
Update on ablative therapy for Barrett’s related dysplasia
Introduction
Barrett’s esophagus (BE) is a metaplastic condition, that occurs, when normal stratified squamous epithelium lining the distal esophagus is replaced by intestinal-type columnar epithelium. BE can arise as a consequence of mucosal injury due to chronic gastroesophageal reflux disease. The clinical importance of BE relates to its role as the only identifiable premalignant lesion for esophageal adenocarcinoma (EAC). Although uncommon, EAC has increased dramatically in incidence over the last decades in western populations. Despite advances in surgical and oncological interventions, the 5-year survival rate remains poor, with only 15% to 20%, and an overall median survival of <1 year in patients with advanced disease (1,2), since diagnosis of EAC is often made in late stage, when the cancer is unlikely to be amenable to any curative modality.
The progression of BE to EAC occurs sequentially through the histopathologic stages of intestinal metaplasia (IM) to low-grade dysplasia (LGD) then high-grade dysplasia (HGD), which can promote to intramucosal carcinoma (IMC) and ultimately invasive EAC (3).
Esophagectomy is the mainstay therapy for submucosal EAC and was the standard of care for BE with HGD and IMC in the past. Despite a low 30-day mortality of 1.7%, it confers high morbidity with overall postoperative complications in 65% of patients and a major complication rate of 29% (4). However, over the last 2 decades, management of BE’s related dysplasia there has been shifted towards eradication therapy (EET).
Endoscopic interventions have been developed to eradicate dysplasia or early neoplasia, to prevent progression of BE into invasive cancer and ultimately to impact the morbidity and mortality associated with EAC (5,6).
EET consists in a multimodal approach comprising endoscopic resection of visible lesions, such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), followed by ablation of any residual BE regardless of dysplasia grade (7,8). The intention of EET is not only to obtain complete eradication of dysplasia (CE-D) or neoplasia, but also complete eradication of IM (CE-IM) considering the 30% risk of metachronous neoplasia (9).
Ablative therapy induces mucosal tissue necrosis by using thermal energy, freezing, or photochemical injury, and eventually results in reepithelization of the damaged mucosa with normal squamous epithelium.
While some ablation modalities including electrocoagulation, photodynamic therapy and stepwise endoscopic resection of the entire BE segment, are abandoned due to poor efficacy or substantial side effect profile, a rising number of studies emerged, evaluating the use of new ablation techniques such as cryotherapy with spray or balloon and (Hybrid-) argon plasma coagulation (APC). However, to date, RFA remains the first-line ablation modality with good efficacy and safe risk profile.
The object of this review is to discuss current approaches and techniques of ablation modalities such as RFA, APC and cryotherapy along with updated data regarding their efficacy and safety profile.
Indication for Barrett’s ablation
Currently, ablative therapy should be performed according to the following two major indications: The first indication is residual flat segment of BE subsequent to successful endoscopic resection of a neoplastic lesion. Endoscopic resection of visible abnormalities followed by surveillance alone yields an inadmissibly high risk of metachronous dysplasia during follow-up endoscopies (9). The risk of recurrence of neoplasia and IM can be decreased to 4% and 8%, respectively, after ablative therapy (10).
The second indication consists in first-line therapy for invisible flat-type dysplasia, LGD or HGD, detected on surveillance biopsies, on the condition that the histological diagnosis of dysplasia is confirmed on two successive endoscopies and verified by pathologist with expertise in gastrointestinal (GI) pathology.
Endoscopic resection is the mainstay in treatment of BE-related neoplasia and it is recommended for all visible abnormalities before application of other ablation methods. Moreover, it enables adequate histological assessment and staging with limited distortion of the invasion depth along with improved interobserver agreement among pathologists using EMR specimens (11). A multicenter cohort study showed that EMR led to a change of diagnosis in 30% of patients with BE with early neoplasia (12). Moreover, incomplete or failed resection of focal abnormalities may put the patient at risk of residual subsquamous neoplasia, given the superficial effect of mucosal ablation modalities (13).
At present, surveillance and treatment decisions are merely based on histopathological evaluation of surveillance biopsies. The degree of dysplasia is the most widely-accepted prediction tool of progression, and consequently the surveillance intervals recommended by current guidelines are based on the grade of dysplasia (13,14).
Histologic diagnosis of LGD in BE is difficult and has an extremely high inter-observer variability, even among expert pathologists (11,15,16). The risk of progression of BE-LGD to EAC amounts to 0.5% annually as reported in a recent meta-analysis (17). In general practice BE-LGD seems to be an overdiagnosed condition as demonstrated in a study by Curvers et al. (18). However, the neoplastic progression of a diagnosis of LGD, when confirmed by more than one expert pathologist, is likely to be underestimated with a significantly higher progression rate to HDG or EAC of 9.1–13.4% (6,18,19). In the latter respect, ablation therapy is warranted in cases of BE-LGD and recommended by current guidelines.
Conversely, prophylactic ablation therapy for non-dysplastic BE (ND-BE) and indefinite dysplasia (IND-BE) is not recommended by GI societies, since the risk of progression to EAC in patients with ND-BE is low, estimated at about 0.3% per year (13,14). Guidelines suggest optimization of acid suppression and surveillance, despite lacking evidence of both in preventing mortality and progression to cancer (20). The estimated prevalence of BE in American adults is 1–5%. Therefore, indiscriminate use of ablative therapies might cause high costs with minimal benefit, in light of the fact that most of the treated patients would never develop EAC (21,22).
Data from a much-anticipated prospective multicenter randomized control trial (RCT) from the UK are awaited, which aim to compare surveillance endoscopy every 2 years with endoscopy only when needed for other clinical indications in a cohort of 3,400 BE patients without HGD or EAC at baseline. The overall survival of these patients will be assessed after 10 years (23).
With regard to flat HGD, it is worth noting that true flat HGD without any visible lesions is a rarity and accounts for less than 20% of patients with HGD. The absence of visible abnormalities in a patient with HGD is often the result of an unnoticed lesion, or an overstaging of the histopathology. Accordingly, the diagnosis of flat HGD as well as flat LGD, has to be confirmed on at least two successive endoscopies before ablation is performed (14).
Radiofrequency ablation (RFA)
Currently, RFA is the most commonly used and studied ablative modality and therefore represents the dominant first-line treatment for dysplastic BE.
The mechanism of RFA consists in delivery of radiofrequency energy to the esophageal mucosa, resulting in tissue necrosis and eventually reconstitution of normal squamous epithelium (24). Due to its limited depth of tissue penetration without submucosa or muscularis propria involvement, RFA bears a low risk of stricture formation or perforation, yet its use is limited in zones of mucosa thickening such as nodularities.
The BarrxTM radiofrequency ablation system (Medtronic, Sunnyvale, California, USA) comprises a bipolar radiofrequency energy generator and two distinct types of ablation catheters: balloon catheters for circumferential RFA (c-RFA) and focal devices for focal RFA (f-RFA) of BE (Figures 1,2).
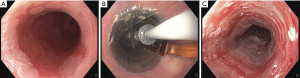
Most patients undergo a first procedure with a stepwise circumferential ablation, followed by additional focal ablation sessions for any residual BE on endoscopic follow-up. In addition to treatment of all visible esophageal columnar mucosa, it is recommended to ablate 5–10 mm proximal to the squamocolumnar junction and 5–10 mm distal to the gastroesophageal junction (GEJ), using a focal device in a circumferential fashion (13).
Circumferential ablation
Prior to the ablation process, cleaning of the esophageal mucosa to remove excessive mucus is recommended by using 1% N-acetylcysteine. Recently, flushing with standard water has proven to be just as effective as randomized trials have demonstrated (25). The extent of BE should be documented according to Prague C&M classification for reference during the sizing and ablation procedures (26). Irregularities of the esophageal wall should be carefully evaluated, including nodularity or strictures, since they may interfere with balloon-based circumferential ablation.
In the past, it was necessary to measure the esophageal inner diameter at different levels using a sizing balloon. Subsequently, an ablation balloon with the appropriate diameter was chosen. However, this process required several introductions and removals of sizing catheter, ablation catheter and endoscope. Moreover, employing an ablation balloon with a fixed diameter to treat the entire BE can be of disadvantage whereas the esophageal inner diameter may differ within the same patient.
Aiming to facilitate the ablation procedure by obviating the sizing process, the BarrxTM 360 express RFA balloon catheter has been introduced, which incorporates the sizing balloon and the ablation balloon into a single device. This balloon catheter inflates with the ability to self-adjust to the esophagus wall, ensuring optimal tissue contact. A pilot study showed that c-RFA with the express RFA balloon using the standard ablation regimen results in shorter procedure time by up to 20% yet maintaining efficacy and safety when compared to ablation with the predecessor model of the BarrxTM 360 system (27).
The ablation process starts with positioning a guidewire into the gastric antrum and removal of the endoscope, followed by the introduction of the balloon catheter over the guidewire (Figure 1). After inflating the balloon, ablation is initiated by pressing the foot switch. Radiofrequency energy is delivered to the electrode, lasting less than 1.5 seconds, after which the balloon automatically deflates. The balloon is then advanced from proximal to distal in 3- to 4-cm intervals (12 J/cm²), allowing for a minimal overlap of less than 1 cm between ablation zones. The process is repeated until the entire length of BE has received one application of RFA.
After the first cycle the devices are removed, in order to clean the surface of the balloon from any remaining coagulum. Subsequently, the endoscope is reinserted with a soft distal cap to push off the necrotic debris from the ablated segments, before a second cycle is applied. This is the common treatment protocol, the so-called “one-clean-one” algorithm.
However, this regimen demands multiple introductions and removals of the endoscope and ablation devices, which is arduous, time consuming and discomforting for the patient. A randomized multicenter trial compared the standard algorithm for c-RFA with simplified regimens in which the cleaning steps are omitted. Efficacy and safety of a simplified regimen was not inferior to the standard regimen, yet twice as fast since it requires fewer introductions of endoscope and devices (25). Therefore, the authors recommend the use of the simplified c-RFA regimen for patients with uncomplicated BE without scarring and stenosis.
Focal ablation
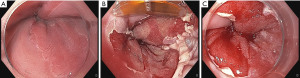
Endoscopic follow-up is recommended in 8- to 12-week intervals to assess the need for further circumferential or focal ablation depending on the residual BE tissue to ablate. Focal ablation can also be used for initial treatment of uncommonly configured BE such as those with elongated tongues (28) (Figure 2).
Various focal ablation catheters are available, depending on the size of the electrode array: the BarrxTM 90 (20 mm × 13 mm), the BarrxTM 60 (15 mm × 10 mm) and the BarrxTM ultra long (40 mm × 13 mm) RFA focal catheter (29). Lately, a f-RFA catheter has been developed that fits into the working channel, obviating the need to remove the endoscope for the cleaning process (BarrxTM Channel RFA endoscopic Catheter; 15.7 mm × 7.5 mm).
For the procedure the focal device is attached to the distal end of the endoscope with the electrode array located at the 12 o’clock position of the endoscopic view. The device and endoscope are then introduced carefully in order to avoid injury or perforation. The targeted residual BE segment is equally orientated to the 12 o’clock position. An upward deflection of the tip of the endoscope puts the electrode array in closer contact with the mucosa. Ablation is initiated via foot switch delivering radiofrequency energy at 15 J/cm². Without detaching the device from the esophageal mucosa, the electrode array is directly activated a second time. All residual segments of BE are treated likewise before removing endoscope and ablation catheter. The cleaning steps consists in cleaning the electrode and sloughing the debris from the ablated areas. Another cycle of treatment is then delivered to all ablated areas with a second “double” application, resulting in a total of four applications of radiofrequency energy at 15 J/cm².
A treatment algorithm incorporating a simplified protocol with a lower energy setting of 3×12 J/cm², with no cleaning, for all f-RFA sessions, is non-inferior to the standard regimen when comparing efficacy. The simplified regimen is associated with a significantly shorter procedure time and appears equally safe, making it favorable to use (30).
In addition to treatment of any visible residual BE, focal ablation in a circumference fashion of the entire GEJ is recommended, even if no evident areas of BE are observed. Gastric folds and widening of the hiatal hernia may result in inefficient mucosal contact of the electrode and make this area difficult to treat with balloon-based devices. The importance of ablation of the GEJ relies on both the difficulty to detect the presence of residual dysplastic BE in this area and neoplastic recurrences, which are commonly found in the cardia during follow-up (31).
Efficacy
The reported rates for CE-IM after a combination of EMR and RFA range between 72% and 97% (32-38). In a landmark trial in 2009, entitled the AIM dysplasia trial, 127 subjects with BE and LGD or HGD were randomized to receive either RFA or a sham procedure. Ninety percent of patients with HGD and 81% of patients with LGD achieved CE-D as compared to <5% in the sham procedure arm. The subjects undergoing RFA also achieved a significantly higher rate of CE-IM, less disease progression and fewer cancers (38).
The benefit of RFA in patients with BE and LGD was highlighted in a RCT in 2014, entitled the SURF Study, in which 136 subjects with confirmed BE and LGD were randomized to either RFA or endoscopic surveillance (6). During a 3-year follow-up since randomization, ablation reduced the risk of progression to HGD or EAC by 25% and risk of progression to EAC by 7.4%. Complete eradication occurred in 92.6% for dysplasia and 88.2% for IM in the ablation group compared with 27.9% for dysplasia and 0.0% for IM among patients in the control group.
Consistent with the latter study, results of a systematic review and meta-analysis showed a significant risk reduction in progression to HGD/EAC among patients with BE-LGD treated with RFA compared with those undergoing surveillance alone (39).
A large prospective multicenter study demonstrated that in patients with HGD or early cancer, intensive multimodality therapy consisting of endoscopic resection (ER) combined with RFA is safe and highly effective, and the treatment effect appears to be durable during mid-term follow-up (2.5 years) (10). Treatment consisted of several sessions of resection and ablation and took a median of 12 months. Eradication rates for neoplasia (intention to treat 92%, per protocol 98%) and IM (intention to treat 87%, per protocol 93%) lie at the upper end of the previously reported spectrum. The authors see an important difference with RFA studies from USA (37,38,40), since circumferential ablation of the GEJ was performed during each focal ablation procedure to ensure optimal treatment.
In spite of RFA’s effectiveness, however, there is a minority of patients in whom CE-IM cannot be achieved. A systematic review and meta-analysis of 18 studies and 3,802 patients showed the pooled CE-IM rate to be 78% (41). Factors associated with poor response or failure of RFA to eradicate IM and dysplasia are active reflux esophagitis, endoscopic resection scar regeneration with BE, narrow pre-RFA esophageal diameters, and longer persistence of dysplasia (42). In addition, increased patient age and length of BE, the presence of a hiatal hernia and incomplete mucosal healing on follow-up endoscopy seem to also contribute to incomplete eradication of dysplasia and metaplasia (43,44).
Recurrences
Despite the fact that RFA has been shown to be effective in achieving CE-D and CE-IM, recurrence of IM and dysplasia after initially successful ablative therapy is a common event and therefore surveillance with biopsy is mandatory. Dysplastic recurrences tend to occur most around the GEJ and the majority were often not visible to the endoscopist but detected on random biopsies (31), thus biopsies in this area have the highest yield. By contrast, recurrences occurring more proximal to the GEJ are often visible to the endoscopist (45).
Outcome data of large prospective cohorts and meta-analyses illustrates a rate of recurrence of approximately 7–10% per patient-year of follow-up (31,45-50).
The final report of the AIM Trial showed that patients treated with RFA for dysplastic BE had >30% chance of developing any recurrent BE (IM, LGD, HGD) within 5 years. Most recurrences were responsive to further endoscopic therapy and almost all recurrences occurred in the first 2 years following therapy (46). In line with this, another study showed that incidence rate of IM, dysplasia, and HGD/EAC detection was higher within the first year than in subsequent years of surveillance (48).
A systematic review and meta-analysis by Krishnamoorthi et al. demonstrated a recurrence rate for IM to be 7.1% per patient year, 1.3% for LGD and 0.8% for HGD/EAC after first-line EET (50).
Another meta-analysis comprising 39 studies aimed to compare recurrence rates between treatment modalities (RFA with or without EMR vs. stepwise complete EMR). The pooled incidence of any recurrence was 7.5/100 PY with an IM recurrence rate of 4.8/100 PY, and dysplasia recurrence rate of 2.0/100 PY. Compared to the stepwise complete EMR group, the RFA group had significantly higher overall (8.6/100 PY vs. 5.1/100 PY, P=0.01) and IM recurrence rates (5.8/100 PY vs. 3.1/100 PY, P<0.01) with no difference in recurrence rates of dysplasia (47).
Factors associated with recurrence of BE after CE-IM are increasing age, length of BE, baseline dysplasia and treatment at low-volume RFA centers (49,50).
Complications of RFA
RFA is an overall safe procedure with a favorable side-effect profile, especially when compared with esophagectomy, stepwise EMR or photodynamic therapy.
Common complications associated with RFA are chest pain and esophageal strictures. Upper GI bleeding, perforation or death occur infrequently.
A pooled adverse event rate of 8.8% was reported in a large meta-analysis including 37 studies and 9,200 patients (51). The majority of these were due to formation of strictures (5.6%), followed by bleeding (1.0%) and perforation (0.6%). There was a significantly higher risk of complications, particularly strictures, when RFA was combined with endoscopic resection. Other factors associated with adverse events were advanced baseline histology as well as augmented length of BE, presumably due to the need for more treatment sessions. Nevertheless, strictures following RFA are generally short and manageable with endoscopic dilation (38).
APC
APC is a clinically established noncontact thermal coagulation technology, in which high frequency energy is transmitted to the target tissue by an ionized and therefore conductive argon gas (argon plasma) with a rate of 1–2 liters/min and energy settings between 30 and 90 W (Figure 3). The plasma beam follows the path of least electrical resistance, which allows the argon plasma to be applied both en-face and tangentially, facilitating treatment of rather difficult-to-access-regions (52). The APC equipment consists of an argon gas source and pump, a flexible probe and a high frequency electrosurgical generator (Erbe Elektromedizin, Tübingen, Germany). Depending on the gas flow and strength of the electric field, the application eventuates in a superficial thermal coagulation and devitalization of pathological mucosa layers, reaching a depth of 2–3 mm (53).
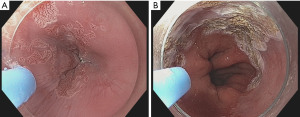
APC is widely available in endoscopy units and commonly used as a subsidiary method to treat small residual or recurrent areas of BE following EMR, ESD or RFA.
Outcome data of RCTs demonstrated that APC has reduced the risk of neoplastic progression (54,55). A study, which compared APC of residual BE after endoscopic resection for HGD or IMC, showed a rate of metachronous lesions in 3% after APC by contrast with 36% after 24 months of surveillance (55).
Efficacy of APC in achieving CE-IM and CE-D has been reported in small case series (56-60), although variable rates of CE were reported ranging from 39–98% in the short-term follow-up (61).
It has been suggested that the differences in efficacy might be explained by APC power settings and the dose of peri-interventional administered proton pump inhibitor (PPI). This hypothesis, however, was not confirmed by a very recent study that demonstrated no impact on the efficacy comparing different APC power settings (90 or 60 W) and PPI doses (120 or 40 mg) (61). The complete ablation rate at 6 weeks was in the 60–78% range and no significant differences in efficacy were observed 2 years after APC treatment and at the end of long-term follow-up. Similar results in CE-IM (rate of 77%) were reported from the multicenter APBANEX study using high-power APC (90 W) in combination with esomeprazole 80 mg per day (59).
Recently, a pilot RCT comparing RFA and APC of BE after endoscopic resection of HGD or EAC showed similar efficacy and safety of these methods (CE-D of 79.4% and CE-IM of 55.8% in the RFA group and CE-D of 83.8% and CE-IM 48.3% in the APC group) (62). However, the cost of APC was substantially lower with a difference of $27,491 per case treated, favoring APC.
Common adverse events of APC are strictures, chest pain following the procedure and bleeding. Symptomatic stricture dilation was required in 8.1% after APC and 8.3% after RFA in the latter RCT. In the APE study the stricture rate after APC was 9% (55). In line with other ablation methods, it is recognized that prior endoscopic resection before APC may lead to an augmented stricture rate (63).
Hybrid-APC
The latest ablation technique in BE treatment is Hybrid-APC. A submucosal injection of saline using a water jet system (Erbejet, Erbe Elektromedizin GmbH, Tübingen, Germany) prior to APC is carried out with the aim to create a submucosal fluid cushion. Subsequently, APC is performed typically at higher wattage than with standard APC. It was shown by means of a randomized ex vivo study using a porcine esophagus model that Hybrid-APC reduces the coagulation depth by half in comparison with standard APC (64). The question arises whether submucosal fluid injection prior to ablation may lower the risk of stricture formation in comparison with RFA and standard APC. In a prospective study of 50 patients who had residual BE after endoscopic resection, 78% achieved histologically CE-IM after a median of 3.5 APC sessions and only 1 of 50 patients (2%) developed a stricture (65). A case series of five BE dysplasia patients refractory to RFA +/− cryoablation showed promising results with achieving CE-IM after 2 sessions with Hybrid-APC (66).
Cryoablation
Mechanism and techniques of cryoablation
Cryotherapy is a novel thermal ablation technique that consists in rapid freezing of the esophageal epithelium by using a cryogen, a liquefied gas such as carbon dioxide (CO2), nitrogen or nitrous oxide, which results in intra- and extracellular ice formation (Figure 4). Subsequently this leads to apoptosis by activation of a rapid, membrane-based response within the core of the cryogenic lesion and a delayed mitochondrial-based apoptotic response in the periphery due to severe oxidative stress. In the last phase, within days or weeks, hypoxia from vascular stasis determine a secondary necrosis (67). Finally, the lining of the esophagus will regenerate with neosquamous epithelium.
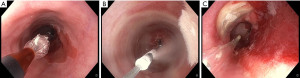
The theoretical benefits of this mechanism are appealing, since cryotherapy may enable deeper ablation than RFA, while preserving the extracellular collagen matrix architecture and therefore may eventuate in lower stricture rates despite the deeper tissue destruction (68).
Cryoablation can be performed using a spray catheter or a balloon (69). For the endoscopic application of cryospray, there were the Trufreeze system (CSA Medical, Baltimore, USA), which used liquid nitrogen cryospray and the other, and the Polar Wand system (GI Supply, Camphill, USA), a CO2 cryospray, which was discontinued by the manufacturing company in 2016. The Trufreeze system utilizes a generator that applies liquid nitrogen at −196 ℃ through a flexible spray catheter to the esophageal wall in a noncontact method (70). A decompression tube with constant suction is necessary to prevent gas accumulation in the stomach. The most frequently used dosimetry is 20-second cycles performed twice at each site of Barrett’s mucosa. Cryoablation by spray may achieve treatment of large areas, but visualization of the targeted mucosa can be hampered by the cryogen obscuring the endoscopic lens. Further limitations of spray therapy are need for sizing, large tanks for the cryogen, a consistent capital investment, and lastly the operator dependency.
The Cryoballoon Focal Ablation System (CbFAS, Pentax Medical, Redwood City, California, USA) has been developed to overcome these limitations. The system consists of a battery-powered hand-held device with a trigger mechanism and a cartridge containing liquid nitrous oxide. The CryoBalloon Ablation Catheter can be deployed into the working channel of a therapeutic endoscope. It has a spray hole in the shaft covered by an inflatable balloon, which is highly compliant and designed to adapt to the esophageal lumen.
Ablation is initiated by releasing the nitrous oxide from the cartridge. While contained within the balloon, the cryogen evaporates inflating the balloon and at the same time reduces the temperature to −85 ℃. The gas is vented back through the catheter into the handle, where it condenses into a sponge, obviating the need for decompression tube. Each cartridge incorporates nitrous oxide for two ablation sites. In case of further ablation treatment, the cartridge in the handle can be exchanged without removing the catheter from the endoscope. The whole system, including the handle, is drafted for single use (71).
Possible advantages of CbFAS comprise its ease of use, the portability and lower costs compared to other ablation systems. Furthermore, the CbFAS may enable more consistent and effective application of cryogen because the nitrous oxide is contained within the balloon and the dose is completely delivered to the targeted area (72).
At first, the CbFAS was designed to treat short segments of BE, as each application results in ice patches of 2 cm². Ablation of larger segments is on the other hand challenging and time-consuming. The novel Cryoballoon Swipe90 Ablation System (CbSAS90) was developed to facilitate treatment of larger BE segments. The difference to the predecessor product lies in its rotatable spray diffuser in the middle of the balloon. Using the foot pedal, a continuous flow of cryogen is delivered to the balloon. The spray diffuser cools the esophageal wall to approximately −80 ℃, resulting in a uniform, 3-cm-long ice patch formation over a quarter of the esophageal circumference in a single application. The controller software enables dose adjustment of the cryogen, by adapting the rate at which the diffuser moves along the axis of the balloon (73).
Efficacy and adverse events
Studies investigating the efficacy and safety of cryoablation using the CbFAS have reported promising CE-IM rates, ranging from 88% for short-segment BE to up to 100% for small BE islands, with acceptable safety profiles (72,74,75).
Previous studies of cryotherapy have suggested several mechanisms that may be associated with better patient tolerance, including an anesthetic effect and blocked nerve conduction by the cooling process (76,77). Additionally, vasoconstriction due to the cooling process is believed to attenuate edema and release of inflammatory mediators (78).
Therefore, improved patient tolerance may be one of the advantages of cryoablation when compared to RFA. This was the aim of a multicenter, nonrandomized cohort study that compared efficacy and tolerability between focal cryoablation and RFA (79). Efficacy did not differ after a single treatment with focal cryoablation and RFA for short-segment BE, but patients noted less pain after focal cryoablation than with RFA and required fewer analgesics. These results suggest a different pain course favoring cryoablation over RFA, but a randomized trial is warranted for definitive conclusions.
A recent meta-analysis analyzed 11 studies including 148 patients treated with cryoablation for persistent dysplasia or IM after RFA. CE-D was obtained in 76.0% and CE-IM in 45.9% of patients (80).
Cryoablation seems to be a possible rescue strategy in cases of refractory BE after first-line EET as suggested in a very recent feasibility cohort study (81). CE-D was observed in 78% and CE-IM in 39% with only one treatment of cryotherapy in patients with different baseline pathologies including LGD, HGD and IMC. At a median follow-up of 19 months, CE-D was maintained in 72% and CE-IM in 33% of patients. Esophageal strictures after cryoablation were noted in two cases (11%), each treated with successful endoscopic dilatation.
A first prospective study was recently conducted to assess feasibility, safety and efficacy of the novel CbSAS90 for eradication of dysplastic BE in 25 patients. Circumferential treatment with CbSAS90 resulted in 93% BE-surface regression. As a reference, BE-surface regression rates after single RFA treatment ranges between 78–90% (25,27,42). The procedures were well tolerated, in accordance with the above-mentioned study on patient tolerance favoring cryoablation over RFA (79). In the confirmation phase with circumferential treatment, strictures were observed in 17% of patients, which was impressive and higher than expected by the authors. However, these percentages should be interpreted with caution since the numbers were low. Further studies to determine the optimal dose are required.
Buried glands
A concern regarding ablation techniques is the potential persistence of buried glands, also referred to as buried metaplasia or subsquamous metaplasia, and has been described in several studies (82-85).
If an ablation procedure does not result in complete destruction of all metaplastic epithelium, and partially-ablated mucosa heals with an overlying stratum of neosquamous epithelium, metaplastic glands may be “buried” in the lamina propria, where they remain undetected from the endoscopist’s eye. This buried metaplasia has malignant potential and therefore may progress to EAC. It is not well established whether neoplasia develops from non-neoplastic glands that were buried by ablation or neoplastic glands that were already subsquamous before ablation (82). Of the ablation techniques available, RFA seems to bear a lower risk of buried metaplasia.
In a study by Sharma et al., 3,007 neosquamous biopsies after RFA were evaluated, with no evidence of buried metaplasia (86). According to a systematic review of 18 studies (6 without dysplasia, and 12 with LGD or HGD), including 1,004 patients after RFA of BE with follow-up intervals ranging from 8 weeks to 5 years, buried glands were detected in only 0.9% (9 of 1,004 patients) compared to 14% (135 of 953 patients) following photodynamic therapy (82).
Outcome data of ablation therapy with APC in a 16-year follow-up study showed buried glands in 6 of 25 patients, who had previously achieved CE-IM (83). In the BRIDE-study observed rates of buried metaplasia were 6.1% in the RFA group compared to 13.3% APC group (62).
However, the risk of progression to malignancy of buried metaplasia is thought to be less than that of unablated BE, as the subsquamous cells have no exposure to gastric reflux (87).
Literature on cryospray therapy reports much higher percentages of buried glands ranging from 0–9.1% (88-91). This might be due to unequal distribution of cryospray. Data regarding balloon-based cryotherapy suggest lower rates of buried glands (74). In the feasibility study of cryotherapy using the CbSAS90 no buried glands were found in follow-up biopsies from endoscopically eradicated areas (73). However, further studies are warranted.
Given the hidden nature of buried glands, surveillance endoscopy with biopsies from the previously ablated BE are endorsed.
Postablation management
EET is efficient in treatment of BE-related dysplasia. Nevertheless, endoscopic surveillance after CE-IM is deemed necessary in light of recurrence rate after achieving endoscopic and histologic CE-IM. The schedule of surveillance intervals depends on the grade of dysplasia before ablation. For baseline diagnosis of HGD/EAC endoscopy should be performed at 3, 6, and 12 months after ablation and annually thereafter. After treatment of baseline LGD, the follow-up is at 1 year after ablation and, if there is no recurrence, at 3 years, as recommended by a very recent expert review (13). Endoscopic surveillance requires the use of high-definition white-light endoscopy and preferably optical chromoendoscopy. It should include a meticulous inspection as well as 4-quadrant biopsies from the GEJ and the esophageal neosquamous mucosa to rule out IM and dysplasia. Optimal acid suppression is essential in treatment with ablative therapy, because it permits healing and squamous reepithelization during and after EET. Uncontrolled acid reflux is associated with a higher number of RFA treatment sessions to achieve CE-IM, and a higher rate of recurrences after EET (92,93). Acid suppression with a PPI twice a day is the standard, whereas an H2-receptor antagonist and sucralfate are administered for a short period after every ablation procedure in European RFA studies (10).
Conclusions
The management of BE’s related dysplasia has seen a substantial change from esophagectomy towards EET in the last decades. The role of RFA as first choice ablation modality lies in its efficacy in treating and preventing neoplastic progression of BE while maintaining an excellent safety profile, which has been proven extensively in literature. Nevertheless, ablation of small areas of residual BE can be treated effectively with APC, which is cost-effective and widely available in endoscopy units. Novel ablation tools, particularly cryotherapy, might offer advantages over heat-based ablation, providing similar safety and effectiveness profile as well as greater patient tolerability, but stronger evidence is required. However, they may be used in RFA-refractory disease, as rescue treatment in patients with persisting lesions that cannot be resected, or in case of stenosis that hinders passage of the RFA catheter.
In contempt of excellent eradication rates of ablative modalities, post-ablation surveillance is pivotal, as recurrences occur commonly.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hon Chi Yip and Philip Wai-Yan Chiu) for the series “Endoscopic Diagnosis and Treatment of Early Esophageal Cancer” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-2020-36/coif). The series “Endoscopic Diagnosis and Treatment of Early Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thrift AP. Barrett's Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Dig Dis Sci 2018;63:1988-96. [Crossref] [PubMed]
- Wani S, Rubenstein JH, Vieth M, et al. Diagnosis and Management of Low-Grade Dysplasia in Barrett's Esophagus: Expert Review From the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822-35. [Crossref] [PubMed]
- Phillips WA, Lord RV, Nancarrow DJ, et al. Barrett's esophagus. J Gastroenterol Hepatol 2011;26:639-48. [Crossref] [PubMed]
- van der Werf LR, Busweiler LAD, van Sandick JW, et al. Reporting National Outcomes After Esophagectomy and Gastrectomy According to the Esophageal Complications Consensus Group (ECCG). Ann Surg 2020;271:1095-101. [Crossref] [PubMed]
- Lenglinger J, Riegler M, Cosentini E, et al. Review on the annual cancer risk of Barrett's esophagus in persons with symptoms of gastroesophageal reflux disease. Anticancer Res 2012;32:5465-73.
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209-17. [Crossref] [PubMed]
- Peters FP, Kara MA, Rosmolen WD, et al. Endoscopic treatment of high-grade dysplasia and early stage cancer in Barrett's esophagus. Gastrointest Endosc 2005;61:506-14. [Crossref] [PubMed]
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012;143:336-46. [Crossref] [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200-6. [Crossref] [PubMed]
- Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut 2016;65:555-62. [Crossref] [PubMed]
- Wani S, Mathur SC, Curvers WL, et al. Greater interobserver agreement by endoscopic mucosal resection than biopsy samples in Barrett's dysplasia. Clin Gastroenterol Hepatol 2010;8:783-8. [Crossref] [PubMed]
- Wani S, Abrams J, Edmundowicz SA, et al. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett's esophagus patients with visible and flat neoplasia: a multicenter cohort study. Dig Dis Sci 2013;58:1703-9. [Crossref] [PubMed]
- Sharma P, Shaheen NJ, Katzka D, et al. AGA Clinical Practice Update on Endoscopic Treatment of Barrett's Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020;158:760-9. [Crossref] [PubMed]
- Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017;49:191-8. [Crossref] [PubMed]
- Coco DP, Goldblum JR, Hornick JL, et al. Interobserver variability in the diagnosis of crypt dysplasia in Barrett esophagus. Am J Surg Pathol 2011;35:45-54. [Crossref] [PubMed]
- Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol 2001;32:368-78. [Crossref] [PubMed]
- Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:897-909.e4; quiz 983.e1, 983.e3.
- Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol 2010;105:1523-30. [Crossref] [PubMed]
- Duits LC, Phoa KN, Curvers WL, et al. Barrett's oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015;64:700-6. [Crossref] [PubMed]
- Watts AE, Cotton CC, Shaheen NJ. Radiofrequency Ablation of Barrett's Esophagus: Have We Gone Too Far, or Not Far Enough? Curr Gastroenterol Rep 2020;22:29. [Crossref] [PubMed]
- Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology 2002;123:461-7. [Crossref] [PubMed]
- Ormsby AH, Kilgore SP, Goldblum JR, et al. The location and frequency of intestinal metaplasia at the esophagogastric junction in 223 consecutive autopsies: implications for patient treatment and preventive strategies in Barrett's esophagus. Mod Pathol 2000;13:614-20. [Crossref] [PubMed]
- Old O, Moayyedi P, Love S, et al. Barrett's Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22:158-64. [Crossref] [PubMed]
- Ganz RA, Utley DS, Stern RA, et al. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc 2004;60:1002-10. [Crossref] [PubMed]
- van Vilsteren FG, Phoa KN, Alvarez Herrero L, et al. Circumferential balloon-based radiofrequency ablation of Barrett's esophagus with dysplasia can be simplified, yet efficacy maintained, by omitting the cleaning phase. Clin Gastroenterol Hepatol 2013;11:491-98.e1. [Crossref] [PubMed]
- Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392-9. [Crossref] [PubMed]
- Belghazi K, Pouw RE, Sondermeijer C, et al. A single-step sizing and radiofrequency ablation catheter for circumferential ablation of Barrett's esophagus: Results of a pilot study. United European Gastroenterol J 2018;6:990-9. [Crossref] [PubMed]
- Visrodia K, Zakko L, Wang KK. Mucosal Ablation in Patients with Barrett's Esophagus: Fry or Freeze? Dig Dis Sci 2018;63:2129-35. [Crossref] [PubMed]
- Medtronic. The Barrx ablation system product catalog. 2020. Available online: https://www.medtronic.com/covidien/en-us/products/gastrointestinal-rf-ablation/barrx-radiofrequency-ablation-system.html
- Pouw RE, Künzli HT, Bisschops R, et al. Simplified versus standard regimen for focal radiofrequency ablation of dysplastic Barrett's oesophagus: a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 2018;3:566-74. [Crossref] [PubMed]
- Guthikonda A, Cotton CC, Madanick RD, et al. Clinical Outcomes Following Recurrence of Intestinal Metaplasia After Successful Treatment of Barrett's Esophagus With Radiofrequency Ablation. Am J Gastroenterol 2017;112:87-94. [Crossref] [PubMed]
- Phoa KN, Pouw RE, van Vilsteren FGI, et al. Remission of Barrett's esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology 2013;145:96-104. [Crossref] [PubMed]
- van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut 2011;60:765-73. [Crossref] [PubMed]
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology 2011;141:460-8. [Crossref] [PubMed]
- Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett's neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy 2008;40:370-9. [Crossref] [PubMed]
- Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23-9. [Crossref] [PubMed]
- Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781-9. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Qumseya BJ, Wani S, Gendy S, et al. Disease Progression in Barrett's Low-Grade Dysplasia With Radiofrequency Ablation Compared With Surveillance: Systematic Review and Meta-Analysis. Am J Gastroenterol 2017;112:849-65. [Crossref] [PubMed]
- Sharma VK, Kim HJ, Das A, et al. A prospective pilot trial of ablation of Barrett's esophagus with low-grade dysplasia using stepwise circumferential and focal ablation (HALO system). Endoscopy 2008;40:380-7. [Crossref] [PubMed]
- Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett's Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245-55. [Crossref] [PubMed]
- van Vilsteren FG, Alvarez Herrero L, Pouw RE, et al. Predictive factors for initial treatment response after circumferential radiofrequency ablation for Barrett's esophagus with early neoplasia: a prospective multicenter study. Endoscopy 2013;45:516-25. [Crossref] [PubMed]
- Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett's esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79-86.e1. [Crossref] [PubMed]
- Bulsiewicz WJ, Kim HP, Dellon ES, et al. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett's esophagus. Clin Gastroenterol Hepatol 2013;11:636-42. [Crossref] [PubMed]
- Cotton CC, Wolf WA, Pasricha S, et al. Recurrent intestinal metaplasia after radiofrequency ablation for Barrett's esophagus: endoscopic findings and anatomic location. Gastrointest Endosc 2015;81:1362-9. [Crossref] [PubMed]
- Cotton CC, Wolf WA, Overholt BF, et al. Late Recurrence of Barrett's Esophagus After Complete Eradication of Intestinal Metaplasia is Rare: Final Report From Ablation in Intestinal Metaplasia Containing Dysplasia Trial. Gastroenterology 2017;153:681-688.e2. [Crossref] [PubMed]
- Fujii-Lau LL, Cinnor B, Shaheen N, et al. Recurrence of intestinal metaplasia and early neoplasia after endoscopic eradication therapy for Barrett's esophagus: a systematic review and meta-analysis. Endosc Int Open 2017;5:E430-49. [Crossref] [PubMed]
- Sawas T, Iyer PG, Alsawas M, et al. Higher Rate of Barrett's Detection in the First Year After Successful Endoscopic Therapy: Meta-analysis. Am J Gastroenterol 2018;113:959-71. [Crossref] [PubMed]
- Tan MC, Kanthasamy KA, Yeh AG, et al. Factors Associated With Recurrence of Barrett's Esophagus After Radiofrequency Ablation. Clin Gastroenterol Hepatol 2019;17:65-72.e5. [Crossref] [PubMed]
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Risk of recurrence of Barrett's esophagus after successful endoscopic therapy. Gastrointest Endosc 2016;83:1090-1106.e3. [Crossref] [PubMed]
- Qumseya BJ, Wani S, Desai M, et al. Adverse Events After Radiofrequency Ablation in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016;14:1086-1095.e6. [Crossref] [PubMed]
- Manner H, Enderle MD, Pech O, et al. Second-generation argon plasma coagulation: two-center experience with 600 patients. J Gastroenterol Hepatol 2008;23:872-8. [Crossref] [PubMed]
- Wahab PJ, Mulder CJ, den Hartog G, et al. Argon plasma coagulation in flexible gastrointestinal endoscopy: pilot experiences. Endoscopy 1997;29:176-81. [Crossref] [PubMed]
- Sie C, Bright T, Schoeman M, et al. Argon plasma coagulation ablation versus endoscopic surveillance of Barrett's esophagus: late outcomes from two randomized trials. Endoscopy 2013;45:859-65. [Crossref] [PubMed]
- Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett's epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6-12. [Crossref] [PubMed]
- Van Laethem JL, Jagodzinski R, Peny MO, et al. Argon plasma coagulation in the treatment of Barrett's high-grade dysplasia and in situ adenocarcinoma. Endoscopy 2001;33:257-61. [Crossref] [PubMed]
- May A, Gossner L, Günter E, et al. Local treatment of early cancer in short Barrett's esophagus by means of argon plasma coagulation: initial experience. Endoscopy 1999;31:497-500. [Crossref] [PubMed]
- Attwood SE, Lewis CJ, Caplin S, et al. Argon beam plasma coagulation as therapy for high-grade dysplasia in Barrett's esophagus. Clin Gastroenterol Hepatol 2003;1:258-63.
- Manner H, May A, Miehlke S, et al. Ablation of nonneoplastic Barrett's mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): a prospective multicenter evaluation. Am J Gastroenterol 2006;101:1762-9. [Crossref] [PubMed]
- Schulz H, Miehlke S, Antos D, et al. Ablation of Barrett's epithelium by endoscopic argon plasma coagulation in combination with high-dose omeprazole. Gastrointest Endosc 2000;51:659-63.
- Wronska E, Polkowski M, Orlowska J, et al. Argon plasma coagulation for Barrett's esophagus with low-grade dysplasia: a randomized trial with long-term follow-up on the impact of power setting and proton pump inhibitor dose. Endoscopy 2021;53:123-32. Erratum in: Endoscopy 2021 Feb;53(2):C2. doi: 10.1055/a-1273-3851. Epub 2020 Oct 1.
- Peerally MF, Bhandari P, Ragunath K, et al. Radiofrequency ablation compared with argon plasma coagulation after endoscopic resection of high-grade dysplasia or stage T1 adenocarcinoma in Barrett's esophagus: a randomized pilot study (BRIDE). Gastrointest Endosc 2019;89:680-9. [Crossref] [PubMed]
- Wani S, Muthusamy VR, Shaheen NJ, et al. Development of Quality Indicators for Endoscopic Eradication Therapies in Barrett's Esophagus: The TREAT-BE (Treatment With Resection and Endoscopic Ablation Techniques for Barrett's Esophagus) Consortium. Am J Gastroenterol 2017;112:1032-48. [Crossref] [PubMed]
- Manner H, Neugebauer A, Scharpf M, et al. The tissue effect of argon-plasma coagulation with prior submucosal injection (Hybrid-APC) versus standard APC: A randomized ex-vivo study. United European Gastroenterol J 2014;2:383-90. [Crossref] [PubMed]
- Manner H, May A, Kouti I, et al. Efficacy and safety of Hybrid-APC for the ablation of Barrett's esophagus. Surg Endosc 2016;30:1364-70. [Crossref] [PubMed]
- Trindade AJ, Wee D, Wander P, et al. Successful treatment of refractory Barrett's neoplasia with hybrid argon plasma coagulation: a case series. Endoscopy 2020;52:812-3. [Crossref] [PubMed]
- Baust JG, Gage AA, Bjerklund Johansen TE, et al. Mechanisms of cryoablation: clinical consequences on malignant tumors. Cryobiology 2014;68:1-11. [Crossref] [PubMed]
- Evonich RF 3rd, Nori DM, Haines DE. A randomized trial comparing effects of radiofrequency and cryoablation on the structural integrity of esophageal tissue. J Interv Card Electrophysiol 2007;19:77-83. [Crossref] [PubMed]
- Overwater A, Weusten BLAM. Cryoablation in the management of Barrett's esophagus. Curr Opin Gastroenterol 2017;33:261-9. [Crossref] [PubMed]
- Ghorbani S, Tsai FC, Greenwald BD, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's dysplasia: results of the National Cryospray Registry. Dis Esophagus 2016;29:241-7. [Crossref] [PubMed]
- Friedland S, Triadafilopoulos G. A novel device for ablation of abnormal esophageal mucosa (with video). Gastrointest Endosc 2011;74:182-8. [Crossref] [PubMed]
- Canto MI, Shaheen NJ, Almario JA, et al. Multifocal nitrous oxide cryoballoon ablation with or without EMR for treatment of neoplastic Barrett's esophagus (with video). Gastrointest Endosc 2018;88:438-446.e2. [Crossref] [PubMed]
- van Munster SN, Overwater A, Raicu MGM, et al. A novel cryoballoon ablation system for eradication of dysplastic Barrett's esophagus: a first-in-human feasibility study. Endoscopy 2020;52:193-201. [Crossref] [PubMed]
- Künzli HT, Schölvinck DW, Meijer SL, et al. Efficacy of the CryoBalloon Focal Ablation System for the eradication of dysplastic Barrett's esophagus islands. Endoscopy 2017;49:169-75. [Crossref] [PubMed]
- Schölvinck DW, Künzli HT, Kestens C, et al. Treatment of Barrett's esophagus with a novel focal cryoablation device: a safety and feasibility study. Endoscopy 2015;47:1106-12. [Crossref] [PubMed]
- Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol 2010;21:S187-91. [Crossref] [PubMed]
- Truesdale CM, Soulen MC, Clark TW, et al. Percutaneous computed tomography-guided renal mass radiofrequency ablation versus cryoablation: doses of sedation medication used. J Vasc Interv Radiol 2013;24:347-50. [Crossref] [PubMed]
- Allaf ME, Varkarakis IM, Bhayani SB, et al. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation--initial observations. Radiology 2005;237:366-70. [Crossref] [PubMed]
- van Munster SN, Overwater A, Haidry R, et al. Focal cryoballoon versus radiofrequency ablation of dysplastic Barrett's esophagus: impact on treatment response and postprocedural pain. Gastrointest Endosc 2018;88:795-803.e2. [Crossref] [PubMed]
- Visrodia K, Zakko L, Singh S, et al. Cryotherapy for persistent Barrett's esophagus after radiofrequency ablation: a systematic review and meta-analysis. Gastrointest Endosc 2018;87:1396-1404.e1. [Crossref] [PubMed]
- Alzoubaidi D, Hussein M, Sehgal V, et al. Cryoballoon ablation for treatment of patients with refractory esophageal neoplasia after first line endoscopic eradication therapy. Endosc Int Open 2020;8:E891-9. [Crossref] [PubMed]
- Gray NA, Odze RD, Spechler SJ. Buried metaplasia after endoscopic ablation of Barrett's esophagus: a systematic review. Am J Gastroenterol 2011;106:1899-908; quiz 1909. [Crossref] [PubMed]
- Milashka M, Calomme A, Van Laethem JL, et al. Sixteen-year follow-up of Barrett's esophagus, endoscopically treated with argon plasma coagulation. United European Gastroenterol J 2014;2:367-73. [Crossref] [PubMed]
- Kohoutova D, Haidry R, Banks M, et al. Esophageal neoplasia arising from subsquamous buried glands after an apparently successful photodynamic therapy or radiofrequency ablation for Barrett's associated neoplasia. Scand J Gastroenterol 2015;50:1315-21. [Crossref] [PubMed]
- Zhou C, Tsai TH, Lee HC, et al. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos). Gastrointest Endosc 2012;76:32-40. [Crossref] [PubMed]
- Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc 2007;65:185-95. [Crossref] [PubMed]
- Basavappa M, Weinberg A, Huang Q, et al. Markers suggest reduced malignant potential of subsquamous intestinal metaplasia compared with Barrett's esophagus. Dis Esophagus 2014;27:262-6. [Crossref] [PubMed]
- Verbeek RE, Vleggaar FP, Ten Kate FJ, et al. Cryospray ablation using pressurized CO2 for ablation of Barrett's esophagus with early neoplasia: early termination of a prospective series. Endosc Int Open 2015;3:E107-12. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Halsey KD, Chang JW, Waldt A, et al. Recurrent disease following endoscopic ablation of Barrett's high-grade dysplasia with spray cryotherapy. Endoscopy 2011;43:844-8. [Crossref] [PubMed]
- Ramay FH, Cui Q, Greenwald BD. Outcomes after liquid nitrogen spray cryotherapy in Barrett's esophagus-associated high-grade dysplasia and intramucosal adenocarcinoma: 5-year follow-up. Gastrointest Endosc 2017;86:626-32. [Crossref] [PubMed]
- Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett's esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576-81. [Crossref] [PubMed]
- Komanduri S, Kahrilas PJ, Krishnan K, et al. Recurrence of Barrett's Esophagus is Rare Following Endoscopic Eradication Therapy Coupled With Effective Reflux Control. Am J Gastroenterol 2017;112:556-66. [Crossref] [PubMed]
Cite this article as: Freund S, Probst A, Messmann H. Update on ablative therapy for Barrett’s related dysplasia. Ann Esophagus 2023;6:6.


