean ± standard deviation. ASA, American Society of Anaesthesiologists; ETS, end to side; ETE, end to end; STS, side to side.
Intrathoracic anastomosis
The intrathoracic anastomosis was assessed using endoscopy in 61 patients. Postoperative CT assessment revealed a supracarinal anastomosis (median 3 cm above the carina) in three patients and an infracarinal anastomosis (6.5 cm below the carina) in one patient, the corresponding endoscopic measurements were adjusted accordingly. Endoscopic measurements revealed a mean level of the anastomosis of 28.2±2.3 cm from the incisors. Subgroup analysis revealed a median of 28.6 cm (IQR, 27.0–30.0 cm) after a linear stapled anastomosis and 28.0 cm (IQR, 24.9–28.8 cm) after a fully circular stapled anastomosis (P=0.041). Preoperative staging endoscopies reported a mean preoperative tumor level of 37.7±3.5 cm from the incisors. Pathology reports documented a mean proximal resection margin of 4.5±1.9 cm. Further pathological and endoscopic measurements are depicted in Table 2 and visualized in Figure 2.
Table 2
| Outcomes | Intrathoracic (n=61) | Cervical (n=147) |
|---|---|---|
| Endoscopic level of anastomosis (cm from the incisors) | 28.2±2.3 | 19.6±1.7 |
| Endoscopic preoperative tumor level (cm from the incisors) | 37.7±3.5 | 33.1±4.3 |
Data are mean ± standard deviation.
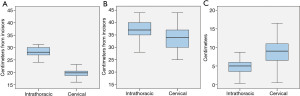
Cervical anastomosis
One hundred and forty-seven patients were subjected to postoperative endoscopic evaluation following a cervical reconstruction. The anastomosis was located at a mean distance of 19.6±1.7 cm from the incisors. Preoperative staging endoscopies revealed a mean tumor level of 33.1±4.3 cm from the incisors. A proximal resection margin of 8.9±3.4 cm was achieved. Stratification for type of procedure revealed a significantly lower tumor level prior to an Orringer esophagectomy (35.5±4.1 cm) compared to a McKeown esophagectomy (31.9±4.0 cm, P<0.001). No significant differences were observed in the level of the anastomosis and proximal resection margins between Orringer and McKeown procedures.
Body length
For patients with a cervical reconstruction a significant correlation was observed between the level of the anastomosis and body length (P<0.001). Linear regression analysis revealed that the level of the cervical anastomosis could be estimated from a patient’s length by the following equation: 4.516 cm +0.086× height (cm). No significant regression was found for patients with an intrathoracic anastomosis.
Discussion
This study reports the postoperative anatomical location of the esophagogastric anastomosis and can be used in clinical practice when compared to preoperative staging endoscopy. This location, determined using postoperative endoscopic assessment, of the intrathoracic and cervical anastomosis guides the treating physicians in planning of radiation field and counseling patients for the level of anastomosis.
As many studies suffice by stating whether a tumor is located in the proximal, mid or distal esophagus, current evidence on the exact location is limited, Walther et al. conducted a randomized controlled trial comparing an intrathoracic anastomosis to a cervical anastomosis, involving 83 patients (11). The level of the anastomosis was determined as a secondary endpoint. The cervical anastomosis was localized at a median of 20 cm (range, 15–25 cm), which is in line with the results presented in this paper. Authors describe a median level of 25 cm (range, 21–28 cm) after an intrathoracic reconstruction, in contrast to a median level of 28.1 cm in this study. Unfortunately, the authors failed to specify the anatomical location of the intrathoracic anastomosis, making it difficult to apply the results to individual practice.
In this study, the intrathoracic anastomosis was consistently created at the level of the tracheal carina and most commonly by a linear stapled side-to-side technique or, alternatively, by a fully circular stapled end-to-end/side approach. The endoscopic distance was measured from the incisors to the upper level of the anastomosis. Due to the side-to-side formation, during which the ventral side of the esophageal remnant is stapled to the posterior wall of the gastric conduit, the most proximal level of the anastomosis is eventually located above the level of transection. As for the circular stapled technique, the anvil is secured using a purse-sting suture, which results in an anastomosis practically at the level of transection. Contrarily, in this study, the linear stapled approach resulted in a slightly lower anastomosis compared to a fully circular stapled approach (median 28.6 and 28.0 cm, respectively). This difference however is clinically irrelevant. For tumors located at or above the tracheal carina, assessed using endoscopy or radiological imaging, a cervical anastomosis is therefore required to achieve safe proximal resection margins.
Preoperative treatment planning is started by assessing the distance from the incisors to the upper border of the tumor during a staging endoscopy. This distance is correlated with CT and proton emission tomography (PET) imaging, providing a more exact anatomical location. These measurements form the single most important factor when selecting the type of reconstruction. The additional benefit of endoscopic evaluation compared to PET/CT imaging is the visualization of mucosal changes, dysplasia or a Barrett’s segment. Therefore, the exact transition from healthy esophagus to the Barrett’s segment contributes to determining the minimally required level of transection. The surgical plan is finalized when radiological re-staging is performed after neoadjuvant chemoradiotherapy.
Trimodality therapy, consisting of neoadjuvant chemoradiotherapy and surgery, is considered the gold standard for the management of esophageal cancer. As irradiation damages microvasculature, anastomotic healing might by impaired when the anastomosis is located within the radiated area. This has been confirmed by some data showing significant higher rates of anastomotic leakage (17). In these patients, knowledge of the endoscopic level of the anastomosis can be useful when deciding whether an intrathoracic anastomosis, preferably outside the radiated area, is technically feasible (18).
Both anastomotic locations have potential benefits and shortcomings. In terms of an intrathoracic anastomosis, the potential benefits are reduced tension at the anastomotic site, increased vascularization of the gastric conduit tip, lower incidence of recurrent laryngeal nerve injury and a lower rate of anastomotic leakage (7,8,19). A cervical anastomosis has lower morbidity associated with a cervical anastomotic leak and a significantly greater proximal resection margin, however, more esophageal strictures are reported. Other postoperative upper gastrointestinal symptoms related to esophagectomy are reflux, nausea, dysphagia, vomiting, dyspepsia, dumping and delayed gastric emptying. Research concluded that 45% of patients had at least one of these symptoms 12 months after surgery (20). The impact of anastomotic location on these postoperative symptoms has not been thoroughly investigated. Present results suggest that, on average, an intrathoracic reconstruction yields a 9-cm longer esophageal remnant. Further research should determine whether an extended esophageal remnant improves functional outcomes after esophagectomy.
This anatomical study was subjected to limitations. First, endoscopic evaluation of the anastomosis was not routinely performed, resulting in a variable postoperative timing of the endoscopy. Second, although attempts were made to compensate for inter- and intra-observer variability, this limitation might have influenced the results. Third, endoscopies were performed for several indications (primarily the assessment of strictures or anastomotic integrity).
Conclusions
In conclusion, this study presents the exact location of the esophagogastric anastomosis using endoscopic evaluation. These insights are valuable during the preoperative workup and should be used next to imaging results to determine radiation fields and to assess whether an intrathoracic anastomosis will not compromise proximal resection margins.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-41/rc
Data Sharing Statement: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-41/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Medical Ethics Committee of the Amsterdam UMC approved the study protocol (No. 2018.595). All living subjects have been provided the opportunity to opt-out and received a written no objection letter.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus. Br J Surg 1976;63:259-62. [Crossref] [PubMed]
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [Crossref] [PubMed]
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. [Crossref] [PubMed]
- Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg 2001;234:581-7. [Crossref] [PubMed]
- Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol 2016;41:88-95. [Crossref] [PubMed]
- van Workum F, Berkelmans GH, Klarenbeek BR, et al. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis 2017;9:S826-33. [Crossref] [PubMed]
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011;28:29-35. [Crossref] [PubMed]
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119-30.
- Gooszen JAH, Goense L, Gisbertz SS, et al. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. Br J Surg 2018;105:552-60. [Crossref] [PubMed]
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. [Crossref] [PubMed]
- D'Journo XB, Martin J, Rakovich G, et al. Mucosal damage in the esophageal remnant after esophagectomy and gastric transposition. Ann Surg 2009;249:262-8. [Crossref] [PubMed]
- Jeon HW, Park JK, Song KY, et al. High Intrathoracic Anastomosis with Thoracoscopy Is Safe and Feasible for Treatment of Esophageal Squamous Cell Carcinoma. PLoS One 2016;11:e0152151. [Crossref] [PubMed]
- Park SY, Lee HS, Jang HJ, et al. The role of one-year endoscopic follow-up for the esophageal remnant and gastric conduit after esophagectomy with gastric reconstruction for esophageal squamous cell carcinoma. Yonsei Med J 2013;54:381-8. [Crossref] [PubMed]
- Wang YC, Deng HY, Wang WP, et al. Positive esophageal proximal resection margin: an important prognostic factor for esophageal cancer that warrants adjuvant therapy. J Thorac Dis 2016;8:2512-8. [Crossref] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Juloori A, Tucker SL, Komaki R, et al. Influence of preoperative radiation field on postoperative leak rates in esophageal cancer patients after trimodality therapy. J Thorac Oncol 2014;9:534-40. [Crossref] [PubMed]
- Spechler SJ. Clinical practice. Barrett's Esophagus. N Engl J Med 2002;346:836-42. [Crossref] [PubMed]
- Pennathur A, Zhang J, Chen H, et al. The "best operation" for esophageal cancer? Ann Thorac Surg 2010;89:S2163-7. [Crossref] [PubMed]
- Bouras G, Markar SR, Burns EM, et al. The psychological impact of symptoms related to esophagogastric cancer resection presenting in primary care: A national linked database study. Eur J Surg Oncol 2017;43:454-60. [Crossref] [PubMed]
Cite this article as: Plat VD, van Toorenburg EL, van Wanrooij RLJ, Heineman DJ, Straatman J, van der Peet DL, Luttikhold J, Daams F. Preoperative anatomic considerations for a cervical or intrathoracic anastomosis: a retrospective cohort study. Ann Esophagus 2023;6:31.



