
Endoscopic management of early esophageal cancer: a literature review
Introduction
Esophageal cancer accounts for approximately 1% of all cancers diagnosed in the United States, but causes 2.6% of all cancer deaths. The American Cancer Society estimates that there will be 19,260 new esophageal cancer cases diagnosed (15,310 men and 3,950 women) and approximately 15,530 deaths from esophageal cancer (12,410 in men and 3,120 in women) in the United States in 2021 (1). Screening and surveillance programs identify premalignant and early stage malignancies and have resulted in declining rates of esophageal cancer and improved outcomes compared to patients diagnosed at later stages. Studies have shown that the outcome for patients with T1 cancer is excellent, with a 5-year disease-specific survival rate exceeding 80%, whereas patients with locally advanced disease (T2 or T3) or distant metastases have 5-year survival rates of 45.2% and 4.8%, respectively (2,3).
Esophageal cancer [squamous cell carcinoma (SCC) and adenocarcinoma (EAC)] is an aggressive disease, in part due to the biological nature of the disease, but also because of anatomical features that readily promote the locoregional and distant spread of tumor cells, such as an extensive network of lymphatics in and around the esophagus. It is imperative that clinicians who are considering curative endoscopic management keep this in mind. From a purely oncologic perspective, surgical esophagectomy is the most definitive modality for treatment of early esophageal cancer, though at the expense of greater treatment-related major morbidity, mortality, and hospital length of stay compared to endoscopic treatment. For example, the mortality rate of surgical esophagectomy is approximately 2% at high-volume centers (4). Minimally invasive, organ-sparing endoscopic interventions like endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have a lower incidence of major complications and essentially no treatment-related mortality, and are therefore attractive options particularly in older patients with comorbid medical conditions. In this review, we discuss the different approaches to endoscopic management of early esophageal cancer. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-30/rc).
Methods
An electronic search and data extraction of literature was performed from databases (MEDLINE, Embase and known textbooks) by the two authors. Studies in English were included. The terms used for data search included “esophageal cancer”, “endoscopic mucosal resection”, “endoscopic submucosal dissection”, “EMR”, and “ESD”. Selected articles and abstracts were evaluated by the two authors to present this narrative review.
Diagnosis and characterization of early esophageal cancer
Endoscopy with advanced imaging and chromoendoscopy
Early diagnosis of esophageal cancer is of paramount importance for treatment purposes. Screening endoscopy is recommended for populations at-risk for esophageal cancer. Once an endoscopic risk factor such as Barrett’s esophagus is identified, longitudinal surveillance or endoscopic treatment (with ablative or respective endoscopic techniques) is recommended based on the presence or absence of dysplasia. Several endoscopic imaging technologies can be used to enhance detection and characterization of suspicious lesions. Some are widely available on commercially available endoscopes and serve to enhance subtle morphologic characteristics of the mucosal surface and vascular pattern, whereas other are not an intrinsic to conventional endoscopes and are therefore less widely available. Examples of the former include high-definition white light optics, optical magnification, and a myriad of proprietary vendor-specific digital chromoendoscopy technologies (e.g., narrow band imaging or NBI, blue light imaging or BLI, linked color imaging or LCI, i-scan). Topical spray-based chromoendoscopy agents are also frequently used, including Lugol’s iodine for improved detection of squamous dysplasia/carcinoma and acetic acid for characterization of Barrett’s neoplasia. All of these image enhancement methods and technologies help augment visualization of normal and abnormal mucosal surface morphology and vascular patterns. Studies have shown that these increase the detection rate of subtle esophageal lesions (5). Less widely available imaging modalities but effective technologies include confocal laser endomicroscopy (CLE), optical coherence tomography (OCT) and volumetric laser endomicroscopy (VLE, a second generation technology based on OCT). Interobserver variations can affect the diagnostic accuracy of each of these methodologies and thus histologic biopsies of suspicious mucosal lesions are often performed prior to embarking upon endoscopic resection (ER). However, aggressive tissue sampling can induce subepithelial fibrosis that can make subsequent ER via EMR or ESD significantly more difficult. Under representative tissue sampling, when small forceps biopsies of a suspicious nodule or lesion fail to confirm the presence of dysplasia or malignant lesion is a potentially catastrophic problem if one relies on forceps biopsies alone of suspicious esophageal lesions. Therefore, ER is sometimes performed without antecedent tissue sampling at centers with extensive experience with enhanced imaging modalities and ER techniques.
Endoscopic ultrasound (EUS)
EUS is the most accurate tool for T-staging for esophageal cancer, but it is highly operator dependent and is more accurate for staging of advanced cancers than it is for differentiating “low-risk” from “high-risk” T1 cancer (explained below). Nevertheless, it is frequently utilized prior to ER, particularly to exclude overt T2-T4 tumors and for assessment of regional lymph node metastases (LNM) (6,7). For example, the ability of EUS to reliably distinguish whether an early esophageal cancer is invading into but not through the muscularis mucosa (T1a or T1m) or into the submucosa (T1b or T1sm) is less than ideal. Because of this limitation, as long as the available endoscopic, endosonographic and radiographic findings do not suggest a T2 or greater T-stage and/or the presence of LNM, many advanced endoscopists then go directly to EMR or ESD because it can serve two purposes – it can provide more accurate histopathologic staging information that guides management decisions (T1a vs. T1b cancer, degree of differentiation, presence or absence of lymphatic or vascular invasion), and if only favorable features are present (more below) the ER can potentially serve as a curative oncologic intervention.
CT/PET
The resolution of computed tomography (CT) and positron emission tomography-computed tomography (PET-CT) scans is inadequate for distinguishing the individual layers of the esophageal wall and are not adequate for T-staging of most esophageal cancer, particularly early esophageal cancer. They are primarily used to assess the relationship or more advanced esophageal cancers and to identify locoregional and distant metastases. One study demonstrated that PET was more accurate than CT in detecting distant metastases, but less than minimally invasive surgical staging or clinical suspicion (8). We obtain CT and/or PET-CT in all patients prior to embarking on ER of a suspected early esophageal cancer.
TNM staging of early esophageal cancer and selection of candidates for ER
Early EAC
T1 esophageal cancers include tumors limited to mucosa (defined as T1a) and those that extend beyond the muscularis mucosa and into the submucosa (T1b) without deeper invasion into the muscularis propria (T2) (9). Only patients with T1 esophageal cancers are potential candidates for curative ER. More specifically, ER with curative intent is primarily reserved for patients with T1a cancers without concomitant high-risk features, such as poorly differentiated histology or lymphovascular invasion or positive deep (vertical) margin following ER; the risk of LNM in T1a EAC patients is <2%.
For surgically fit candidates, the presence of confirmed or highly suspected submucosal invasion (T1b) should prompt multidisciplinary discussion and serious consideration of surgical esophagectomy because it carries an amplified risk of LNM, even in the absence of radiologically or endosonographically enlarged lymph nodes. In other words, surgical esophagectomy is the preferred treatment for all but a small, select group of T1b cancer patients (usually those for whom the risk of surgical esophagectomy is deemed to be unacceptably high) because of the risk of concomitant LNM (up to 45% of T1b EACs).
As discussed below, ER alone may be appropriate for some patients with T1b cancers, however. Patients with T2 (invading the muscularis propria), T3 (invading adventitia) and T4 (invading adjacent structures) cancers are never candidates for curative endoscopic management. Similarly, patients with proven or highly suspected lymph node involvement or distant metastasis are not candidates for endoscopic therapy with curative intent.
ER for early esophageal cancer, increasingly performed, is best suited for superficial, small, flat cancers less than 2 cm in size. Patients and providers considering ER with curative intent must carefully weigh the advantages of ER (organ-preservation with lower major morbidity and mortality than surgery, shorter hospital LOS) against the disadvantages of definitive surgical esophagectomy. Randomized controlled studies directly comparing oncologic outcomes of endoscopic treatment to surgical esophagectomy for early esophageal cancers are lacking; practice is guided by primarily by observational series and systematic reviews. A systematic review and meta-analysis assessing treatment outcomes of endoscopic eradication therapies or esophagectomy for patients with high-grade dysplasia (HGD) and intramucosal EAC found comparable complete eradication rates and overall survival. Endoscopic eradication was associated with a minimally higher neoplasia recurrence rate but significantly lower adverse event rate (10). A study of 1,000 intramucosal cancers with long-term follow up, in which cases of local recurrence were treated with repeat endoscopic treatment, demonstrated complete local remission rate of 93.8% (11). Guidelines from the American Society for Gastrointestinal Endoscopy and the European Society for Gastrointestinal Endoscopy recommend ER for patients with HGD and well or moderately differentiated (grade 0 or 1) intramucosal (T1a) EAC without LVI, since it has lower morbidity and mortality than surgery (4,12).
A limited body of data from small studies suggests that acceptable outcomes can also be achieved with endoscopic treatment for early EAC patients with very superficial (≤500 microns into SM) T1b tumors. This is acknowledged in a recent clinical practice update in Gastroenterology which proposes that T1b EACs that have minimal submucosal invasion (<500 microns) may suffice as a curative option for high operative risk patients, as long as the cancer is not poorly differentiated and lacks lymphovascular invasion, since these features are associated with increased risk for LNM and hence a failure of endoscopic cure (13). In one such study, LNM in only developed in 1 of 53 patients (1.9%) with these “low-risk” T1b cancers over a 49-month period (14). In another series, EMR followed by endoscopic ablation resulted in 87% complete endoscopic remission. Of those that achieved complete remission initially, 19% developed metachronous cancers that could be treated endoscopically if detected early. One patient (2%) developed LNM, and the estimated 5-year survival was 84% (15).
The National Comprehensive Cancer Network (NCCN) currently lists ER as the preferred treatment option for low-risk pTis and pT1a EACs, and indicates that for superficial pT1b EACs either ER followed by ablation or esophagectomy are acceptable. Esophagectomy is the only recommended option for other pT1bN0 cancers. As stated above, the decision to utilize only endoscopic therapy without esophagectomy or adjunctive interventions for early esophageal EAC, particularly superficial pT1b cancers, should involve multidisciplinary discussion and shared decision making with the affected patient. Complete endoscopic eradication of all Barrett’s epithelium, not just an area of early Barrett’s-related EAC, is a commonly accepted treatment goal since there is a significant risk of metachronous EACs when residual “at-risk” Barrett’s epithelium is left in situ. The American Society for Gastrointestinal Endoscopy recommends achieving this goal by first performing ER of all visible lesions within the Barrett’s segment (e.g., nodules) followed by radiofrequency ablation (RFA) of all remaining Barrett’s epithelium (4). Circumferential ER of all Barrett’s is often feasible and can be considered in lieu of RFA, but harbors significant risk of esophageal stenosis, particularly if circumferential or near-circumferential ER is performed. The total length of the Barrett’s segment should be factored into decisions about endoscopic versus surgical management, particularly for surgically fit patients for whom curative treatment is desired, since treatment failures (inability to completely eradicate Barrett’s) is more common with very long segments of Barrett’s. For example, in one study, the hazard ratio for failing to achieve this goal using EMR and RFA was 0.46 for persons with 3–10 cm and 0.22 for those with great than 10 cm long segments of Barrett’s epithelium (16).
Early esophageal SCC
Lymph node metastasis appears to occur more readily and with lesser depths of submucosal invasion in early SCC than EAC. In SCC, the risk of LNM is as low as 0% for M1 and M2 cancers (involving epithelium or lamina propria), 8–18% for M3 cancers (involving but not going through the muscularis mucosa), 11–53% with invasion ≤200 microns, and 30–54% with deeper submucosal invasion (17). For this reason, the Japanese Esophageal Society advocates for ER as curative treatment for T1 M1 or M2 tumors but recommends additional therapy (esophagectomy, chemoradiation) for T1 M3 tumors and T1b tumors (18).
The NCCN considers endoscopic therapy or esophagectomy to be acceptable primary treatments for pathologic Tis or T1a SCCs, but advocates esophagectomy for T1bN0 tumors (9). An alternative strategy, advocated by some, is to separate early SCC into those that can be considered to have “absolute indications” for en bloc resection via ESD (T1a, M1-M2 cancers, involving two-thirds or less of the esophageal circumference) and others which have “expanded indications” (T1a M2 cancers or T1b cancers with submucosal invasion <200 microns that are clinical N0 status and without LVI), since rates of LNM as low as 4.7% have been reported in the latter group following ESD (17,19). En bloc ESD with curative intent for patients meeting the expanded criteria is more appropriate for suboptimal rather than good operative candidates.
For a person with SCC who undergoes ER and is found to have concerning histologic features (e.g., T1b and/or LVI), it has been long accepted that esophagectomy offers the best opportunity for cure. Recent studies have compared chemoradiation (CRT) to esophagectomy for patients with unfavorable histology following ER. In one study of 83 patients with pT1b SCC confirmed by ER specimens, there were no significant differences in overall or relapse-free survival between CRT and esophagectomy (20). In another study of 175 patients (non-randomized), those with either pT1b with negative resection margin or pT1a with LVI were treated with 40.4 Gy RT, and all patients with a positive vertical/deep resection margin were treated with definitive CRT. The 3-year overall survival was >90%, comparable to that of a historical surgical cohort, suggesting that non-operative management might be a viable option (21).
Other predictors of LNM
As discussed previously, the risk of LNM is influenced by the cancer’s depth of invasion and the histologic subtype (EAC versus SCC). Additional factors that portend a greater risk for LNM include tumor size (larger than 2 cm), morphology (Paris type 0-I protruding lesions and Paris type 0-III excavated lesions have greater risk and are less well suited for curative ER than the relatively flat Paris 0-II lesions), poorly differentiated histology, presence of lymphatic or vascular invasion in the resected specimen, and biological markers such as E-cadherin and Cyclin D1 (22,23).
Some of the technical aspects of EMR and ESD for esophageal neoplasia, and the rationales for considering one technique over the other, are discussed below.
Technical aspects of ER
EMR
In the esophagus neoplasms are frequently flatter and less adequately raised with submucosal injection of solution. Therefore, esophageal EMR typically utilizes one of several varieties of “EMR caps” which are affixed to the distal end of the endoscope insertion tube. Through different mechanisms, these caps create “pseudopolyps” that can then be grasped and resected by a snare. Various devices are available for “cap-based” EMR, and are commonly broken down into two main types: cap-only EMR and multiband mucosectomy (24,25).
Cap-only EMR
This term refers to the use of a transparent distal attachment cap specially designed for EMR. Within the distal-most edge of the cap exists a shallow “ridge” or “shelf”. The endoscopist inserts a proprietary crescent-shaped snare down the working channel of the endoscope and maneuvers the open wire loop of this snare until it sits, almost invisibly, inside the ridge (a process that can be frustrating and time consuming). After injecting a solution to provide a submucosal cushion, the open end of the cap is positioned against the target tissue and endoscope suction is applied, effectively creating a pseudopolyp of target tissue inside the cap/snare assembly. While the suction is being applied, the endoscopy assistant closes the snare, effectively capturing the tissue within then snare. The tissue is then transected using electrosurgical energy. Additional mucosal resection can be performed if the entire specimen was not resected during the first attempt (26).
Multiband mucosectomy/EMR
This method was developed after the advent of cap EMR, and now dominates many endoscopic marketplaces because of its safety and greater ease of use (does not require seating of a crescent snare, as described above). Various proprietary multiband EMR kits are available. They are akin to variceal band ligation kits. They consist of a transparent cap around which several rubber bands have been stretched/applied. The rubber bands can be “fired” off the cap by rotation of a specialized handle attached to endoscope accessory channel; rotating this wheel applies tension to a string or wire that runs down the endoscope working channel and connects to the cap/band assembly applied to the distal end of the endoscope, resulting in release of the rubber band. After the target lesion has been identified, it is suctioned into the cap, and a rubber band is released to effectively create a pseudopolyp within the rubber band. A snare is then advanced through the working channel and used to resect the pseudopolyp using electrosurgical energy (Figure 1).
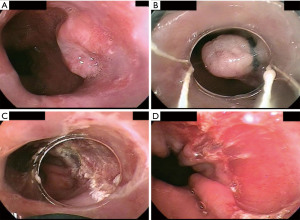
In general, either cap-based EMR allows removal of approximately 15 mm of target tissue at a time (slightly more with the cap-only than the multiband kits), and can potentially achieve en bloc R0 resection of small lesions less than 15 mm. Cap-based EMR techniques offer significant time-savings compared to ESD, and lesions greater than 15 mm can be resected in a piecemeal fashion. However, piecemeal resection is disadvantageous in the setting of early esophageal cancer since it hinders optimal histopathologic assessment of the specimen and can make it difficult or impossible to assess margin status.
ESD
ESD is a significantly more time-consuming and technically demanding technique with greater risk of complications (particularly perforation) than EMR. Its major advantage is that it allows en bloc resection of larger lesions and better control over circumferential resection margins. ESD may also be feasible, albeit more challenging, for fibrotic lesions that are not amenable to EMR or for neoplastic recurrences following prior EMR. ESD is more widely available in Asia where the technique originated as a means to reduce the need for surgical gastrectomy for early gastric cancers. ESD is still not universally available in the United States, but its popularity and opportunities for training are expanding. Many factors impede ESD use in the United States, including lack of awareness about the technique or its benefits, the steep learning curve, significantly longer procedure times compared to EMR, previous lack of Food and Drug Administration approval of ESD devices (though this has improved significantly in recent years) and training required for by western pathology providers to learn how to optimally process ESD specimens (27).
Multiple through-the-scope tools for ESD now exist, many of which have become available for use in the United States, including knife-type (insulated tip and non-insulated tip, straight and hook-knife varieties) and scissor-type devices. The ESD technique is akin to en bloc removal of the skin from a fillet of fish. At the onset of ESD, the margins of the target lesion are carefully characterized and demarcated, usually with thermal markings. The markings facilitate resection because the evolving mucosal “flap” created during ESD can become mobile and twist, obscuring anatomic orientation. Submucosal injection of either saline or one of several viscous solutions lifts the target lesion away from the muscularis propria, protecting against transmural perforation. In classic ESD, a circumferential mucosal incision is made through the epithelium and muscularis mucosa, around the lesion (ensuring a several millimeter margin of non-malignant mucosa), exposing the fluid-filled submucosal plane (the so called “third space”). The distal tip of the endoscope affixed with a transparent distal attachment cap (different than those used for cap-based EMR) is then delivered into the submucosal plane, and dissection of the submucosal fibers and coagulation/transection of penetrating submucosal vessels is carefully and meticulously performed using electrosurgical energy until the entire lesion is removed en bloc. The specimen is then affixed to paraffin wax, cork, or other material for histopathologic inspection (Figure 2). Numerous technical variations and methods have been described (variations in the different ESD steps, electrosurgical generator settings, use of adjuvant methods to apply traction to the mucosal flap during ESD in order to improve visibility of the submucosal structures and expedite the resection).
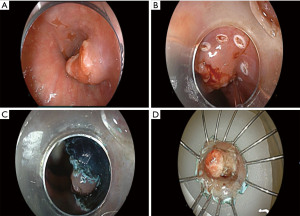
Comparison of EMR and ESD
A meta-analysis of 8 studies assessed the effectiveness ESD and EMR for treating superficial esophageal cancer. Patients who underwent ESD had significantly higher en bloc and curative resection rates and lower local recurrence rates (28). In a study by Ishihara et al., (29) ESD was compared to 2 major EMR methods for esophageal cancers ≤20 mm in size. ESD was found to have the highest rates of en bloc (100%) and curative resection (97%). Cap EMR was considered a good alternative for lesions <15 mm (en bloc and curative resection rates of 100% and 86%, respectively). In our opinion, EMR is an acceptable option for small lesions where there is a reasonable chance for en bloc resection in a single piece. Piecemeal EMR can be curative of early esophageal cancer if, despite the piecemeal nature, the pathologists can confidently confirm T1a disease, clear vertical/deep margins, and absence of LVI or poorly differentiated histology. Otherwise, the advantages (en bloc R0 resection) should be balanced against the disadvantages (time-consuming, greater higher risk of complications) of ESD. There is a large pool of prospective and retrospective studies on EMR and ESD and more prospective studies with larger cohorts are being done to confirm the results of these techniques.
Complications of esophageal EMR and ESD
The most common complication after esophageal ESD is formation of a stricture, the incidence of which is 5% to 17% (higher in some series) and is directly proportional to the extent of the resected area. The greatest risk occurs when more than two-thirds to three-quarters of the mucosal circumference are resected. Strictures are also a major limitation of wide-field piecemeal EMR involving similarly extensive resection. In 2015, a study by Chevaux et al. (30) showed that in 75 patients who underwent ESD with a median specimen diameter of approximately 52 mm, 60% developed esophageal strictures, all of which could be treated endoscopically. The exact mechanisms for stricture formation in this setting are not completely understood. These resections result in temporary loss of the epithelial barrier function, which exposes the submucosa to chemical and mechanical injury from acid and food in the esophagus. This activates an inflammatory infiltrate and myofibroblast proliferation leading to a hypertrophic scar formation (31). Various modalities have been used to try to prevent strictures with varying degrees of success, including glucocorticoid preparations aimed at reducing inflammation and fibrosis (local submucosal triamcinolone injection, topical oral budesonide, or, systemic oral glucocorticoids) serial prophylactic endoscopic balloon dilations, temporary stent placement or use of other novel antimitotic and ant fibrotic agents.
Relative to some other portions of the gastrointestinal tract, the esophagus has a thin muscularis propria layer and lacks serosa. Despite this, esophageal perforation during multiband mucosectomy/EMR is uncommon; it is believed that the rubber bands in the commercially available kits do not easily grasp/hold the muscularis propria of the esophagus. The risk of transmural perforation with ESD is greater than that of EMR. Park et al. (32) described 225 patients who underwent ESD for 261 esophageal lesions. Adverse events occurred in 33 cases (12.6%), including bleeding (1.5%), perforation (4.6%), and stricture (6.5%). Kim et al. (33) reported on ER for 147 superficial esophageal neoplasms in 129 patients; adverse events occurred in 22 patients (17.1%), including bleeding (n=2, 1.6%), perforation (n=12, 9.3%), and stricture (n=8, 6.2%). If detected during the ESD procedure, endoscopic closure may be feasible and temporary endoscopic stent placement should be considered. However, life-threatening sequelae such as mediastinitis or tension pneumothorax or pneumomediastinum can occur. It is common practice to use carbon dioxide for luminal insufflation during ER, since it is more rapidly reabsorbed and eliminated from the body than regular air.
Summary and conclusions
ER is an effective, minimally invasive treatment option with the potential for organ-preserving curative for some patients with early esophageal cancer. The key to achieving oncologic outcomes comparable to surgical esophagectomy lies in careful selection of patients who are deemed to have a very low risk of lymph node or distant metastasis. Of the ER options for early esophageal cancer, EMR is acceptable for smaller lesions and has the advantages of faster learning curve, shorter procedure time, and fewer complications compared to ESD. ESD is much more challenging and requires significantly greater technical proficiency but is the best endoscopic strategy when en bloc resection of larger mucosal lesions is desired, as this optimizes the ability of the pathologist to provide the most accurate histopathologic diagnosis and staging.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Abbas E. Abbas and Roman V. Petrov) for the series “New Technologies in Esophageal Surgery and Endoscopy” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-30/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-30/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. JLT reports no reception of significant/actual COI with the article, but reports receiving consulting fees from Fujifilm Endoscopy, USA; honoraria to American Society for Gastrointestinal Endoscopy (ASGE); prior chair of ASGE Publication Committee, and past-president of Delaware Valley Society for Gastrointestinal Endoscopy. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Key Statistics for Esophageal Cancer. Available online: https://www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html
- Tachibana M, Kinugasa S, Dhar DK, et al. Prognostic factors in T1 and T2 squamous cell carcinoma of the thoracic esophagus. Arch Surg 1999;134:50-4. [Crossref] [PubMed]
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Standards of Practice Committee. Endoscopic eradication therapy for patients with Barrett's esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907-931.e9. [Crossref] [PubMed]
- Pohl J, May A, Rabenstein T, et al. Computed virtual chromoendoscopy vs. conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett's esophagus: a prospective randomized crossover study. Gastrointest Endosc 2007;65:AB348. [Crossref]
- Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001;49:534-9. [Crossref] [PubMed]
- Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 2008;14:1479-90. [Crossref] [PubMed]
- Li Z, Rice TW. Diagnosis and staging of cancer of the esophagus and esophagogastric junction. Surg Clin North Am 2012;92:1105-26. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett's esophagus: a meta-analysis. Gastrointest Endosc 2014;79:233-241.e2. [Crossref] [PubMed]
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-660.e1. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Sharma P, Shaheen NJ, Katzka D, et al. AGA Clinical Practice Update on Endoscopic Treatment of Barrett's Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020;158:760-9. [Crossref] [PubMed]
- Manner H, May A, Pech O, et al. Early Barrett's carcinoma with "low-risk" submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol 2008;103:2589-97. [Crossref] [PubMed]
- Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol 2013;11:630-5; quiz e45. [Crossref] [PubMed]
- Shimamura Y, Iwaya Y, Kobayashi R, et al. Clinical and pathological predictors of failure of endoscopic therapy for Barrett's related high-grade dysplasia and early esophageal adenocarcinoma. Surg Endosc 2021;35:5468-79. [Crossref] [PubMed]
- Draganov P, Gotoda T, Yang, D. Advanced Techniques for Endoscopic Resection in the GI Tract (Textbook). 1st edition. Publisher Slack Incorporated, 2019.
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus 2019;16:25-43.
- Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16-25.e1. [Crossref] [PubMed]
- Tanaka T, Ueno M, Iizuka T, et al. Comparison of long-term outcomes between esophagectomy and chemoradiotherapy after endoscopic resection of submucosal esophageal squamous cell carcinoma. Dis Esophagus 2019;32:doz023. [Crossref] [PubMed]
- Minashi K, Nihei K, Mizusawa J, et al. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2019;157:382-390.e3. [Crossref] [PubMed]
- Bollschweiler E, Baldus SE, Schröder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006;38:149-56. [Crossref] [PubMed]
- Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy 2007;39:41-5. [Crossref] [PubMed]
- Inoue H, Endo M. Endoscopic esophageal mucosal resection using a transparent tube. Surg Endosc 1990;4:198-201. [Crossref] [PubMed]
- Inoue H, Endo M, Takeshita K, et al. Endoscopic resection of early-stage esophageal cancer. Surg Endosc 1991;5:59-62. [Crossref] [PubMed]
- Inoue H, Endo M, Takeshita K, et al. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC) Surg Endosc 1992;6:264-5. [Crossref] [PubMed]
- Draganov PV, Gotoda T, Chavalitdhamrong D, et al. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013;78:677-88. [Crossref] [PubMed]
- Guo HM, Zhang XQ, Chen M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol 2014;20:5540-7. [Crossref] [PubMed]
- Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [Crossref] [PubMed]
- Chevaux JB, Piessevaux H, Jouret-Mourin A, et al. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett's neoplasia. Endoscopy 2015;47:103-12. [PubMed]
- Barret M, Beye B, Leblanc S, et al. Systematic review: the prevention of oesophageal stricture after endoscopic resection. Aliment Pharmacol Ther 2015;42:20-39. [Crossref] [PubMed]
- Park HC, Kim DH, Gong EJ, et al. Ten-year experience of esophageal endoscopic submucosal dissection of superficial esophageal neoplasms in a single center. Korean J Intern Med 2016;31:1064-72. [Crossref] [PubMed]
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and Oncologic Outcomes of Endoscopic Resection for Superficial Esophageal Neoplasm. Gut Liver 2015;9:470-7. [Crossref] [PubMed]
Cite this article as: Wander P, Tokar JL. Endoscopic management of early esophageal cancer: a literature review. Ann Esophagus 2023;6:16.


