
Clinical applications of esophageal stents
Introduction
It is generally considered that the word “stent” is attributed to Dr. Charles Stent, an English dentist from Brighton, England in 1856 (1). Although in 1845 there was the first report of use of an esophageal prosthesis (2). Since that time the materials from which they are constructed evolved from ivory to silastic to nickel-titanium alloy (i.e., Nitinol) to 3-D printed and even biodegradable. In 1969, Dr. Charles Dotter reported using coaxial tubes made of a nickel-titanium alloy for intra-arterial stenting (3). This technology was eventually applied to esophageal stents, which until then had been silastic tubes. Now, nitinol is the most commonly used material and is found in all esophageal self-expanding metal stents (SEMS). Its unique property of being able to be coiled to a very small size that expands to an even greater diameter upon warming of the stent to greater than 30 ℃ and at the same time increases in stiffness with increasing temperature is particularly beneficial with malignant dysphagia. Additionally, synthetic polymers have been developed to cover the stent, which initially was to prevent in-growth of tumor, but has also helped to greatly expanded the clinical uses.
Since an initial experience published in the New England Journal of Medicine in 1993 on the use of esophageal stents for the palliation of obstruction in inoperable esophageal cancer (4), their use in treatment of other conditions, such as esophageal perforation, has also expanded (5). Numerous studies on the use of stents based upon type and indication have been published (6).
Nowadays, endoluminal stents are routinely placed for the treatment of esophageal perforations, fistula, strictures, and intrathoracic anastomotic leaks. While all the uses, pitfalls, and successes of endoluminal esophageal stents is not able to be fully covered in one publication, we hope to provide an overview of their use for various clinical scenarios, the pros and cons, as well as technical aspects of stent placement. Finally, advances in technology will undoubtedly continue to broaden the use of endoluminal esophageal stents for the coming years. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-29/rc).
Stent types
Regardless of why a stent is placed, the primary goal is to restore patency and allow oral alimentation. There are numerous stent options currently in use stemming from various companies around the world. Each one has purported advantages to include stent coating to inhibit tumor ingrowth, maintain positioning, ease extraction, and various other benefits. In recent history, the surgical community has seen the development of self-expanding plastic stents (SEPS) and self-expanding metal stents (SEMS) with the latter being more commonly used nowadays. These self-expanding models allow for appropriate implantation without the need for prior esophageal dilation which can pose further risk of complication.
Beyond these two main types of self-expanding stents, there are three different types of SEMS separated by the extent of a silicone or polyurethane coverage: fully, partially, and uncovered. The most common coverage material used is polytetrafluoroethylene. The main difference between fully covered and partially covered stents is that partially covered ones have a minimal amount of metal or plastic exposed at the proximal and distal ends of the stent. This allows for increased imbedding into the wall of the esophagus and thus inhibits migration compared to fully covered stents. Stents with no coverage have all metal or plastic base structure exposed along the entirety of the stent and have less risk of migration but allow for tumor ingrowth or do not cover a perforation or fistula opening in the esophagus. While there is no “one type fits all” answer to esophageal stenting, consideration of the goals of treatment in addition to oral alimentation may help to guide stent choice.
Esophageal stents in benign disease
Self-expanding stents have consistently increased in use for benign esophageal diseases since the early 2000’s. Compared to malignant esophageal disease; benign disease commonly requires a more nuanced approach. Due to the varying cause of benign esophageal disease, conservative and surgical care varies greatly depending on each individual patient presentation. This coincides with the use of stents as a treatment option, but positive long-term outcomes are seen in the use of stents in such cases specifically when stent coverage and type is chosen carefully (5).
A stricture can develop from inflammatory, congenital, iatrogenic, or neuromuscular causes. Sometime, the etiology is multifactorial (7). These present clinically as dysphagia when the narrowing of the luminal diameter is 12 mm or less. This is usually treated with simple dilation. However, strictures can be more complex or recur and sometimes stents are used to maintain the esophageal lumen after dilation as part of the treatment strategy in hopes the stent decreases the risk of stricture recurrence while it is healing and remodeling after the dilation. However, practical results have been less predictable (8).
Current recommendations actually recommend AGAINST the use of SEMS as a first-line therapy for benign strictures mainly due to adverse events (9). However, for refractory strictures, as defined by Kochman (10), that are unable to reach 14 mm diameter after biweekly dilations over 5 weeks or failure to maintain an internal lumen of 14 mm for 4 weeks since the last dilation, temporary use of a SEMS can be considered. Finally, data combining treatment technique of a dilation with corticosteroid injection or endoscopic incision therapy with a stent does not show a clear benefit (11).
Esophageal stents in malignant disease
Esophageal cancer is currently the 6th leading cause of cancer-related deaths globally and accounted for over 544,000 deaths worldwide (12). In the United States, it is estimated that there will be 19,260 new cases and 15,530 deaths in 2021 (13). As much as 50% of cases are metastatic at the time of presentation. The presumed reason for this is that dysphagia is usually not reported until roughly 50% of the luminal diameter has been compromised. As such, dysphagia often suggests more advanced disease (14).
Malignant dysphagia can be associated with a partial or complete obstruction of the esophagus. This obstruction significantly impairs a patient’s ability to maintain appropriate nutrition while the neoplastic disease process further depletes patient’s reserves. Therefore, the presentation of patients with obstructive esophageal tumors often are plagued by severe malnutrition and weight loss. Regardless of clinical stage of the disease, the malnutrition must be addressed first. This brings the decision for stent early in the evaluation of a patient.
A comprehensive discussion with the patient to include attempts to objectively characterize the dysphagia prior to the endoscopy will eliminate the “surprise finding” of a near completely obstructing tumor and allows for an appropriate discussion about stent placement (15). Patients with grade 3 or greater dysphagia (Table 1) are very likely to require an early intervention to address nutrition status.
Table 1
| Score | Description |
|---|---|
| 0 | No dysphagia, normal swallowing |
| 1 | Able to swallow some solid foods |
| 2 | Able to swallow only semi-solid foods |
| 3 | Able to swallow only liquids |
| 4 | Unable to swallow anything |
Adapted from Ogilvie et al. Gut 1982 (15).
At the time of initial endoscopy, if the scope is unable to pass the tumor, a stent should be immediately considered and placed. The other option would be some type of feeding access, either transnasal or enteral, however their limitations have to be weighed against their benefits. These can include delays due to healing or complications, leading to postponements in initiation systemic therapy. Compared to esophageal stents which provide an almost immediate solution that allows for natural enteral feeding.
In patients undergoing radiation as part of their treatment, the dysphagia from mucositis seems to be less in patients with stents in place (16). However, stents can migrate which is mainly due to “melting away” of the luminal portion of the tumor restricting the stent in place, prompting retrieval prior to esophagectomy. Additionally, irritation, discomfort, and perforation have been reported in esophageal cancer patients with stents.
It is important to note that once a stent is placed, endoscopic ultrasound (EUS) cannot be successfully performed through the stent (14). After a tissue diagnosis is established, the remainder of the oncologic workup proceeds along standard guidelines. In patients with dysphagia were an EUS cannot be passed easily, the added value from the information provided by EUS may not change the overall treatment strategy (14). Determination of the presence or absence of metastatic disease should occur prior to any physiological testing to determine if a patient is fit for surgery.
Patients with dysphagia and known metastatic esophageal cancer will be treated with definitive chemoradiotherapy. In these patients with severe dysphagia with the inability to partake in oral alimentation, the placement of a self-expanding metal stent (SEMS) quickly restores their ability to eat. By prioritizing nutritional maintenance, patients have better outcomes, less complications, and improved overall quality of life (17,18).
Specific situations
Anastomotic leaks
Anastomotic leak after esophagectomy is one of the most concerning complications and is reported to occur 7–12% of the time (19) and a contributing factor in up to 40% of post-operative deaths (20). While there is no substitute for excellent surgical technique, even in the best hands this complication occurs. Now with the use of covered SEMS, as soon as a leak is detected a stent can be placed. This immediately limits contamination of the mediastinum and may allow for resumption of oral alimentation. The patient is then followed clinically and after 4–6 weeks they return for removal of the stent and evaluation of healing of the anastomosis (21).
Initial reports (22) demonstrated very high success rates of treatment of these leaks approaching 100%. Since then, subsequent analyses demonstrate the clinical success of esophageal stents in the management of anastomotic leak is around 80% (23).
One of the questions that arises is the timing of the removal of the stent. While the standard recommendation is 4–6 weeks, a review of a prospectively collected database demonstrated that for anastomotic leak, removal of the stent 2 weeks after placement did not compromise clinical success and reduced the risk of complications by 56%, compared to those left in for greater than 2 weeks (24).
Perforation of the Esophagus
The reported incidence of esophageal perforation is between 3–6 cases per one million people per year based upon estimates from single institution reviews and national databases (25-28). Perforations can occur anywhere along the esophagus, but the middle and distal esophagus account for upwards of 80% of all perforations (25). It is estimated that up to a third of patients do not have an underlying pathology or may be due to a benign stricture or may be the result of excessive retching or vomiting (i.e., Boerhaave syndrome). In the remaining instances it may be iatrogenic, due to underlying malignancy, foreign body (e.g., dentures), food impaction, or trauma. Regardless of the cause, esophageal perforation requires urgent management as it is associated with a mortality rate as high as 25% even with treatment and even higher without, mainly due to a sepsis (29-32).
Iatrogenic perforations usually occur during esophageal dilation or instrumentation. It is reported to occur at a rate of between 0.1–3% (33-35). Balloon dilation for achalasia has the highest risk of iatrogenic esophageal perforation. If perforation is immediately recognized, a covered SEMS can be immediately utilized to decrease the risk of mediastinal soilage. While the treatment paradigm of esophageal perforations has changed with the advent of covered SEMS, it is still imperative to consult early with a thoracic surgeon. The patient should be started on appropriate antibiotics, antifungals, and admitted to the hospital (29).
Utilization of covered SEMS has decreased the need for surgery to repair iatrogenic perforations of the esophagus (30) (Figure 1). In cases that are successfully managed in this fashion, the stent is usually left in place for about 4–6 weeks. However, a recent paper questions this dogma. Patient with acute perforations managed with a stent had them removed prior to 4 weeks (mean of 19+/-16 days) with a 95% leak resolution rate (24). This did not statistically effect resolution of the leak compared to stents left in for greater than 28 days but did decrease stent related complications by 39%. It is our practice to admit the patient to the hospital for observation on the day of their stent removal. If same day contrast radiographs do not demonstrate a leak and the patient remains afebrile while resuming a regular diet they are discharged to home. While some authors feel that an overnight observation is not required, it is felt to take into account the false negative rate of contrast esophagram, which is reported to be around 5–10% (21,36).

Esophageal fistula
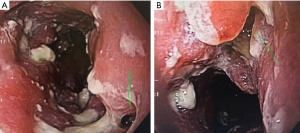
An esophageal fistula is rare but can occur in both the benign and malignant settings (Figure 2A,2B). They can also be the resulting complication of other treatments such as radiation, dilation, or prior stent placement (37). Regardless of the etiology, a stent can often be utilized as a bridge to palliative or definitive care.
The first consideration is to determine if the underlying condition is malignant and if there are definitive surgical options that can be undertaken urgently thus obviating the need for a stent. A stent that was placed prior to induction therapy for esophageal cancer that erodes through the wall of the esophagus with perforation into the pleural space, peritoneal space, mediastinum, or pericardium have all been described (38,39). In these scenarios, the stent may have been the nidus for the fistula and a replacement stent may not be a viable option. In cases where fistula occur with esophageal cancer and a stent is not involved, it is considered “acquired” and occur in about 5–15% of all esophageal cancer patients (40,41). By definition these patients are T4 tumors and are most likely stage 4, based upon the current 8th AJCC staging manual, and very likely have metastatic spread. However, placement of a palliative stent may allow time for additional workup and discussion with the patient about the limited options available. These are very challenging cases with poor overall survival (42). In our experience, even without gross evidence of metastatic dissemination, it becomes apparent within weeks to months.
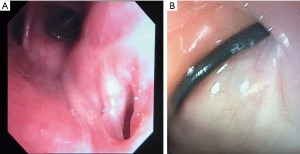
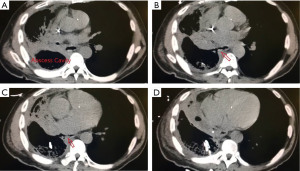
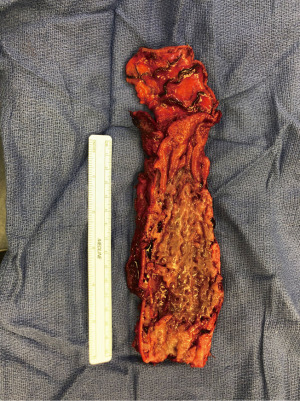
The other group is where an esophageal fistula develops in the face of benign disease. Reports of fistula between the esophagus and the lung, airways (Figure 3A,3B), and pleural space (Figure 4A-4D) have all been published with etiologies that range from Crohn’s disease (Figure 5), infectious agents, and complications of radiation. In these cases, the stent can be used to stop or limit soiling of extra-esophageal organs or tissue. It may allow for resumption of oral alimentation. Oral alimentation is usually confined to “comfort eating” and should not be relied on for complete caloric intake. As such, these patients usually require placement of surgical feeding access, with jejunostomy tubes the preferred type. Swallow studies should be performed to ensure that the oral feeds do not reflux into the esophagus and leak, similar to a type 1 vascular retrograde endoleak (Figure 6). Some etiologies (e.g., Crohn’s Disease) are able to undergo standard therapy that may result in healing of the fistula and not require esophagectomy. However, halting soilage of adjunct tissue and organs with a stent must be part of the treatment paradigm. In cases where there are no medical therapies or there has been failure of medical treatment, the patient should be nutritionally optimized and then esophagectomy can be undertaken.
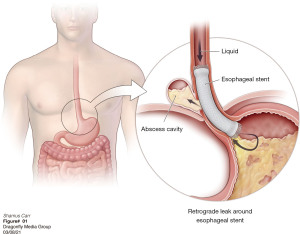
In patients with an esophageal fistula regardless of it being related to malignant or benign disease, esophagectomy with or without reconstruction may eventually be required. There is a limited amount of literature detailing issues with this challenging scenario, and some support immediate reconstruction in select circumstances (43).
Techniques for stent placement
There are currently three common methods for placement of an esophageal stent: fluoroscopy assisted, side-by-side technique, or through the scope (TTS). There are no studies that demonstrate superiority of any technique each with pros and cons. The first step of any esophageal stent placement is to perform an upper endoscopy. This allows for identification of the concerning pathology and its location. This is important as issues in the very proximal esophagus are sometimes not amenable to stenting. A good rule of thumb is to never have the proximal end of the stent above (proximal) to the upper esophageal sphincter (UES). In fact, in the packaging information of every esophageal stent there is a warning about placement near the UES. For example, Boston Scientific specifically states in the package information of the WallflexTM esophageal stent that a contraindication is “within 2 cm of the cricopharyngeal muscle”.
Once the pathology in question is identified, a guidewire is placed through the working channel of the endoscope. Most commonly a 0.035-inch guidewire is used, in some cases a wire is not required with the TTS technique. If not performing the TTS technique, the endoscope is removed while leaving the guidewire in place. Below are the commonly used techniques to place a stent over a wire. Familiarity with all are important but having one technique being almost “second nature” is invaluable. There is also a hybrid approach combing the fluoroscopy with the side-by-side technique. The most recently developed option is TTS technique (44), where the stent delivery device is placed directly through the working channel of the endoscope.
Fluoroscopy assisted
With the aid of an endoscope, fluoroscopy is utilized for placement of external markers at the site of the pathology in question. While many use a paperclip or other instrument as the external marker, the authors utilize a 1.4 mm Kirschner wire cut to 7 cm with rubber shod over each end (Figure 7). The marker can then be taped to the skin so that it directly overlies the end of the inserted endoscope. After placement of all markers, the endoscope is now removed leaving the guidewire with the distal end of it well past the pathology to be stented. The stent is then brought over the wire in the standard fashion utilizing real-time fluoroscopy to place it at the desired position in the esophagus. Then it is deployed under real-time imaging using the external markers to ensure proper position of the esophageal stent. Once the stent is completely deployed, the delivery device and guidewire are removed. Repeat endoscopy can be done to visually confirm the fluoroscopic images and further check stent placement.
Side-by-side technique
With the guidewire in place the stent is brought into position in the esophagus. The endoscope is re-inserted into the esophagus. Utilizing the external markers on the stent delivery device, the delivery device is placed in the desired position and the stent is deployed under direct endoscopic visualization. Adjustments to the stent can be made based upon what the endoscopist visualizes. Once fully deployed, the stent delivery device and guidewire are removed. As the endoscope is in the esophagus, visualization and confirmation of correct placement of the stent is obtained. Slight adjustments can be made as necessary.
Through the scope technique (TTS)
Depending on the brand of TTS stent, a wire may or may not be able to be used. Either way, as opposed to the traditional over the wire deployment, a TTS esophageal stent is deployed from a sheath that is passed through the working channel of the endoscope. The purported benefit is that similar to the side by side technique (see above) the proximal end of the stent can be visualized directly from the endoscope during deployment. This in theory allows for more precise placement of the stent. There are very limited studies since the initial report in an animal model of this technology (44).
In one published multicenter trial, placement of esophageal TTS stents was feasible in patients with malignant dysphagia, but was only able to be done without a guidewire or fluoroscopy a third of the time and complications still occurred in about two-thirds of patients (45). The most common complication being recurrent dysphagia due to stent migration (16%), tissue overgrowth (13%), or stent deformation (13%). Another slight downside of a TTS stent is the maximal outer diameter is 20 mm, compared to 23 mm for traditional SEMS. While the overall implication of this small difference is unclear, it does warrant future studies looking at stent migration rates, relief of dysphagia, associated retrosternal pain, and need for additional procedures to see if there is an objective difference.
Hybrid technique
One can see how fluoroscopy can be combined with either the side-by-side or TTS technique to allow for both real-time endoscopic and fluoroscopic visualization during stent deployment.
Complications of esophageal stents and postoperative management
Complications after stent placement are classified as early or delayed presentation. Early complications usually arise between either immediately after placement, or within 1–2 weeks postoperatively. Minimal postoperative bleeding is not uncommon while more severe early complications such as chest pain, fever, perforation, reflux difficulties, and migration do arise. Delayed complications are found to be more common than early ones and range anywhere from four weeks to several months postop (46). These issues include occlusion, recurrent strictures, stent invasion by the present tumor, erosion through the esophagus, and the most common complication being overall stent migration within the esophagus. Stent migration has a reported rate of between 24–40% (8,47). This rate differs based upon type of sent (SEPS versus SEMS) with SEMS have the lowest reported average of any migration rate of 26%. However, clinically relevant migration occurs at about 17% (48).
In attempts to reduce the risk of stent migration, various endoscopic techniques have been investigated to improve fixation of the esophageal stent (49-54). This is mainly done with either endoscopic suturing or over-the-stent clips. All recent studies show a benefit to either or the two techniques and reduce migration. A recent meta-analysis of 14 studies confirms these smaller studies results. However, it remains unclear of the benefit of routine fixation on initial placement of a stent. Current guidelines recommend stent fixation only in patients with prior migration who still require a stent (9).
Patients can also complain of constant pressure or pain in the chest but can also note globus or bleeding. Generally, it is self-limited and either become tolerable by the patient or resolves. Replacing the current stent with a slightly smaller diameter stent can be a first step. The risk being a higher migration rate. However, sometimes the pain will be unrelenting, and the stent must be removed (55).
While rare, stents have been reported to erode through the wall of the esophagus with perforation into the pleural space, peritoneal space, mediastinum, or pericardium have all been described (46,56). This can lead to a multitude of complications depending on what in addition to the esophagus is injured. Generally, these types of complications are treated emergently and carry increased rates of morbidity and mortality. They may require complete revision of the stent placement or attempted repair of the fistula, which may entail esophagectomy with diversion or without reconstruction and eventual extraanatomical reconstruction.
Future applications
Biodegradable stents
A recent advancement in stent technology is the creation of biodegradable esophageal stents (BDS). They are generally constructed from a woven polydioxanone monofilament that degrades by hydrolysis that deploys similar to SEMs. Since they eventually completely degrade, these stents are recommended for use with benign esophageal strictures so that the patient does not require a second procedure to remove the stent. While these stents fully disintegrated in 3–6 months after deployment, the radial tension that maintains the stents patency does begin to decrease after about 4 weeks. Currently, there is only one BDS available on the market, the SX-ELLA BDS (ELLA-CS, Hradec Králové, Czech Republic) (Figure 8).

There have been a small number of studies and 2 meta-analysis that compare BDS to other treatment modalities for benign esophageal strictures (8,47,57-61). The general conclusion of these studies is that various interventions combined with non-biodegradable stents and BDS both offer similar moderate long-term dysphagia relief, and both are superior to interventions when a stent is not part of the treatment paradigm. However, BDS required significantly fewer re-interventions and lower migration rates, but may have a slightly higher complications rate associated with bleeding (Table 2). While the currently available literature possibly demonstrates an apparent role for BDS, high quality studies are lacking, and more robust evidence is required to firmly establish the role of BDS in the treatment of esophageal pathologies.
Table 2
| Study | Study type | Participants (n) | Clinical success | Complication | Migration | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate | P | Rate | P | Rate | P | |||||
| van Boeckel et al. (60) | Single center, retrospective | BDS: 18 | 33% | 0.83 | 38.8% | 0.09 | 22.2% | 0.85 | ||
| SEPS: 20 | 30% | 15% | 25% | |||||||
| Canena et al. (61) | Multi-center prospective, observational | BDS: 10 | 30% | 0.27 | 50% | 0.38 | 20% | 0.16 | ||
| SEPS: 10 | 10% | 70% | 60% | |||||||
| SEMS: 10 | 40% | 60% | 30% | |||||||
| Fuccio et al. (8) | Meta-analysis (18 studies) | Total: 444 | 40.5% | 20.6% | 28.6% | |||||
| BDS: 77 | 32.9% | 21.9% | 15.3% | |||||||
| SEPS: 140 | 46.2% | 19.4% | 33.3% | |||||||
| SEMS: 227 | 40.1% | 21.9% | 31.5% | |||||||
| van Halsema et al. (47) | Pooled analysis (8 studies) | Total: 232 | 24.2% | 31.0% | 24.6% | |||||
| BDS: 77 | 32.9% | 38.9% | 14.3% | |||||||
| SEPS: 70 | 27.1% | 25.7% | 27.1% | |||||||
| SEMS: 85 | 14.1% | 28.2% | 31.8% | |||||||
Clinical success did not have a universal definition, but generally was described as resolution of dysphagia (Grade 0−1) at study completion at follow-up (median 3–15 months). BDS, biodegradable stent; SEPS, self-expanding plastic stent; SEMS, self-expanding metal stent.
Anti-reflux self-expanding stent
As stents are now the first-choice treatment for palliation of dysphagia and the majority of pathology is in the distal esophagus, it is quite common for the esophageal stent to cross the gastroesophageal junction. While this relieves dysphagia, a common complaint is symptomatic acid reflux. This has been shown to occur in 45% of patients with unresectable esophageal cancer treated with an esophageal stent (62).
The same company that invented the biodegradable stent had previously produced a commercially available anti-reflux stent (ARS) in an attempt at addressing the dysphagia in these patients while preventing symptomatic reflux (63). There have been numerous small studies that have shown varied results (64-67). Some studies did demonstrate a benefit to ARS (65), while others did not find significant difference in reflux symptoms. This may have been due to varied design of the trials or the small size of patients resulting in an underpowered study. An early meta-analysis demonstrated did not observe a difference in reflux between ARS and SEMS (68). More recently, a larger pooled analysis (69) validated similar earlier studies with the addition of additional data and continued to show no significant difference between ARS and a covered SEMS, especially when anti-reflux medication was added (66). In fact, variations in stent length may have more of an impact then the type of stent used (67), with the conclusion being longer stents are associated with less reflux symptoms compared to shorter stents.
3D-printed stents
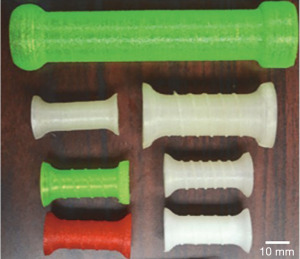
The initial patent for three-dimensional printing (3D-printing) was in 1986. Since then, advances in technology and increased access have turned 3D-printing from an exotic manufacturing strategy by specialized companies to easily attainable technology. 3D-printing has been successfully utilized for central airway stents in the treatment of obstruction (70). More recently the same technology has been used for esophageal stents (Figure 9). Additional small pilot studies have further shown the proof of concept for 3D-printed esophageal stenting (71,72). The clear benefits allow for individualized design for unique problems that can accommodate limitless sizes and shapes. However, further studies are required to see if this technology can be widely applicable and economical.
Drugs/tissue engineered stents
While using esophageal stents for palliation of dysphagia caused by esophageal cancer is common, the use of anticancer drugs incorporated into the stent is a new development. The idea it to try and improve palliation and overall survival of these patients with direct drug delivery to the tumor. Studies have found that customized 3D-printed stents may be incorporated at the time of fabrication with specific drugs that may provide a route for direct drug delivery within the esophagus (73). Because the concept of drug treatment as an adjunct to stents is fairly novel, additional development and research is required to understand their costs and benefits. These therapies are expected to advance parallel to the development of 3D stents.
The other area is the use of bio-3D printing system for the construction of tubular structures. This is done by growing specific cells in culture and creating multicellular spheroids. The spheroids are then organized into a tubular structure and placed into a bioreactor to mature over a few weeks. The mature organ has been successfully transplanted into small animal models (74). Finally, stents have been shown to be able to incorporate a decellularized extracellular matrix hydrogel to aid in healing of esophageal mucosa, but again this has only been done in small animal models (75).
Conclusions
Esophageal stent usage has significantly expanded since their introduction. Now they are routinely utilized for both malignant and benign esophageal disease with a proportionate increase in the evaluation of clinical outcomes. The introduction of self-expanding metal and plastic stents has greatly expanded the treatment paradigms for several diseases. At times, even supplanting surgery as the initial option. With the further introduction of biodegradable, adjunct imbedded, and 3D printed stents, the clinical outcomes of esophageal stent usage is expected to further improve. Yet, it is imperative that well designed studies be carried out to allow for objective measures to be evaluated then indiscriminate use. With both a detailed work-up and specific stent choice, self-expanding esophageal stents present as an attractive option for patients with all types of esophageal disease.
Acknowledgments
The authors would like to thank Dr. Eric Krause, JD, MD for his assistance obtaining many of the figures.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Abbas E. Abbas and Roman V. Petrov) for the series “New Technologies in Esophageal Surgery and Endoscopy” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-29/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-29/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-29/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. SRC reports personal fees (royalties as an author and reviewer) from UpToDate, Inc., outside the submitted work; and Associate Research Physician at National Institutes of Health, National Cancer Institute, Thoracic Surgery Branch. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ring ME. How a dentist's name became a synonym for a life-saving device: the story of Dr. Charles Stent. J Hist Dent 2001;49:77-80. [PubMed]
- Vermeulen BD, Siersema PD. Esophageal Stenting in Clinical Practice: an Overview. Curr Treat Options Gastroenterol 2018;16:260-73. [Crossref] [PubMed]
- Payne MM. Charles Theodore Dotter. The father of intervention. Tex Heart Inst J 2001;28:28-38. [PubMed]
- Knyrim K, Wagner HJ, Bethge N, et al. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med 1993;329:1302-7. [Crossref] [PubMed]
- Thornblade LW, Cheng AM, Wood DE, et al. A Nationwide Rise in the Use of Stents for Benign Esophageal Perforation. Ann Thorac Surg 2017;104:227-33. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Dake M, et al. An analysis of esophageal stent placement for persistent leak after the operative repair of intrathoracic esophageal perforations. Ann Thorac Surg 2014;97:1715-9; discussion 1719-20. [Crossref] [PubMed]
- Ravich WJ. Endoscopic Management of Benign Esophageal Strictures. Curr Gastroenterol Rep 2017;19:50. [Crossref] [PubMed]
- Fuccio L, Hassan C, Frazzoni L, et al. Clinical outcomes following stent placement in refractory benign esophageal stricture: a systematic review and meta-analysis. Endoscopy 2016;48:141-8. [PubMed]
- Spaander MCW, van der Bogt RD, Baron TH, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy 2021;53:751-62. [Crossref] [PubMed]
- Kochman ML, McClave SA, Boyce HW. The refractory and the recurrent esophageal stricture: a definition. Gastrointest Endosc 2005;62:474-5. [Crossref] [PubMed]
- Wilson JL, Louie BE, Farivar AS, et al. Fully covered self-expanding metal stents are effective for benign esophagogastric disruptions and strictures. J Gastrointest Surg 2013;17:2045-50. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Ripley RT, Sarkaria IS, Grosser R, et al. Pretreatment Dysphagia in Esophageal Cancer Patients May Eliminate the Need for Staging by Endoscopic Ultrasonography. Ann Thorac Surg 2016;101:226-30. [Crossref] [PubMed]
- Ogilvie AL, Dronfield MW, Ferguson R, et al. Palliative intubation of oesophagogastric neoplasms at fibreoptic endoscopy. Gut 1982;23:1060-7. [Crossref] [PubMed]
- Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119-26. [PubMed]
- Bower M, Jones W, Vessels B, et al. Nutritional support with endoluminal stenting during neoadjuvant therapy for esophageal malignancy. Ann Surg Oncol 2009;16:3161-8. [Crossref] [PubMed]
- Miller KR, Bozeman MC. Nutrition therapy issues in esophageal cancer. Curr Gastroenterol Rep 2012;14:356-66. [Crossref] [PubMed]
- Groth SS, Burt BM. Minimally invasive esophagectomy: Direction of the art. J Thorac Cardiovasc Surg 2021;162:701-4. [Crossref] [PubMed]
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71-5. [PubMed]
- Hu Z, Wang X, An X, et al. The Diagnostic Value of Routine Contrast Esophagram in Anastomotic Leaks After Esophagectomy. World J Surg 2017;41:2062-7. [Crossref] [PubMed]
- Schubert D, Scheidbach H, Kuhn R, et al. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc 2005;61:891-6. [Crossref] [PubMed]
- Dasari BV, Neely D, Kennedy A, et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 2014;259:852-60. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Dake M, et al. An Assessment of the Optimal Time for Removal of Esophageal Stents Used in the Treatment of an Esophageal Anastomotic Leak or Perforation. Ann Thorac Surg 2015;100:422-8. [Crossref] [PubMed]
- Søreide JA, Konradsson A, Sandvik OM, et al. Esophageal perforation: clinical patterns and outcomes from a patient cohort of Western Norway. Dig Surg 2012;29:494-502. [Crossref] [PubMed]
- Ryom P, Ravn JB, Penninga L, et al. Aetiology, treatment and mortality after oesophageal perforation in Denmark. Dan Med Bull 2011;58:A4267. [PubMed]
- Bhatia P, Fortin D, Inculet RI, et al. Current concepts in the management of esophageal perforations: a twenty-seven year Canadian experience. Ann Thorac Surg 2011;92:209-15. [Crossref] [PubMed]
- Vidarsdottir H, Blondal S, Alfredsson H, et al. Oesophageal perforations in Iceland: a whole population study on incidence, aetiology and surgical outcome. Thorac Cardiovasc Surg 2010;58:476-80. [Crossref] [PubMed]
- Søreide JA, Viste A. Esophageal perforation: diagnostic work-up and clinical decision-making in the first 24 hours. Scand J Trauma Resusc Emerg Med 2011;19:66. [Crossref] [PubMed]
- Minnich DJ, Yu P, Bryant AS, et al. Management of thoracic esophageal perforations. Eur J Cardiothorac Surg 2011;40:931-7. [PubMed]
- Mavroudis CD, Kucharczuk JC. Acute Management of Esophageal Perforation. Curr Surg Rep 2013;2:34. [Crossref]
- Port JL, Kent MS, Korst RJ, et al. Thoracic esophageal perforations: a decade of experience. Ann Thorac Surg 2003;75:1071-4. [Crossref] [PubMed]
- Hagel AF, Naegel A, Dauth W, et al. Perforation during esophageal dilatation: a 10-year experience. J Gastrointestin Liver Dis 2013;22:385-9. [PubMed]
- Vanuytsel T, Lerut T, Coosemans W, et al. Conservative management of esophageal perforations during pneumatic dilation for idiopathic esophageal achalasia. Clin Gastroenterol Hepatol 2012;10:142-9. [Crossref] [PubMed]
- Ghoshal UC, Kumar S, Saraswat VA, et al. Long-term follow-up after pneumatic dilation for achalasia cardia: factors associated with treatment failure and recurrence. Am J Gastroenterol 2004;99:2304-10. [Crossref] [PubMed]
- Giménez A, Franquet T, Erasmus JJ, et al. Thoracic complications of esophageal disorders. Radiographics 2002;22:S247-58. [Crossref] [PubMed]
- Kachaamy T, Pannala R. Esophageal stents: when and how. Minerva Gastroenterol Dietol 2016;62:155-66. [PubMed]
- Saha BK. Few and Far Between: A Case of Aortoesophageal Fistula in Locally Advanced Esophageal Cancer. Am J Med Sci 2021;361:e3-4. [Crossref] [PubMed]
- Javaid T, Khan Z, Hasan S, et al. Esophago-pericardial fistula with development of hydro-pneumo-pericardium resulting in hemodynamic instability: an unusual complication of esophageal cancer. Intern Emerg Med 2018;13:959-60. [Crossref] [PubMed]
- Balazs A, Galambos Z, Kupcsulik PK. Characteristics of esophagorespiratory fistulas resulting from esophageal cancers: a single-center study on 243 cases in a 20-year period. World J Surg 2009;33:994-1001. [Crossref] [PubMed]
- Tsushima T, Mizusawa J, Sudo K, et al. Risk Factors for Esophageal Fistula Associated With Chemoradiotherapy for Locally Advanced Unresectable Esophageal Cancer: A Supplementary Analysis of JCOG0303. Medicine (Baltimore) 2016;95:e3699. [Crossref] [PubMed]
- Guan X, Liu C, Zhou T, et al. Survival and prognostic factors of patients with esophageal fistula in advanced esophageal squamous cell carcinoma. Biosci Rep 2020;40:BSR20193379. [Crossref] [PubMed]
- Altorjay A, Kiss J, Vörös A, et al. The role of esophagectomy in the management of esophageal perforations. Ann Thorac Surg 1998;65:1433-6. [Crossref] [PubMed]
- Cheon YK, Lee TY, Sung IK, et al. Clinical feasibility of a new through-the-scope fully covered esophageal self-expandable metallic stent: an in vivo animal study. Dig Endosc 2014;26:32-6. [Crossref] [PubMed]
- Vermeulen BD, Reijm AN, van der Bogt RD, et al. Through-the-scope placement of a fully covered metal stent for palliation of malignant dysphagia: a prospective cohort study (with video). Gastrointest Endosc 2019;90:972-9. [Crossref] [PubMed]
- Wang MQ, Sze DY, Wang ZP, et al. Delayed complications after esophageal stent placement for treatment of malignant esophageal obstructions and esophagorespiratory fistulas. J Vasc Interv Radiol 2001;12:465-74. [Crossref] [PubMed]
- van Halsema EE, van Hooft JE. Clinical outcomes of self-expandable stent placement for benign esophageal diseases: A pooled analysis of the literature. World J Gastrointest Endosc 2015;7:135-53. [Crossref] [PubMed]
- Thomas S, Siddiqui AA, Taylor LJ, et al. Fully-covered esophageal stent migration rates in benign and malignant disease: a multicenter retrospective study. Endosc Int Open 2019;7:E751-6. [Crossref] [PubMed]
- Watanabe K, Hikichi T, Nakamura J, et al. Feasibility of esophageal stent fixation with an over-the-scope-clip for malignant esophageal strictures to prevent migration. Endosc Int Open 2017;5:E1044-9. [Crossref] [PubMed]
- Yang J, Siddiqui AA, Kowalski TE, et al. Esophageal stent fixation with endoscopic suturing device improves clinical outcomes and reduces complications in patients with locally advanced esophageal cancer prior to neoadjuvant therapy: a large multicenter experience. Surg Endosc 2017;31:1414-9. [Crossref] [PubMed]
- Bick BL, Imperiale TF, Johnson CS, et al. Endoscopic suturing of esophageal fully covered self-expanding metal stents reduces rates of stent migration. Gastrointest Endosc 2017;86:1015-21. [Crossref] [PubMed]
- Irani S, Baron TH, Gluck M, et al. Preventing migration of fully covered esophageal stents with an over-the-scope clip device (with videos). Gastrointest Endosc 2014;79:844-51. [Crossref] [PubMed]
- Ngamruengphong S, Sharaiha RZ, Sethi A, et al. Endoscopic suturing for the prevention of stent migration in benign upper gastrointestinal conditions: a comparative multicenter study. Endoscopy 2016;48:802-8. [Crossref] [PubMed]
- Vanbiervliet G, Filippi J, Karimdjee BS, et al. The role of clips in preventing migration of fully covered metallic esophageal stents: a pilot comparative study. Surg Endosc 2012;26:53-9. [Crossref] [PubMed]
- Forootan M, Tabatabaeefar M, Mosaffa N, et al. Investigating Esophageal Stent-Placement Outcomes in Patients with Inoperable Non-Cervical Esophageal Cancer. J Cancer 2018;9:213-8. [Crossref] [PubMed]
- Homann N, Noftz MR, Klingenberg-Noftz RD, et al. Delayed complications after placement of self-expanding stents in malignant esophageal obstruction: treatment strategies and survival rate. Dig Dis Sci 2008;53:334-40. [Crossref] [PubMed]
- Dhar A, Close H, Viswanath YK, et al. Biodegradable stent or balloon dilatation for benign oesophageal stricture: pilot randomised controlled trial. World J Gastroenterol 2014;20:18199-206. [Crossref] [PubMed]
- Walter D, van den Berg MW, Hirdes MM, et al. Dilation or biodegradable stent placement for recurrent benign esophageal strictures: a randomized controlled trial. Endoscopy 2018;50:1146-55. [Crossref] [PubMed]
- Repici A, Vleggaar FP, Hassan C, et al. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc 2010;72:927-34. [Crossref] [PubMed]
- van Boeckel PG, Vleggaar FP, Siersema PD. A comparison of temporary self-expanding plastic and biodegradable stents for refractory benign esophageal strictures. Clin Gastroenterol Hepatol 2011;9:653-9. [Crossref] [PubMed]
- Canena JM, Liberato MJ, Rio-Tinto RA, et al. A comparison of the temporary placement of 3 different self-expanding stents for the treatment of refractory benign esophageal strictures: a prospective multicentre study. BMC Gastroenterol 2012;12:70. [Crossref] [PubMed]
- Włodarczyk JR, Kużdżał J. Stenting in Palliation of Unresectable Esophageal Cancer. World J Surg 2018;42:3988-96. [Crossref] [PubMed]
- Köcher M, Dlouhy M, Neoral C, et al. Esophageal stent with antireflux valve for tumors involving the cardia: work in progress. J Vasc Interv Radiol 1998;9:1007-10. [Crossref] [PubMed]
- Blomberg J, Wenger U, Lagergren J, et al. Antireflux stent versus conventional stent in the palliation of distal esophageal cancer. A randomized, multicenter clinical trial. Scand J Gastroenterol 2010;45:208-16. [Crossref] [PubMed]
- Power C, Byrne PJ, Lim K, et al. Superiority of anti-reflux stent compared with conventional stents in the palliative management of patients with cancer of the lower esophagus and esophago-gastric junction: results of a randomized clinical trial. Dis Esophagus 2007;20:466-70. [Crossref] [PubMed]
- Sabharwal T, Gulati MS, Fotiadis N, et al. Randomised comparison of the FerX Ella antireflux stent and the ultraflex stent: proton pump inhibitor combination for prevention of post-stent reflux in patients with esophageal carcinoma involving the esophago-gastric junction. J Gastroenterol Hepatol 2008;23:723-8. [Crossref] [PubMed]
- Wenger U, Johnsson E, Arnelo U, et al. An antireflux stent versus conventional stents for palliation of distal esophageal or cardia cancer: a randomized clinical study. Surg Endosc 2006;20:1675-80. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. The use of self-expanding stents in esophageal and gastroesophageal junction cancer palliation: a meta-analysis and meta-regression analysis of outcomes. Dig Dis Sci 2010;55:3018-30. [Crossref] [PubMed]
- Pandit S, Samant H, Morris J, et al. Efficacy and safety of standard and anti-reflux self-expanding metal stent: A systematic review and meta-analysis of randomized controlled trials. World J Gastrointest Endosc 2019;11:271-80. [Crossref] [PubMed]
- Cheng GZ, Folch E, Wilson A, et al. 3D Printing and Personalized Airway Stents. Pulm Ther 2017;3:59-66. [Crossref]
- Kang Y. A Review of Self-Expanding Esophageal Stents for the Palliation Therapy of Inoperable Esophageal Malignancies. Biomed Res Int 2019;2019:9265017. [Crossref] [PubMed]
- Lin M, Firoozi N, Tsai CT, et al. 3D-printed flexible polymer stents for potential applications in inoperable esophageal malignancies. Acta Biomater 2019;83:119-29. [Crossref] [PubMed]
- Fouladian P, Kohlhagen J, Arafat M, et al. Three-dimensional printed 5-fluorouracil eluting polyurethane stents for the treatment of oesophageal cancers. Biomater Sci 2020;8:6625-36. [Crossref] [PubMed]
- Takeoka Y, Matsumoto K, Taniguchi D, et al. Regeneration of esophagus using a scaffold-free biomimetic structure created with bio-three-dimensional printing. PLoS One 2019;14:e0211339. [Crossref] [PubMed]
- Ha DH, Chae S, Lee JY, et al. Therapeutic effect of decellularized extracellular matrix-based hydrogel for radiation esophagitis by 3D printed esophageal stent. Biomaterials 2021;266:120477. [Crossref] [PubMed]
Cite this article as: Wilson MK, Carr SR. Clinical applications of esophageal stents. Ann Esophagus 2023;6:18.



