A narrative review of endoscopic therapy for Barrett’s esophagus
Introduction
Barrett’s esophagus (BE) is a pathological condition in which specialized columnar epithelium replaces stratified squamous epithelium that normally delineates the distal part of the esophagus. The condition usually develops due to chronic gastroesophageal reflux disease (GERD). BE is considered a precancerous condition that can possibly progress to low-grade dysplasia (LGD), high-grade dysplasia (HGD) and subsequently adenocarcinoma. Factors such as age, race and gender increase the risk for development of abnormal specialized epithelium and possible progression to malignancy.
Historically, patients with BE were followed up closely by repeat endoscopies and biopsies in order to detect HGD and/or early cancer. Previously, HGD and cancer had one treatment option, which was esophagectomy.
Due to significant morbidity and mortality associated with esophagectomy, minimally invasive interventions have developed to hinder progression or to achieve curative resection, particularly in early stages of the disease. These interventions include radiofrequency ablation (RFA), cryoablation, endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD) or a combination. The choice of treatment is dependent on the grade of dysplasia and the morphology of BE. For LGD and HGD, EMR is indicated for the removal of any visible lesion smaller than 2 cm in size; ESD is indicated for the removal of any visible lesions larger than 2 cm in size. This should be followed by RFA of the residual flat Barrett’s epithelium with the goal of complete eradication of BE. If no visible lesions were seen in BE with LGD or HGD, then RFA is the modality of choice. For T1a esophageal cancer, EMR is indicated for lesions smaller than 2 cm in size; if the endoscopist is confident that the lesion can be removed entirely en bloc with clean margins. ESD is the preferred modality for resection of T1a esophageal cancer 2 cm or larger given the higher curative resection rate of ESD. Selective cases with T1b esophageal cancer can be treated by endoscopic resection if favorable pathologic features such as submucosal invasion <500 µm [sm1], well or moderately differentiated tumor, absent lymphovascular invasion are present. Endoscopic resection of T1b esophageal cancer should be determined on a case-by-case basis after multi-disciplinary tumor board discussion (Table 1). In this article, we will discuss in detail various endoscopic modalities for the treatment of BE. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-18/rc).
Table 1
| Non-dysplastic Barrett’s esophagus | Surveillance endoscopy every 3–5 years |
|---|---|
| Indefinite for dysplasia | Acid suppression therapy and repeat EGD in 3–6 months. |
| Low-grade dysplasia | EMR of visible lesions less than 20 mm and ESD of any visible lesions larger than 20 mm. |
| Endoscopic ablation therapy of residual flat BE segment with the goal of complete eradication on subsequent sessions. | |
| OR: EGD every 6–12 months with biopsies, tailor treatment to patient preferences. | |
| High-grade dysplasia | EMR of visible lesions less than 20 mm and ESD of any visible lesions larger than 20 mm. |
| Endoscopic ablation therapy of residual flat BE segment with the goal of complete eradication on subsequent sessions. | |
| T1a esophageal cancer | EMR of visible lesions less than 20 mm and ESD of any visible lesions larger than 20 mm. |
| Endoscopic ablation therapy of residual flat BE segment with the goal of complete eradication on subsequent sessions. | |
| T1b esophageal cancer | EMR or ESD of the visible lesion should be considered. If T1b confirmed and favorable pathologic features (negative margins, submucosal invasion <500 µm [sm1], well or moderately differentiated, absent lymphovascular invasion), can consider EET on case-by-case basis after multidisciplinary tumor board discussion. |
| If T1b sm2-3 (deeper submucosal invasion) or poor pathologic features, referral to surgical oncology for esophagectomy. |
BE, Barrett’s esophagus; EET, endoscopic eradication therapy; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; EGD, esophagogastroduodenoscopy; HGD, high-grade dysplasia; LGD, low-grade dysplasia.
Methods
Using PubMed, we performed a literature review of all published articles (from 1993 to 2021) focusing on endoscopic treatment of BE. The following terms were used: Barrett’s esophagus, radiofrequency ablation, endoscopic mucosal resection, endoscopic submucosal dissection. The search was limited to English language and excluded case reports.
Discussion
Radiofrequency ablation (RFA)
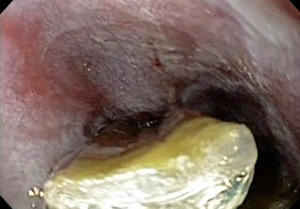
RFA is an endoscopic intervention to eradicate BE. A bipolar electrode mounted on a balloon or the scope tip is used to apply energy to the lesion to ablate (burn) the epithelium in a circumferential (using the balloon) or targeted manner (using the electrode tip, focal ablation) (1,2). Figure 1 illustrates esophageal mucosa post circumferential ablation.
Indications for RFA
High-grade flat lesions (HGD)
RFA requires adequate contact with the lesion to achieve the best outcome, thus RFA is usually used to ablate flat lesions in patients with BE and HGD. If successful, RFA prevents BE progression to cancer (3). RFA is highly effective and safe for flat lesions; however, nodular and visible lesions limit efficacy (4). If the patient has visible lesions, RFA can be combined with other modalities such as EMR or ESD to create a flat surface that is ideal for RFA application. This hybrid approach is used to ensure optimal outcome (5). In the landmark sham-controlled trial by Shaheen et al., complete eradication of HGD was achieved in 81% of patients with HGD randomized to the ablation arm compared to 19% of the control group (3). A retrospective study of 169 patients with BE and advanced neoplasia undergoing RFA for flat lesions with intramucosal cancer found focal endoscopic mucosal resection before radiofrequency ablation was equally effective and safe compared with radiofrequency ablation alone for the eradication of BE with advanced neoplasia (6).
Low-grade dysplasia (LGD)
Endoscopic treatment of LGD is associated with decreased progression to HGD and/or adenocarcinoma (7). Alternatively, surveillance of LGD could be implemented. Other factors including patient preference, age , comorbid conditions or length of Barrett’s segment should be considered before determining surveillance versus RFA in BE with LGD (8).
In a meta-analysis of 19 studies including a total of 2,746 patients, RFA of LGD was found to be safe and effective in limiting disease progression with absolute risk reduction of 10.9%. The cumulative rate of progression to HGD/Early Esophageal Adenocarcinoma (EAC) was lower in RFA compared with surveillance (1.7% vs. 12.6%, P<0.001) (7).
Cost-analysis studies found that RFA is the preferred approach for LGD (9). It should also be noted that treatment of dysplasia improved quality of life and patient perception (10).
Outcomes
Several studies, including well designed randomized studies, found that RFA is safe and effective in BE with an 80–100% complete eradication rate (2,3,5,6,11-25). One of these studies assessed a 3-year follow-up of 106 patients and found complete eradication of dysplasia at a rate of 95%, and no recurrence in 91% of patients (19).
Effect on quality of life
Quality of life following RFA of dysplastic BE assessed in a randomized trial of 127 patients, who received either ablation or sham therapy found improvement of patients’ quality of life secondary to perceived decreased of risk of cancer (10).
Recurrence and cancer risk
BE patients with LGD and HGD are at low risk of developing esophageal adenocarcinoma (EAC) (19). In a multi-center registry of 4,982 patients who underwent RFA of BE, 100 patients (2%) developed EAC and 0.2% died of EAC (26). Another retrospective study of 306 patients who were treated with RFA for dysplastic BE, found that only 4 patients developed esophageal adenocarcinoma which translates to an incidence rate of 0.65% person/year (27). In this trial, progression to EAC was related to certain factors including male sex, older age, longer BE segment length, and a higher pathology grade at baseline.
Adverse events
RFA adverse events are usually mild, including chest pain stricture and hemorrhage (3,5).
Stricture rates range from 0–6% and relate to technique and operator (3,5,18,20,24,28,29).
In one meta-analysis of 18 studies including 3,802 patients who underwent RFA for BE, 1% had hemorrhage, 3% had chest pain while 5% had esophageal stricture (18).
Combining RFA with other procedures increased risk of complications (5). It is recommended to prescribe the patient a mild analgesic post-ablation for the treatment of possible chest pain which usually starts within a few hours to 24 hours post procedure and can last for a few days.
Follow-up
Follow-up after RFA is recommended to detect recurrence of HGD and/or progression to cancer.
High-resolution endoscopy 8 to 12 weeks post-ablation with careful examination of the neo-squamocolumnar junction is recommended, since that is the area with the highest risk of recurrence (11,12). It is important to inform the patient that on average, two to three sessions of ablation are needed to achieve complete eradication of BE. Follow-up endoscopy after complete eradication of BE depends on the degree of dysplasia. For HGD, follow-up endoscopy should be scheduled in 3, 6 and 12 months then annually; however, for LGD, EGD should be after 1 year and 3 years following eradication of BE (30).
Endoscopic cryotherapy (EC)
Endoscopic cryotherapy is another intervention aimed at the eradication of BE mucosa. A cryogen such as liquid nitrogen or liquid nitrous oxide is applied by endoscopy to the targeted lesion resulting in abrupt disruption of the cell membrane and coagulation of nearby blood vessels through cycles of freezing and thawing (31-33). Theoretically, cryotherapy can achieve deeper ablation with minimal chest pain due to its anesthetic effect. RFA remains the preferred modality of ablation over cryotherapy due to the technical difficulty of cryotherapy and limited data.
In a study of 60 patients with BE and HGD who completed all planned cryotherapy sessions, 97% had complete eradication of HGD, 87% had eradication of all dysplasia; buried dysplastic mucosa was found in 3% on follow-up, adverse events were noted in 3% of patients which included stricture, chest pain, or bleeding (31). In a prospectively collected cohort of 46 patients with BE who underwent either RFA or cryoablation, BE regression was similar in both groups (88% vs. 90 %, P=0.62) but pain level, as measured by pain scale, and pain duration were significantly lower in the cryoablation group (34). The exact role of cryoablation in the management of BE and whether it should be a first line treatment for flat BE or reserved for cases which failed RFA is currently being investigated in an ongoing prospective trial.
Endoscopic resection techniques (EMR and ESD)
EMR and ESD offer an alternative option to surgical resection when it comes to removing mucosal and submucosal lesions including superficial neoplastic tumors.
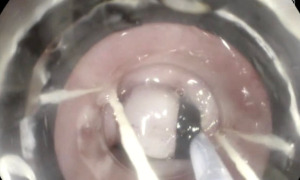
EMR is usually the method of choice for the removal of small mucosal lesions using a cap and snare resection (Figure 2), although it can also be used in a piecemeal fashion to remove larger mucosal lesions. Drawbacks of piecemeal EMR include the inability to assess the resected specimen margins for complete removal. This may increase the risk of recurrence and result in fibrosis at the site of the lesion making subsequent interventions more difficult. This practice would overall worsen the outcome of endoscopic therapy and limit future treatment options.
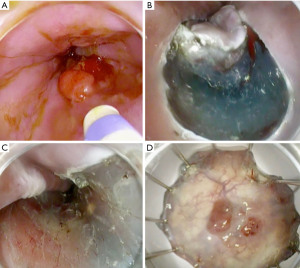
ESD is the method of choice for larger lesions despite a steep learning curve and length of the intervention. Figure 3 shows the steps of performing esophageal ESD. EMR and ESD can be used separately or combined, the best approach should be tailored based on patient status and lesion stage.
Lesion classification
Endoscopic preassessment of BE and its associated lesions is crucial to determine the need for endoscopic resection. Visual inspection of any nodularity within Barrett’s epithelium should be classified based on Paris classification as the following (35):
- Type 0-I lesions that are polypoid are subcategorized as:
- Type 0-Ip: protruded, pedunculated;
- Type 0-Is: protruded, sessile.
- Type 0-II: lesions that are nonpolypoid are subcategorized as:
- Type 0-IIa: slightly elevated;
- Type 0-IIb: flat;
- Type 0-IIc: slightly depressed.
- Type 0-III: lesions are excavated.
After determining the lesion’s topographic morphology, detailed examination of the lesion’s surface using normal white light endoscopy, chromoendoscopy or digital chromoendoscopy is recommended. Acetic acid chromoendoscopy was proven in several trials to aid in detecting dysplastic epithelium within BE and its use is encouraged due to high cost-effective value (36). Portsmouth acetic acid classifications system is novel classification which increased the sensitivity of detecting dysplasia in BE up to 98%. Loss of acetowhiting and irregular and crowded pit patterns are indicative of dysplasia in this classification (37). Several other classifications based on Narrow Band Imaging (NBI) or other advanced imaging modalities exists, but they are beyond the topic of this review.
Indications for endoscopic resection
Standard EMR technique can be applied to treat esophageal cancer if the lesion is less than 2 cm, involving less than one-third of the circumference of the esophageal wall and limited to the mucosa (38,39). Lesions larger than 2 cm which are limited to the mucosa can be managed by ESD (40). A subset of patients with T1b lesions can be managed endoscopically, provided that the lesion is limited to the upper third of the mucosa, the tumor is well-differentiated and there is no evidence of lymphovascular invasion (41). Endoscopic resection can be combined with RFA and cryotherapy to improve patients outcome (35).
Endoscopic resection outcomes
Studies that assessed the overall outcome of endoscopic resection suggested that ESD has better outcomes compared with EMR in the management of early esophageal cancer (42,43). A meta-analysis of 15 studies, including 2,758 patients, found ESD had better en bloc and curative resection rates with lower recurrence of premalignant and malignant conditions (OR 0.09, 95% CI: 0.04–0.18) (42).
For esophageal lesions, EMR and ESD have favorable outcomes with definitive curative resection in select patients. Low morbidity and low mortality rates were published in numerous reports following endoscopic resection with 5-year survival rates over 90 percent. Survival rates are lower in patients with lesions spreading beyond the lamina propria and recurrence can be treated with repeated intervention.
In a prospective study of 53 patients with adenocarcinoma of the gastroesophageal junction treated with ESD, the 5-year overall survival rate was 94% and 92% of the patients had no recurrence during a median follow up period of 6.1 year (44).
A retrospective study using the National Cancer Database included 5,390 patients who underwent endoscopic resection vs. surgery for the treatment of superficial esophageal cancer from 2004–2010. In this study, 1,427 underwent endoscopic resection and 3,963 underwent surgical resection. Patients treated surgically had a lower 30-day survival rate compared with patients treated endoscopically (96.5% vs. 99.5% respectively). After excluding all patients who died during the initial 30 days post-surgery, the 5 years modified survival rate was better for patients who underwent surgical intervention in comparison with endoscopic resection (88% vs. 77%, respectively). This could be explained by poor patients’ selection in a subset of patients in the ER group, since most patients who died within 5 years after ER had a high risk for lymph nodes metastasis (T1b tumor on presentation) (45).
For BE, endoscopic resection achieved eradication of intestinal metaplasia at a rate of 59–100% and dysplasia at a rate of 86–100% (46-48). Endoscopic resection was performed in 349 patients with BE-HGD and mucosal adenocarcinoma. The mean follow-up period was 63.6 (SD 23.1) months. Complete response was achieved in 96.6% patients and surgery was necessary in 13 patients (3.7%) who failed endoscopic therapy. Metachronous lesions developed during the follow-up period in 74 patients (21.5%); 56 died of concomitant disease, but none died of esophageal cancer. The calculated 5-year survival rate was 84%. Risk factors most frequently associated with recurrence in this study were piecemeal resection, long-segment BE, no ablative therapy of BE, time until complete response was achieved (>10 months) and multifocal neoplasia (49).
Adverse events of endoscopic resection
Severe complications following endoscopic resection are relatively rare and most of the time can be managed with endoscopy (50-52). Piecemeal resection and large involvement of the mucosa can result in higher risk of complications (51,53). Endoscopic resection adverse events include perforation, bleeding and strictures. Adverse events are more common in ESD than EMR.
The risk of bleeding after endoscopic resection varied from 0 to 45% in published series (4,54,55). In a retrospective series of 681 patients who underwent EMR, bleeding occurred in 0.01% (8 patients) and were addressed with endoscopy except in one patient which required surgery (56). The rate of symptomatic strictures after EMR in this series 1.0% (7 cases). All strictures were successfully treated with endoscopic dilation (56). In ESD, bleeding was noted in 0–0.7% of patients, in three retrospective studies including a total of 771 patients (57-59).
Perforations rate is around 0 to 5% in published series (52,55,57,59). Most perforations encountered during ESD are microperforations which can be treated endoscopically by clip placement or endoscopic suturing.
Strictures are more common after ESD, especially with a mucosal defect involving more than three fourths of the esophageal lumen circumference (60). Strictures are usually treated with endoscopic balloon dilation (EBD). In a retrospective study of 23 patients, oral steroid therapy dramatically reduced the need for EBD (61). In a case series of 41 patients, local injection with triamcinolone decreased incidence of stricture and frequency of required EBD (62).
Conclusions
Endoscopic treatment of BE includes ablative and resection techniques. Ablative procedures such as radiofrequency ablation or cryoablation can be used to treated dysplastic flat Barrett’s epithelium. Resection techniques such EMR or ESD should be used for nodular dysplastic Barrett’s epithelium or early esophageal adenocarcinoma limited to the mucosa. EMR should be used for the treatment of lesions smaller than 2 cm (preferably 1.5 cm or less). ESD ensures higher en bloc and curative resection rates for lesions larger than 2 cm but has a steep learning curve and higher adverse events. Future research should be focused on methods to improve training and adoption of ESD in everyday practice.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Madhav Desai) for the series “Endoscopic Therapy for Barrett’s Esophagus” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-18/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-18/coif). The series “Endoscopic Therapy for Barrett’s Esophagus” was commissioned by the editorial office without any funding or sponsorship. M.O.O. reports personal fees from Olympus, personal fees (consultant) from Boston Scientific Corporation, personal fees (consultant) from Lumendi, personal fees (consultant) from CondMed, grants from Lumendi, Abbvie and ConMed, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Stepwise circumferential and focal ablation of Barrett's esophagus with high-grade dysplasia: results of the first prospective series of 11 patients. Endoscopy 2008;40:359-69. [Crossref] [PubMed]
- Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett's neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy 2008;40:370-9. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14:1085-91. [Crossref] [PubMed]
- Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23-9. [Crossref] [PubMed]
- Kim HP, Bulsiewicz WJ, Cotton CC, et al. Focal endoscopic mucosal resection before radiofrequency ablation is equally effective and safe compared with radiofrequency ablation alone for the eradication of Barrett's esophagus with advanced neoplasia. Gastrointest Endosc 2012;76:733-9. [Crossref] [PubMed]
- Qumseya BJ, Wani S, Gendy S, et al. Disease Progression in Barrett's Low-Grade Dysplasia With Radiofrequency Ablation Compared With Surveillance: Systematic Review and Meta-Analysis. Am J Gastroenterol 2017;112:849-65. [Crossref] [PubMed]
- Wani S, Rubenstein JH, Vieth M, et al. Diagnosis and Management of Low-Grade Dysplasia in Barrett's Esophagus: Expert Review From the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822-35. [Crossref] [PubMed]
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett's esophagus. Gastroenterology 2012;143:567-75. [Crossref] [PubMed]
- Shaheen NJ, Peery AF, Hawes RH, et al. Quality of life following radiofrequency ablation of dysplastic Barrett's esophagus. Endoscopy 2010;42:790-9. [Crossref] [PubMed]
- Sampliner RE, Camargo E, Prasad AR. Association of ablation of Barrett's esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus 2006;19:277-9. [Crossref] [PubMed]
- Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett's esophagus ablation. Gastrointest Endosc 2005;61:515-21. [Crossref] [PubMed]
- Alvarez Herrero L, van Vilsteren FG, Pouw RE, et al. Endoscopic radiofrequency ablation combined with endoscopic resection for early neoplasia in Barrett's esophagus longer than 10 cm. Gastrointest Endosc 2011;73:682-90. [Crossref] [PubMed]
- Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut 2016;65:555-62. [Crossref] [PubMed]
- van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut 2011;60:765-73. [Crossref] [PubMed]
- Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781-9. [Crossref] [PubMed]
- Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett's esophagus. Clin Gastroenterol Hepatol 2014;12:1840-7.e1. [Crossref] [PubMed]
- Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett's Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245-55. [Crossref] [PubMed]
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology 2011;141:460-8. [Crossref] [PubMed]
- Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett's esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy 2010;42:272-8. [Crossref] [PubMed]
- Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc 2008;68:35-40. [Crossref] [PubMed]
- Sharma VK, Jae Kim H, Das A, et al. Circumferential and focal ablation of Barrett's esophagus containing dysplasia. Am J Gastroenterol 2009;104:310-7. [Crossref] [PubMed]
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209-17. [Crossref] [PubMed]
- Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett's esophagus: a multicenter study with 2.5-year follow-up. Gastrointest Endosc 2008;68:867-76. [Crossref] [PubMed]
- Phoa KN, Pouw RE, van Vilsteren FGI, et al. Remission of Barrett's esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology 2013;145:96-104. [Crossref] [PubMed]
- Wolf WA, Pasricha S, Cotton C, et al. Incidence of Esophageal Adenocarcinoma and Causes of Mortality After Radiofrequency Ablation of Barrett's Esophagus. Gastroenterology 2015;149:1752-1761.e1. [Crossref] [PubMed]
- Guthikonda A, Cotton CC, Madanick RD, et al. Clinical Outcomes Following Recurrence of Intestinal Metaplasia After Successful Treatment of Barrett's Esophagus With Radiofrequency Ablation. Am J Gastroenterol 2017;112:87-94. [Crossref] [PubMed]
- Beaumont H, Gondrie JJ, McMahon BP, et al. Stepwise radiofrequency ablation of Barrett's esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy 2009;41:2-8. [Crossref] [PubMed]
- Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett's esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc 2014;79:718-731.e3. [Crossref] [PubMed]
- Sharma P, Shaheen NJ, Katzka D, et al. AGA Clinical Practice Update on Endoscopic Treatment of Barrett's Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020;158:760-9. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett's esophagus. Endoscopy 2015;47:582-91. [Crossref] [PubMed]
- Gosain S, Mercer K, Twaddell WS, et al. Liquid nitrogen spray cryotherapy in Barrett's esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc 2013;78:260-5. [Crossref] [PubMed]
- van Munster SN, Overwater A, Haidry R, et al. Focal cryoballoon versus radiofrequency ablation of dysplastic Barrett's esophagus: impact on treatment response and postprocedural pain. Gastrointest Endosc 2018;88:795-803.e2. [Crossref] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Bhandari P, Kandaswamy P, Cowlishaw D, et al. Acetic acid-enhanced chromoendoscopy is more cost-effective than protocol-guided biopsies in a high-risk Barrett's population. Dis Esophagus 2012;25:386-92. [Crossref] [PubMed]
- Kandiah K, Chedgy FJQ, Subramaniam S, et al. International development and validation of a classification system for the identification of Barrett's neoplasia using acetic acid chromoendoscopy: the Portsmouth acetic acid classification (PREDICT). Gut 2018;67:2085-91. [Crossref] [PubMed]
- Takeshita K, Tani M, Inoue H, et al. Endoscopic treatment of early oesophageal or gastric cancer. Gut 1997;40:123-7. [Crossref] [PubMed]
- Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology 2000;118:670-7. [Crossref] [PubMed]
- Ning B, Abdelfatah MM, Othman MO. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer. Ann Cardiothorac Surg 2017;6:88-98. [Crossref] [PubMed]
- Othman MO, Lee JH, Wang K. Clinical Practice Update on the Utility of Endoscopic Submucosal Dissection in T1b Esophageal Cancer: Expert Review. Clin Gastroenterol Hepatol 2019;17:2161-6. [Crossref] [PubMed]
- Cao Y, Liao C, Tan A, et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751-7. [Crossref] [PubMed]
- Park YM, Cho E, Kang HY, et al. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc 2011;25:2666-77. [Crossref] [PubMed]
- Yamada M, Oda I, Nonaka S, et al. Long-term outcome of endoscopic resection of superficial adenocarcinoma of the esophagogastric junction. Endoscopy 2013;45:992-6. [Crossref] [PubMed]
- Merkow RP, Bilimoria KY, Keswani RN, et al. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst 2014;106:dju133. [Crossref] [PubMed]
- Seewald S, Akaraviputh T, Seitz U, et al. Circumferential EMR and complete removal of Barrett's epithelium: a new approach to management of Barrett's esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc 2003;57:854-9. [Crossref] [PubMed]
- Saligram S, Chennat J, Hu H, et al. Endotherapy for superficial adenocarcinoma of the esophagus: an American experience. Gastrointest Endosc 2013;77:872-6. [Crossref] [PubMed]
- Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett's esophagus. Gastrointest Endosc 2000;52:328-32. [Crossref] [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200-6. [Crossref] [PubMed]
- Chung A, Bourke MJ, Hourigan LF, et al. Complete Barrett's excision by stepwise endoscopic resection in short-segment disease: long term outcomes and predictors of stricture. Endoscopy 2011;43:1025-32. [Crossref] [PubMed]
- Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett's esophagus. Gastrointest Endosc 2011;74:753-60. [Crossref] [PubMed]
- Gerke H, Siddiqui J, Nasr I, et al. Efficacy and safety of EMR to completely remove Barrett's esophagus: experience in 41 patients. Gastrointest Endosc 2011;74:761-71. [Crossref] [PubMed]
- Soetikno RM, Gotoda T, Nakanishi Y, et al. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567-79. [Crossref] [PubMed]
- Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology 2009;137:815-23. [Crossref] [PubMed]
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-660.e1. [Crossref] [PubMed]
- Tomizawa Y, Iyer PG, Wong Kee Song LM, et al. Safety of endoscopic mucosal resection for Barrett's esophagus. Am J Gastroenterol 2013;108:1440-7; quiz 1448. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860-6. [Crossref] [PubMed]
- Isomoto H, Yamaguchi N, Minami H, et al. Management of complications associated with endoscopic submucosal dissection/ endoscopic mucosal resection for esophageal cancer. Dig Endosc 2013;25:29-38. [Crossref] [PubMed]
- Tsujii Y, Nishida T, Nishiyama O, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy 2015;47:775-83. [Crossref] [PubMed]
- Katada C, Muto M, Manabe T, et al. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003;57:165-9. [Crossref] [PubMed]
- Sato H, Inoue H, Kobayashi Y, et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc 2013;78:250-7. [Crossref] [PubMed]
- Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011;74:1389-93. [Crossref] [PubMed]
Cite this article as: Ahmed Y, Othman MO. A narrative review of endoscopic therapy for Barrett’s esophagus. Ann Esophagus 2023;6:45.