Management of Barrett’s esophagus: a narrative review
Introduction
The management of Barrett’s esophagus (BE) is a rapidly evolving field of study with numerous technologies and management strategies continuously falling in and out of favor due to the rapid pace of research and development in this arena. This review aims to distill and synthesize the vast amount of available primary data and society guidelines in order to present the most current and widely used practices and therapies available today. Below, we aim to discuss the diagnosis, screening, surveillance, and medical and interventional therapies. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-31/rc).
Methods
For this review, we utilized the PubMed database to search for English language articles from 2014 through April, 2021. Key search terms included “Barrett’s” in combination with “esophagus”, “oesophagus”, “guidelines”, “diagnosis”, “surveillance”, “treatment”, “ablation”, “eradication”, “radio frequency”, “chemoprevention”, and “endoscopic mucosal resection”. Additionally, the websites of the major US GI societies including the American College of Gastroenterology (ACG), American Gastroenterological Association (AGA), and the American Society for Gastrointestinal Endoscopy (ASGE) were also queried with these search terms. Abstracts were not included for review. Lastly the websites of the medical device manufacturers discussed in this review were accessed for technical information and to ensure accuracy/availability of mentioned devices.
Background on BE
BE is characterized by the metaplasia of esophageal stratified squamous epithelium into columnar epithelium with goblet cells (1). This metaplasia is thought to occur due to long-standing, repetitive injury from acid-bile reflux (1). Both acidic refluxate from the stomach and non-acidic bile salt refluxate from the duodenum are thought to contribute to this process. However, bile salts, which become more soluble as the pH rises in the esophagus, have been shown to be particularly strong inducers of CDX2 expression and goblet cell proliferation (2).
BE is a precancerous condition and is the only known precursor of esophageal adenocarcinoma (EAC). Once metaplasia occurs, BE usually follows a stepwise progression from non-dysplastic BE (NDBE) to low grade dysplasia (LGD) followed by high-grade dysplasia (HGD) and lastly EAC, although non-stepwise progression can occur. NDBE has been estimated to have a 0.33% annual incidence of progression to EAC (1). For patients with LGD, this incidence is considered to be 0.54–0.7% (3,4). Finally, patients with HGD have been shown to have about a 6–7% annual risk of progression to EAC (4-7). If a patient’s biopsies are indefinite for dysplasia (IND), they can be considered to be at similar risk as patients with LGD (4,8).
The prevalence of BE in the general population is about 1–2% (9,10). Risk factors for the development of BE include increasing age, early age of onset of GERD symptoms, male sex, cigarette smoking, central obesity, first-degree family history of BE, and Caucasian race (11-17).
The need for appropriate screening, surveillance, and management of BE arises in order to prevent the progression to EAC, and its associated morbidity and mortality. The incidence of EAC is rapidly increasing in Western countries, including the United States (18). The five-year survival rate of all patients with EAC is just 21.4% (19,20). This increases to 52.9% in patients with only localized disease, highlighting the need for early detection through appropriate BE screening and surveillance (20). Fortunately, treatment of BE with dysplasia has been shown to successfully reverse dysplasia and often BE itself, thus decreasing disease progression (6,21-23).
Diagnosis
The diagnosis of BE in the United States requires endoscopic visualization of at least 1 cm of typical BE appearing mucosa, described as salmon-colored or metaplastic columnar epithelium in the esophagus, with biopsies revealing intestinal metaplasia (IM), the hallmark of which is goblet cells. Of note, the British Society of Gastroenterology does not require evidence of IM (24). This is based on the notion that biopsies may miss diagnosing IM, and even patients without IM may progress to EAC (24-26). If the segment observed is <1 cm, it is referred to as specialized IM of the esophagogastric junction, as opposed to BE because of its significantly lower risk of progression to EAC (4,27). If the segment of BE is >3 cm, it is considered to be long-segment BE (LSBE), and if <3 cm, it is short-segment (SSBE) (4). Notably, patients with non-dysplastic LSBE have a greater risk of progression to EAC compared to SSBE patients (0.31% vs. 0.06% annual risk) (28).
When performing a high quality endoscopic exam, if LA Grade B esophagitis or worse is found on endoscopy, biopsies for BE should not be taken at that time, and a proton pump inhibitor (PPI) should be used for 8–12 weeks to allow for healing (4). This should be followed by repeat endoscopy to rule out underlying BE, as 12% of patients with erosive esophagitis had BE on repeat endoscopy after PPI therapy (29). When documenting endoscopy findings, the Prague C&M Criteria should be used, which describes the affected area using its circumferential extent and maximum length from the gastroesophageal junction in centimeters (29). Additionally, the positions of the diaphragmatic pinch, gastroesophageal junction, and squamocolumnar junction should be documented.
Once suspected BE is identified endoscopically, ideally at least eight biopsies should be taken to increase yield of diagnosis if the segment is <2 cm (26). If that is not feasible, then at least four biopsies per centimeter of circumferential BE and at least one biopsy per centimeter of BE tongues should be obtained (4). Otherwise, Seattle protocol sampling should be followed, which includes four quadrant biopsies every two centimeters in suspected LSBE or every 1 cm if there is prior history of dysplasia (which is typical during post-therapy surveillance) (4). Once biopsies are obtained, any specimen identified as dysplastic should be reviewed by a second pathologist with gastrointestinal expertise to confirm the diagnosis, as dysplasia, especially LGD, is often over-diagnosed (30,31).
Screening
Deciding on whom to screen for BE has been controversial and inconsistent. While there are no studies directly comparing screening vs non-screening patients at risk for BE, expert recommendations remain in place to screen at risk individuals. The American College of Gastroenterology (ACG) recommends screening males with chronic or frequent GERD symptoms, with two or more additional risk factors for BE or EAC (see Table 1) (20). The American Society of Gastrointestinal Endoscopy (ASGE) recommends that if screening is performed, it is for patients with a family history of BE or EAC or patients with GERD plus one other risk factor (32). Lastly, the American Gastroenterological Association (AGA) recommends screening individuals with multiple risk factors (7).
Table 1
| Society | Screening criteria for BE |
|---|---|
| American College of Gastroenterology | GERD symptoms >5 years and/or at least weekly and; male and; at least one of the following: (I) >50 years old, (II) Caucasian race, (III) central obesity (>102 cm waist circumference or waist: hip ratio >0.9), (IV) current or previous smoker, (V) first degree relative with BE or EAC |
| American Society of Gastrointestinal Endoscopy | Family history of BE or EAC or; GERD diagnosis and at least one of the following: (I) >50 years old, (II) obesity/central adiposity, (III) current or previous smoker, (IV) male |
| American Gastroenterological Association | Multiple risk factors for EAC: >50 years old; male sex; Caucasian race; chronic GERD; hiatal hernia; elevated BMI; intra-abdominal distribution of body fat |
BE, Barrett’s esophagus; GERD, gastroesophageal reflux disease; EAC, esophageal adenocarcinoma.
High-definition white light upper endoscopy (HDWLE) with biopsies remains both the gold standard and the most common modality for BE screening and surveillance. However, there are limitations to conventional upper endoscopy, including operator dependence in endoscopy, inter-observer variability among pathologists, risks associated with invasive procedures, and cost. Newer modalities aim to decrease these limitations by increasing diagnostic yield or by decreasing risk and cost with less invasive techniques.
Transnasal endoscopy (TNE) uses a thinner endoscope than standard upper endoscopy, eliminating the need for sedation, and its associated risk and cost. As a result, patients appear more willing to undergo TNE for BE screening than standard upper endoscopy (30). TNE had a respective sensitivity and sensitivity of 98% and 100% when compared with standard per-oral sedated upper endoscopy in a small prospective study (33). TNE is limited by the decreased success in taking biopsies, as this requires a larger sheath, which patients sometimes could not tolerate (34). Furthermore, gastroenterologists and primary care physicians have limited access to and experience with unsedated TNE to perform screening.
Esophageal capsule endoscopy (ECE) is another screening tool that does not require sedation. The capsule, which is a small indigestible disposable capsule with a camera on each end and takes images of the esophagus at a combined rate of 14 frames per second (7 frames per second from each side) is swallowed by the patient and can capture esophageal changes consistent with BE as well as other esophageal or gastric pathology (35). In a 2009 meta-analysis, ECE was found to be safe and preferred by patients, with a respective sensitivity and specificity of 78% and 90% compared to standard upper endoscopy (36).
Tethered capsule endomicroscopy (TCE) is a distinct modality that uses optical coherence tomography (OCT) to obtain high resolution images of the whole esophagus. In a recent clinical trial, the correlation between TCE and standard endoscopy for detection of BE was 0.77–0.79 (37). While this modality is still young, it shows potential to quickly and safely screen BE.
Cytosponge is a screening technology gaining momentum, which involves a swallowed gelatin capsule housing a compressed mesh attached to a string. Once the gelatin capsule dissolves in the gastric cardia, the sponge is released. It is then retrieved via the string and collects a cytologic specimen through abrasion of the esophagus as it is pulled up from the mouth. The specimen is then measured for trefoil factor-3, which is a cellular marker for BE (38). When compared to standard upper endoscopy, the respective sensitivity and specificity of Cytosponge was 73% and 94% for detecting at least 1 cm of circumferential BE, and 90% and 94% for detecting clinically relevant segments at least 2 cm (39). In a recent randomized control trial, Cytosponge was used in a primary care setting for screening patients aged 50 years or older on a PPI. BE was ultimately diagnosed in 2% of the intervention arm and 0.2% of the control arm (P<0.0001), demonstrating its feasibility and effectiveness (40).
Surveillance
Once a diagnosis of BE is made, surveillance is typically performed despite conflicting data on its effect on mortality (41,42). Patients with nondysplastic BE are recommended to undergo surveillance every 3–5 years to monitor for progression to dysplastic BE or EAC, but there is little data to support this recommendation (4,32). The ACG recommends that patients with BE with newly diagnosed BE with IND undergo repeat endoscopy in 3–6 months on optimized acid suppression, followed by annual surveillance if diagnosis is confirmed (4). Patients with BE with LGD are also recommended to undergo annual endoscopic surveillance if no endoscopic therapy is performed (4). However, progression from LGD to EAC is reduced when ablative strategies are employed, so when possible, endoscopic treatment should be offered to patients with BE with LGD (43). If HGD is found, endoscopic eradication therapy (EET) should be pursued, which is discussed further below.
As stated, conventional HDWLE remains the gold standard for BE screening and surveillance. Alternative or adjunctive technologies are being studied and are often utilized in practice, with the goal of increasing diagnostic yield or minimizing the need for random biopsies which can be time-consuming as well as inaccurate. Recently, wide-area transepithelial sampling with computer-assisted three-dimensional analysis (WATS3D) has been used in addition to upper endoscopy with biopsies. WATS3D utilizes a brush that is able to take full thickness, intact samples of the esophageal epithelium, as compared to traditional biopsies which do not preserve the cellular structure. This allows a greater area of the BE mucosa to be sampled, as traditional forceps biopsies only cover a small area, and dysplasia may often be missed. A computer software then analyzes these samples, identifying areas of abnormal cells for pathologists to further review (32). The ASGE currently recommends using WATS3D in conjunction with standard WLE with biopsies in patients with known or suspected BE. Their systematic review found a relative increase in dysplasia detection of 52% for patients with or without a history of BE with dysplasia, with minimal incidence of adverse events (32).
Chromoendoscopy (CE) and more so, virtual chromoendoscopy (VCE) are also among the most prevalent of such surveillance modalities. CE, used less commonly, utilizes a staining solution applied topically to the esophagus to help identify mucosal abnormalities. Solutions include methylene blue, Lugol’s solution, and acetic acid. VCE does not use a stain and instead uses blue light with narrow band filters. This allows for improved visualization of the superficial mucosa and vascular structures, as hemoglobin absorbs blue light. On most standard endoscopes, VCE is available to be instantly enabled during the procedure. Varying versions of VCE are available based on endoscope manufacturer and include narrow band imaging (NBI), Fuji intelligent color enhancement (FICE), and i-Scan. CE and VCE can help endoscopically identify areas of BE and dysplasia to allow for more targeted, rather than random, biopsies. The ASGE recommends using CE or VCE in addition to WLE with biopsies for BE surveillance. This is based on their meta-analysis, which found that employing these techniques increased detection of dysplasia by 30.3% (32).
Autofluorescence imaging (AFI) uses fluorescence, which is emitted by endogenous fluorophores in esophageal epithelium and stroma after undergoing neoplastic changes (44). This imaging can allow for endoscopists to take targeted biopsies of abnormal areas, similar to CE and VCE. However, AFI has been found to have a high false positive rate for HGD of 49% (45). As a result, it is sometimes used in conjunction with WLE and even CE/VCE.
Confocal laser endomicroscopy (CLE) visualizes the esophagus on a cellular level in real time via a probe passed through an endoscope. Prior to the procedure, the patient must first undergo intravenous injection of a fluorescence agent (46). While there is an advantage in CLE’s ability to make a diagnosis of BE with or without dysplasia during the procedure, the ASGE’s review did not find a statistical significance in the relative increase in diagnostic yield (32).
Volumetric laser endomicroscopy (VLE) employs OCT via an inflated balloon in the esophageal lumen and provides a real time image of the esophageal wall magnified similarly to low-power microscopy. The newest version helps identify areas of dysplasia using an artificial intelligence software termed intelligent real-time image segmentation or IRIS (47). With this information, endoscopists can make visible laser marks on the esophageal mucosa to target biopsies and direct therapy (48,49). VLE with laser marking for targeted biopsies has a higher diagnostic yield than WLE with biopsies alone, at 33.7% compared to 9.6% (50). However, the ASGE could not recommend for or against the use of VLE, calling for more evidence (32). Unfortunately, NinePoint Medical, the maker of VLE filed for bankruptcy in October, 2020, calling into question the future of this technology.
Artificial intelligence in the form of computer-aided detection is in its infancy. A system has been studied in the Netherlands that detected BE or EAC with 90% sensitivity and 88% specificity and outperformed non expert endoscopists (51,52). While this technology is nascent, the potential to revolutionize screening and surveillance is present.
TissueCypher is being studied as a method of analyzing biopsy specimens using their epithelial, stromal, and morphometric components. It provides a numeric score to categorize the risk of progression to EAC and can thus help guide decisions to perform endoscopic eradication therapies. In several small studies, it has been validated to predict risk of progression (53-55). Larger studies are needed before it can be widely implemented.
Non-endoscopic based programs, such as a serum assays have been studied as well and could potentially remove the risk associated with invasive surveillance regimens. Several different miRNAs have been found to be circulating in patients with BE and EAC compared to patients with just esophagitis (56,57). With continued studies and validation, these tools can potentially be used as non-invasive forms of screening and surveillance of BE patients.
Treatment
Chemoprevention
Therapy of BE begins with medical management, of which PPIs are a mainstay, especially in the case of erosive esophagitis, which can confer a five-fold risk of BE (58). However, there has been notable controversy regarding their chemopreventive value in the past. Although data from the 2000’s and 2010’s was largely favorable, two high quality observational studies out of Denmark and the UK actually suggested an increased odds-ratio between EAC and PPI use (59). Now the AspECT trial, a randomized factorial trial involving about 2,500 patients published in 2018, demonstrated that in fact high dose PPI in combination with aspirin therapy provided the greatest overall benefit (60).
Current guidelines, which predate the AspECT trial, only support the use of daily PPI and actually recommend against high dose PPI unless needed for refractory reflux. They also do not endorse aspirin or NSAIDs for chemoprevention at this time unless indicated for another medical co-morbidity (4). However, given this new data, this recommendation could change.
EET
EET, defined as the complete destruction and replacement of BE mucosa with neosquamous epithelium via endoscopic modality, has revolutionized the care of BE and is the first-line therapy for patients diagnosed with confirmed LGD, HGD and EAC T1a (7). Especially in the case of EAC T1a, EET when compared to esophagectomy has been shown to be comparably effective in its therapeutic success while superior in terms of morbidity, mortality and cost (61).
In the case of LGD, EET was previously considered controversial due to high interobserver variability of pathological diagnosis as well as a low rate of progression of 0.5%. However, in LGD that is confirmed by expert pathologist and found to be persistent on repeat EGD after 3–6 months of acid suppression, the annual progression rate was found in fact to be 13.4% (7). The SURF trial compared EET of LGD to surveillance alone and found an absolute risk reduction of progression to HGD/EAC by 25% (43). Given this data, recent guidelines suggest both EET and yearly surveillance are valid treatment strategies (7). Lastly, in those with NDBE plus a family history of EAC, EET could reasonably be offered on a case-by-case basis, though in general, EET of NDBE is not considered cost-effective (7,60). As stated, all cases of dysplasia/neoplasia should be confirmed by an expert pathologist prior to initiation of any advanced therapeutic regimen.
Because BE is largely considered to proceed (though not always) in a stepwise fashion to EAC, early intervention with EET can prevent progression to invasive cancer with low risk of lymph node metastasis (0–2%) (7,62). The goal of EET is the complete eradication of dysplasia (CE-D) and neoplasia (CE-N) with an end point of complete eradication of intestinal metaplasia (CE-IM). The process begins with a thorough endoscopic examination, followed by resection of all nodules/visible abnormalities, then ablation of any remaining flat BE, and ends with a surveillance program.
There is often a temptation when assessing nodules to utilize endoscopic ultrasound (EUS) to assess the depth of invasion. However, due to cost, risk of perforation, and false positive rate (6–10%), it is not recommended to do so unless attempting to examine a lymph node for metastasis (33).
Endoscopic resection
If any suspicious mucosal irregularities or nodularities are identified, the lesion should undergo endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). If the endoscopist cannot perform EMR, then preferably, the patient should be referred to an expert rather than performing biopsy, as this can make future resection more challenging due to scarring (7). If HGD is identified on biopsy of presumably flat BE, a repeat endoscopy should be performed within 6–8 weeks to reexamine for nodules and irregularities as truly flat HGD is uncommon (60). EMR is not only therapeutic but also allows for superior staging of the lesion, as compared to biopsy (62). It does this by providing larger and deeper specimens with preserved architecture, resulting in greater interobserver agreement among pathologists and often in an upgraded histopathologic diagnosis from original (63).
While multiple EMR devices are available, the multiband mucosectomy (MBM) technique is currently favored due to its lower cost, learning curve and procedure time (64). There are currently two commercially available devices: the established Duette® Multi-Band Mucosectomy device (Cook Medical) and the newer Captivator (Boston Scientific Ltd.). In general terms, MBM devices consist of a transparent cap preloaded with multiple rubber bands, which is affixed over the distal end of the endoscope; a triggering cord that traverses through the suction channel; and a hand-cranked ligator handle to which the triggering cord attaches. The operator will suction the mucosal abnormality into the suction channel and then turn the ligator handle, firing the rubber band over and around the lesion and causing it to be cinched at its base, thus producing a pseudopolyp. The effect of the deployed rubber band is to safely separate the mucosa and submucosa from the muscularis propria, allowing resection to proceed with a lowered risk of perforation while obviating the need for submucosal injection. A snare is then passed down the scope, placed around the pseudopolyp and below the rubber band, and then is cinched closed. Lastly electrocautery is applied to resect the tissue. Up to 6 rubber bands can be placed at a time per MBM device.
Both the Duette and Captivator have been shown to be equally efficacious in a recent retrospective study of 40 patients (65). However, the authors did report an increased mucosal resection size and depth with the Captivator, leading them to conclude it may be more favorable for larger lesions or when fewer resections are preferred (65). Other authors have also reported improved visibility and suction capability with the Captivator device (66). In combination with ablative therapies of flat BE, EMR has proven to be a successful modality with a CE-N rate of 92% and CE-IM rate of 87% and recurrence rates of 4% and 8% respectively (67). Nonetheless, EMR does have its pitfalls, primarily of which is the limited rate of R0 resection due to piecemeal resections. This limitation results in missed areas of advanced neoplasia as well as a higher incidence of recurrence (68).
Overcoming this disadvantage of EMR is ESD, an alternative resection technique, albeit one that requires much more intensive training as well as intraprocedural time to perform. Developed in the 1990’s in Japan, ESD quickly established itself in Asia due to the high rates of gastric cancer with adoption slowly emerging next in Europe and now in the US (69). Compared to EMR, ESD is able to resect larger lesions, especially those >2 cm, and achieve a higher rate of R0 resection (69). In its most basic form, ESD consists of thermally marking the borders of a lesion, then using a submucosal injection to lift an adjacent portion of the lesion, followed by incision with an endoscopic knife to gain entry into the submucosal space. Use of a transparent cap on the endoscope tip is essential to create traction and visualization in this space and allow continued submucosal dissection until the lesion is entirely separated from the wall and can be removed en bloc (69). While ESD does provide more R0 resections, it also has higher incidence of adverse events without necessarily improving rates of CE and elective surgery (7). As mentioned, it also takes significantly longer to perform and is not yet widely available in the US due to the rigorous training required to safely and adequately perform the procedure. ESD is specifically recommended over EMR in the following scenarios: lesions >15 mm, morphology indicating submucosal invasion such as ulceration or central depression, and intramucosal carcinoma (61).
Once the lesion is successfully removed with no evidence of residual tissue on pathology, ablative therapy, as detailed below, should be performed on the remaining flat BE mucosa in order to prevent metachronous and recurrent disease, which can range from 14.5–36.7% (7). If pathology reveals there is submucosal involvement (stage T1b), then a multi-disciplinary approach should be considered, as these lesions can have up to a 45% chance of lymphatic spread (4,70). Further management of early EAC is beyond the scope of this review but is found elsewhere in this series.
Endoscopic ablation
After successful resection or if starting with flat, confirmed dysplastic BE, ablation is performed next. Several modalities are currently available including photodynamic therapy (PDT), argon plasma coagulation (APC), hybrid APC, spray cryotherapy, balloon-based cryotherapy, and radiofrequency ablation (RFA). RFA is the most widely used modality followed by cryotherapy. Currently, given the level I evidence documenting its efficacy and safety, RFA is recommended by societal guidelines as the first line ablation method (7).
The first pre-clinical RFA human trials were done in 2006 and to date, RFA is the most well-studied ablative modality (62). RFA is performed every 2–3 months until CE-IM is achieved using the Barrx system (Medtronic, Minneapolis, Minn, USA), which delivers controllable RF (450 to 500 kHz) energy to BE mucosa to the depth of the muscularis mucosa (700–800 µm), inducing tissue necrosis and resulting in regeneration of neosquamous epithelium (71,72). Generally speaking, the treatment regimen typically begins with a circumferential 360 balloon, followed by a focal 60 or 90 ablation catheter to address any residual BE tissue. There are currently two available versions of the 360 balloon: the first-generation 360 balloon, which has a 3 cm long bipolar RF electrode wrapped around a 4 cm non-compliant, cylindrical balloon; and a newer version called the 360 Express, which features a 4 cm long electrode around a self-sizing balloon that can adjust its diameter endoluminally from 18 to 31 mm (71).
The procedure begins with instilling 1% N acetylcysteine into the esophageal lumen to remove any excess mucous. With the traditional balloon, the next step is sizing of the esophageal inner diameter at multiple levels with a sizing balloon catheter placed via guidewire and then selecting the smallest appropriate sized RFA balloon catheter. Once the RFA catheter is in place, the endoscope is reinserted for visualization and a “One-Clean-One” algorithm is typically employed, whereby the first round of energy is delivered, the sloughed coagulum is scraped off with a transparent tip cap, and then a second round of energy is delivered. However, the traditional balloon has two main disadvantages; first, it required multiple, time-consuming passes of the endoscope and sizing balloon catheters and second, the fixed balloon diameter does not always produce optimal mucosal contact due to the compliant and luminally variable nature of the esophagus (73).
With the self-sizing balloon, the procedure remains identical except that multiple passes with sizing balloons are no longer needed. While this does lead to a significantly decreased procedure time without sacrificing efficacy, there was concern for increased stricture formation at the traditional energy density setting of 12 J/cm2 due to larger electrode and better electrode-mucosa apposition. As a result, a setting of 10 J/cm2 is now recommended (73). In an attempt to reduce the procedure time of the first generation balloon, some studies investigated whether cleaning the sloughed coagulum was in fact a necessary step and found that the efficacy and safety were not reduced by skipping this step (74). Interestingly in the case of the self-sizing balloon, Belghazi et al. found that the cleaning step was essential to preventing high rates of stricture formation (73). They theorized that the cleaning phase allows for a “cool-down” period in between treatments as well as time for the development of submucosal edema which further protects the underlying tissue.
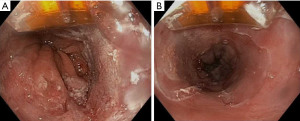
Focal RFA with the Barrx 90, 60, Ultra Long and Channel endoscopic catheter is utilized for either limited areas of dysplastic BE or in follow up after initial circumferential RFA (Figure 1) (72). Excluding the channel catheter which passes through the working channel, the devices attach on the outside to the distal tip of the endoscope with the electrode oriented at the 12 o’ clock position. For the 90 and 60 device, the electrode is apposed against the lesion and 2 applications of 12 J/cm2 are delivered, followed by a cleaning phase and then 2 more applications, also known as a “Two-Clean-Two” algorithm. For the Ultra Long, “One-Clean-One” algorithm is performed (72).
Overall, RFA is a proven and successful modality with recent meta-analysis demonstrating pooled CE-D and CE-IM of 93.4% and 73.1%, respectively (75). It typically takes two to three sessions to achieve CE. RFA is also considered safe with a pooled stricture rate of 5.6%, bleeding rate of 1%, and perforation rate of 0.7% (7). Only about 4% of patients experienced significant post-procedure chest pain, although most experienced at least some chest discomfort (7).
Cryotherapy is the next most frequently utilized modality and proposes to address some of the drawbacks of RFA such as stricture formation, post-procedure pain, and failure of CE-D (71). The use of cold therapy dates back to the Egyptians around 3000BC (76). Modern application starts with a cryogen, a substance that can be cooled to extremely low temperatures and then applied to target tissue, typically liquefied gas such as nitrogen or carbon dioxide (77). When the cryogen is applied to BE mucosa, it triggers death via several mechanisms, including cell membrane disruption, protein denaturation, and destructive osmotic gradients due to ice crystal formation as well as the upregulation of apoptosis mediated by cytochrome C release (77). The thawing process is also important for tissue destruction because during thawing, ice crystals fuse and further damage cell membranes. Additionally, vascular stasis due to endothelial damage, platelet aggregation, and microthrombi result in local ischemic injury (77). Because cryotherapy does not utilize thermal therapy, it has been suggested that it preserves more of the extracellular matrix architecture, resulting in less stricture formation (71).
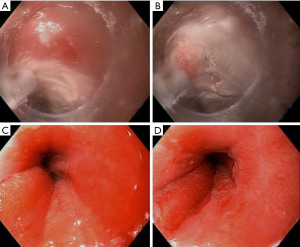
There are two types of cryotherapy systems currently commercially available: spray cryotherapy (truFreeze, CSA Medical, Lexington, Mass) and a more recently developed balloon cryotherapy (Coldplay CryoBalloon Focal Ablation System, C2 Therapeutics, Redwood City, Calif) (77). Spray cryotherapy delivers liquid nitrogen at −196 ℃ to esophageal mucosa via a catheter passed through the working channel of a standard endoscope (Figure 2). Liquid nitrogen rapidly converts to a gas so prior to spray initiation, a 20F dual channel decompression tube is positioned coaxially with the endoscope to aid in active venting so as to prevent perforation due to high pressure. The tip of the catheter is positioned about 0.5 to 1 cm away from the target and then the spray is discharged and “painted” on to the areas of interest. The dosimetry timer (20 seconds) is started once a white frost is formed. The tissue is then allowed to thaw before applying a second dose. The balloon cryotherapy device is also a focal and not circumferential device, but it does not require a decompression tube or a large console storing a liquid nitrogen tank. Instead, the disposable, hand-held, and self-contained device utilizes a battery powered handle housing a 23.5 g liquid nitrous oxide cartridge, attached to a long catheter with a self-sizing balloon at the tip. When the handle is triggered, the catheter delivers a preset perpendicularly oriented focal spray at −85 ℃ inside the balloon, the exterior of which is contacting the mucosa. This catheter can be rotated inside the balloon to target different areas. Areas are treated only once for about 6–10 seconds and each cartridge can treat 2–3 lesions. When the balloon is deflated, the rest of the gas is vented back into the handle (77).
Cryotherapy has shown promising efficacy and impressive safety. A 2016 multicenter, prospective open label registry demonstrated that for LGD, cryotherapy had CE-D and CE-IM rates of 91% and 61%, respectively. For HGD, CE-D and CE-IM were 81% and 65%, respectively (78). Cryotherapy’s first retrospective cohort study in 2010 showed CE-D of 97% and CE-IM of 87% (79). No perforation has yet been reported, the stricture rate has been almost zero, and patients report significantly less procedure-related pain (71). While cryotherapy is well-studied, it does not have the robust level I evidence of RFA or a head-to-head comparison trial. Additionally, its performance does appear to be more operator dependent than RFA, so for these reasons, RFA is still considered first line (72). However, for the approximately 20% of patients who fail RFA, cryotherapy is emerging as a salvage therapy, achieving CE-D and CE-IM rates of approximately 75% and 30–50%, respectively (71).
PDT was a more widely used modality in the past but has recently fallen out of favor due to its safety profile, which includes unfavorable stricture rate and phototoxicity (80). Prior to the procedure, patients must receive a photosensitizer drug, either orally with 5-aminolevulinic acid (not available in the US) 4 hours before or intravenously with sodium porfimer 48 hours before (81). This drug is preferentially taken up by dysplastic and neoplastic tissue. A light fiber is then passed through the endoscope and then photoradiation can be applied, typically at “red” wavelengths of 630–635 nm, which activate the drug resulting in cell death via reactive oxygen species (81).
APC (Erbe Elektromedizin, Tuebingen, Germany) is a long-standing therapeutic option for BE, but like RFA, had a stricture rate from 5–10% (82). Proposing to address this drawback, Hybrid APC combines APC’s thermal therapy with submucosal lift via isotonic saline injection. Both of these functions are performed by a single integrated catheter (82). However, being a focal ablation device, its success is still much more operator dependent than RFA. While early studies show promising data, further study is required.
Post-treatment surveillance
Once CE-IM has been achieved, post-treatment surveillance begins. Surveillance must be diligent because recurrence of BE is not uncommon post-therapy with reported rates between 10–20% over 2 years and up to 49% at 8.6 years (73,83). The surveillance regimen depends upon the initial staging of the lesion as LGD or HGD/IMC as well as which society guideline the provider chooses to follow. For LGD, the ACG recommends surveillance every 6 months for 1 year and then annually thereafter, while the AGA recommends surveillance at 1 and 3 years. For HGD/IMC, the ACG recommends surveillance every 3 months for the first year, then every 6 months in year two, and then annually. The AGA recommends surveillance at 3 months, 6 months, 1 year and then annually (83). As previously discussed, Seattle protocol biopsies should be obtained and strong consideration of the use of advanced imaging and sampling techniques, such as NBI, WATS3D, and possibly VLE if available, should be made (32).
Surgical management
Anti-reflux surgery has been used in BE as well, with laparoscopic fundoplication as the mainstay surgical option. However, data do not show that it decreases the progression of BE to EAC (84,85). A meta-analysis did show an association between surgery and regression of BE though (84). Regardless, the ACG does not recommend it as a therapy for BE itself, but validates its continued use for refractory GERD (4). The Society of American Gastrointestinal and Endoscopic Surgeons recommends considering surgical options for patients with BE with symptomatic GERD, noting it remains controversial in patients with asymptomatic BE, noting the data is too inconclusive to comment on the resolution or improvement of BE after anti-reflux surgery (86).
In regards to dysplastic BE and early EAC, esophagectomy is increasingly becoming a treatment of last resort. However, it is still the mainstay therapy of submucosal EAC (T1b). It carries a morbidity and mortality rate of 30% and 6%, respectively (7). The detailed management of early esophageal cancer is discussed elsewhere in this series.
Conclusions
The field of BE is a rapidly evolving area of study with numerous technological innovations either in development or quickly reaching maturity. Even with so much progress, numerous controversies still exist from screening and diagnosis to surveillance and eradication therapy. With the incidence of EAC rising decade over decade, ongoing research to clarify these management decisions will take on ever increasing importance, particularly in regards to targeted screening as well as progression risk stratification in order to reduce the burden of unnecessary endoscopic procedures. Clinical studies evaluating the integration of acid testing, endoscopic findings, biomarkers and pathology to develop a robust model of BE progression would be especially helpful in addressing controversies in screening and surveillance. As both advanced imaging and ablation techniques become more advanced and refined, hopefully rates of missed lesions and recurrence will further diminish. The study of BE will continue to provide dramatic and meaningful impact on the health and longevity of patients for years to come.
Acknowledgments
An abstract of this study was accepted for presentation at Digestive Disease Week (DDW) 2020 and published in Gastroenterology.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Abbas E. Abbas and Roman V. Petrov) for the series “New Technologies in Esophageal Surgery and Endoscopy” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-31/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-31/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-31/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut 2012;61:970-6. [Crossref] [PubMed]
- Quante M, Graham TA, Jansen M. Insights Into the Pathophysiology of Esophageal Adenocarcinoma. Gastroenterology 2018;154:406-20. [Crossref] [PubMed]
- Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:897-909.e4; quiz 983.e1, 983.e3.
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol 2016;111:30-50; quiz 51. [Crossref] [PubMed]
- Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394-8. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Sharma P, Shaheen NJ, Katzka D, et al. AGA Clinical Practice Update on Endoscopic Treatment of Barrett's Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020;158:760-9. [Crossref] [PubMed]
- Montgomery E, Goldblum JR, Greenson JK, et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol 2001;32:379-88. [Crossref] [PubMed]
- Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology 2005;129:1825-31. [Crossref] [PubMed]
- Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut 2008;57:1354-9. [Crossref] [PubMed]
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett's esophagus by endoscopy indication. Gastrointest Endosc 2010;71:21-7. [Crossref] [PubMed]
- Thrift AP, Kramer JR, Qureshi Z, et al. Age at onset of GERD symptoms predicts risk of Barrett's esophagus. Am J Gastroenterol 2013;108:915-22. [Crossref] [PubMed]
- Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol 2005;162:1050-61. [Crossref] [PubMed]
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett's esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013;28:1258-73. [Crossref] [PubMed]
- Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1399-1412.e7. [Crossref] [PubMed]
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett's oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002;51:323-8. [Crossref] [PubMed]
- Abrams JA, Fields S, Lightdale CJ, et al. Racial and ethnic disparities in the prevalence of Barrett's esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol 2008;6:30-4. [Crossref] [PubMed]
- Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol 2012;23:3155-62. [Crossref] [PubMed]
- Sikkema M, de Jonge PJ, Steyerberg EW, et al. Risk of esophageal adenocarcinoma and mortality in patients with Barrett's esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2010;8:235-44; quiz e32. [Crossref] [PubMed]
- Adenocarcinoma of the esophagus SEER 5-year relative survival rates, 2010-2016. SEER Web site. Available online: https://seer.cancer.gov/explorer/application.html?site=600&data_type=4&graph_type=5&compareBy=stage&chk_stage_101=101&chk_stage_104=104&chk_stage_105=105&chk_stage_106=106&chk_stage_107=107&series=9&sex=1&race=1&age_range=1&advopt_precision=1. Accessed March 19, 2021.
- Canto MI, Trindade AJ, Abrams J, et al. Multifocal Cryoballoon Ablation for Eradication of Barrett's Esophagus-Related Neoplasia: A Prospective Multicenter Clinical Trial. Am J Gastroenterol 2020;115:1879-90. [Crossref] [PubMed]
- Manner H, May A, Kouti I, et al. Efficacy and safety of Hybrid-APC for the ablation of Barrett's esophagus. Surg Endosc 2016;30:1364-70. [Crossref] [PubMed]
- Thota PN, Arora Z, Dumot JA, et al. Cryotherapy and Radiofrequency Ablation for Eradication of Barrett's Esophagus with Dysplasia or Intramucosal Cancer. Dig Dis Sci 2018;63:1311-9. [Crossref] [PubMed]
- Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7-42. [Crossref] [PubMed]
- Gatenby PA, Ramus JR, Caygill CP, et al. Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus. Scand J Gastroenterol 2008;43:524-30. [Crossref] [PubMed]
- Harrison R, Perry I, Haddadin W, et al. Detection of intestinal metaplasia in Barrett's esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol 2007;102:1154-61. [Crossref] [PubMed]
- Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population-based study. Am J Gastroenterol 2011;106:1447-55; quiz 1456. [Crossref] [PubMed]
- Chandrasekar VT, Hamade N, Desai M, et al. Significantly lower annual rates of neoplastic progression in short- compared to long-segment non-dysplastic Barrett's esophagus: a systematic review and meta-analysis. Endoscopy 2019;51:665-72. [Crossref] [PubMed]
- Hanna S, Rastogi A, Weston AP, et al. Detection of Barrett's esophagus after endoscopic healing of erosive esophagitis. Am J Gastroenterol 2006;101:1416-20. [Crossref] [PubMed]
- Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol 2010;105:1523-30. [Crossref] [PubMed]
- Duits LC, Phoa KN, Curvers WL, et al. Barrett's oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015;64:700-6. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc 2019;90:335-359.e2. [Crossref] [PubMed]
- Shariff MK, Bird-Lieberman EL, O'Donovan M, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett's esophagus. Gastrointest Endosc 2012;75:954-61. [Crossref] [PubMed]
- Sami SS, Dunagan KT, Johnson ML, et al. A randomized comparative effectiveness trial of novel endoscopic techniques and approaches for Barrett's esophagus screening in the community. Am J Gastroenterol 2015;110:148-58. [Crossref] [PubMed]
- Waterman M, Gralnek IM. Capsule endoscopy of the esophagus. J Clin Gastroenterol 2009;43:605-12. [Crossref] [PubMed]
- Bhardwaj A, Hollenbeak CS, Pooran N, et al. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett's esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol 2009;104:1533-9. [Crossref] [PubMed]
- Dong J, Grant C, Vuong B, et al. Feasibility and Safety of Tethered Capsule Endomicroscopy in Patients With Barrett's Esophagus in a Multi-Center Study. Clin Gastroenterol Hepatol 2022;20:756-65.e3. [Crossref] [PubMed]
- Spechler SJ, Katzka DA, Fitzgerald RC. New Screening Techniques in Barrett's Esophagus: Great Ideas or Great Practice? Gastroenterology 2018;154:1594-601. [Crossref] [PubMed]
- Kadri SR, Lao-Sirieix P, O'Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ 2010;341:c4372. [Crossref] [PubMed]
- Fitzgerald RC, di Pietro M, O'Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet 2020;396:333-44. [Crossref] [PubMed]
- Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett's esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am J Gastroenterol 2014;109:1215-22. [Crossref] [PubMed]
- Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett's esophagus-associated esophageal adenocarcinomas. Gastroenterology 2013;145:312-9.e1. [Crossref] [PubMed]
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209-17. [Crossref] [PubMed]
- Thekkek N, Anandasabapathy S, Richards-Kortum R. Optical molecular imaging for detection of Barrett's-associated neoplasia. World J Gastroenterol 2011;17:53-62. [Crossref] [PubMed]
- Kara MA, Peters FP, Ten Kate FJ, et al. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett's esophagus. Gastrointest Endosc 2005;61:679-85. [Crossref] [PubMed]
- Li H, Hou X, Lin R, et al. Advanced endoscopic methods in gastrointestinal diseases: a systematic review. Quant Imaging Med Surg 2019;9:905-20. [Crossref] [PubMed]
- Trindade AJ, McKinley MJ, Fan C, et al. Endoscopic Surveillance of Barrett's Esophagus Using Volumetric Laser Endomicroscopy With Artificial Intelligence Image Enhancement. Gastroenterology 2019;157:303-5. [Crossref] [PubMed]
- Suter MJ, Gora MJ, Lauwers GY, et al. Esophageal-guided biopsy with volumetric laser endomicroscopy and laser cautery marking: a pilot clinical study. Gastrointest Endosc 2014;79:886-96. [Crossref] [PubMed]
- Houston T, Sharma P. Volumetric laser endomicroscopy in Barrett's esophagus: ready for primetime. Transl Gastroenterol Hepatol 2020;5:27. [Crossref] [PubMed]
- Alshelleh M, Inamdar S, McKinley M, et al. Incremental yield of dysplasia detection in Barrett's esophagus using volumetric laser endomicroscopy with and without laser marking compared with a standardized random biopsy protocol. Gastrointest Endosc 2018;88:35-42. [Crossref] [PubMed]
- de Groof AJ, Struyvenberg MR, van der Putten J, et al. Deep-Learning System Detects Neoplasia in Patients With Barrett's Esophagus With Higher Accuracy Than Endoscopists in a Multistep Training and Validation Study With Benchmarking. Gastroenterology 2020;158:915-929.e4. [Crossref] [PubMed]
- Pannala R, Krishnan K, Melson J, et al. Artificial intelligence in gastrointestinal endoscopy. VideoGIE 2020;5:598-613. [Crossref] [PubMed]
- Frei NF, Konte K, Bossart EA, et al. Independent Validation of a Tissue Systems Pathology Assay to Predict Future Progression in Nondysplastic Barrett's Esophagus: A Spatial-Temporal Analysis. Clin Transl Gastroenterol 2020;11:e00244. [Crossref] [PubMed]
- Frei NF, Khoshiwal AM, Konte K, et al. Tissue Systems Pathology Test Objectively Risk Stratifies Barrett's Esophagus Patients With Low-Grade Dysplasia. Am J Gastroenterol 2021;116:675-82. [Crossref] [PubMed]
- Davison JM, Goldblum J, Grewal US, et al. Independent Blinded Validation of a Tissue Systems Pathology Test to Predict Progression in Patients With Barrett's Esophagus. Am J Gastroenterol 2020;115:843-52. [Crossref] [PubMed]
- Cabibi D, Caruso S, Bazan V, et al. Analysis of tissue and circulating microRNA expression during metaplastic transformation of the esophagus. Oncotarget 2016;7:47821-30. [Crossref] [PubMed]
- Bus P, Kestens C, Ten Kate FJ, et al. Profiling of circulating microRNAs in patients with Barrett's esophagus and esophageal adenocarcinoma. J Gastroenterol 2016;51:560-70. [Crossref] [PubMed]
- Ronkainen J, Talley NJ, Storskrubb T, et al. Erosive esophagitis is a risk factor for Barrett's esophagus: a community-based endoscopic follow-up study. Am J Gastroenterol 2011;106:1946-52. [Crossref] [PubMed]
- Snider EJ, Kaz AM, Inadomi JM, et al. Chemoprevention of esophageal adenocarcinoma. Gastroenterol Rep (Oxf) 2020;8:253-60. [Crossref] [PubMed]
- Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): a randomised factorial trial. Lancet 2018;392:400-8. [Crossref] [PubMed]
- Kolb JM, Wani S. Endoscopic eradication therapy for Barrett's oesophagus: state of the art. Curr Opin Gastroenterol 2020;36:351-8. [Crossref] [PubMed]
- Komanduri S, Muthusamy VR, Wani S. Controversies in Endoscopic Eradication Therapy for Barrett's Esophagus. Gastroenterology 2018;154:1861-1875.e1. [Crossref] [PubMed]
- Wani S, Mathur SC, Curvers WL, et al. Greater interobserver agreement by endoscopic mucosal resection than biopsy samples in Barrett's dysplasia. Clin Gastroenterol Hepatol 2010;8:783-8. [Crossref] [PubMed]
- Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointest Endosc 2011;74:35-43. [Crossref] [PubMed]
- Alzoubaidi D, Graham D, Bassett P, et al. Comparison of two multiband mucosectomy devices for endoscopic resection of Barrett's esophagus-related neoplasia. Surg Endosc 2019;33:3665-72. [Crossref] [PubMed]
- Schölvinck DW, Belghazi K, Pouw RE, et al. In vitro assessment of the performance of a new multiband mucosectomy device for endoscopic resection of early upper gastrointestinal neoplasia. Surg Endosc 2016;30:471-9. [Crossref] [PubMed]
- Phoa KN, Rosmolen WD, Weusten BLAM, et al. The cost-effectiveness of radiofrequency ablation for Barrett's esophagus with low-grade dysplasia: results from a randomized controlled trial (SURF trial). Gastrointest Endosc 2017;86:120-129.e2. [Crossref] [PubMed]
- Terheggen G, Horn EM, Vieth M, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett's neoplasia. Gut 2017;66:783-93. [Crossref] [PubMed]
- Nishizawa T, Yahagi N. Endoscopic mucosal resection and endoscopic submucosal dissection: technique and new directions. Curr Opin Gastroenterol 2017;33:315-9. [Crossref] [PubMed]
- Nentwich MF, von Loga K, Reeh M, et al. Depth of submucosal tumor infiltration and its relevance in lymphatic metastasis formation for T1b squamous cell and adenocarcinomas of the esophagus. J Gastrointest Surg 2014;18:242-9; discussion 249. [Crossref] [PubMed]
- Visrodia K, Zakko L, Wang KK. Mucosal Ablation in Patients with Barrett's Esophagus: Fry or Freeze? Dig Dis Sci 2018;63:2129-35. [Crossref] [PubMed]
- ASGE Technology Committee. Radiofrequency ablation devices. VideoGIE 2017;2:252-9. [Crossref] [PubMed]
- Belghazi K, Pouw RE, Koch AD, et al. Self-sizing radiofrequency ablation balloon for eradication of Barrett's esophagus: results of an international multicenter randomized trial comparing 3 different treatment regimens. Gastrointest Endosc 2019;90:415-23. [Crossref] [PubMed]
- van Vilsteren FG, Phoa KN, Alvarez Herrero L, et al. Circumferential balloon-based radiofrequency ablation of Barrett's esophagus with dysplasia can be simplified, yet efficacy maintained, by omitting the cleaning phase. Clin Gastroenterol Hepatol 2013;11:491-98.e1. [Crossref] [PubMed]
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett's esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017;85:482-495.e4. [Crossref] [PubMed]
- Frederiks CN, Canto MI, Weusten BLAM. Updates in Cryotherapy for Barrett's Esophagus. Gastrointest Endosc Clin N Am 2021;31:155-70. [Crossref] [PubMed]
- ASGE Technology Committee. Cryotherapy in gastrointestinal endoscopy. VideoGIE 2017;2:89-95. [Crossref] [PubMed]
- Ghorbani S, Tsai FC, Greenwald BD, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's dysplasia: results of the National Cryospray Registry. Dis Esophagus 2016;29:241-7. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Wu H, Minamide T, Yano T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig Endosc 2019;31:508-16. [Crossref] [PubMed]
- Wang KK, Lutzke L, Borkenhagen L, et al. Photodynamic therapy for Barrett's esophagus: does light still have a role? Endoscopy 2008;40:1021-5. [Crossref] [PubMed]
- Manner H, Neugebauer A, Scharpf M, et al. The tissue effect of argon-plasma coagulation with prior submucosal injection (Hybrid-APC) versus standard APC: A randomized ex-vivo study. United European Gastroenterol J 2014;2:383-90. [Crossref] [PubMed]
- Dam AN, Klapman J. A narrative review of Barrett's esophagus in 2020, molecular and clinical update. Ann Transl Med 2020;8:1107. [Crossref] [PubMed]
- Chang EY, Morris CD, Seltman AK, et al. The effect of antireflux surgery on esophageal carcinogenesis in patients with barrett esophagus: a systematic review. Ann Surg 2007;246:11-21. [Crossref] [PubMed]
- Parrilla P, Martínez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett's esophagus. Ann Surg 2003;237:291-8. [Crossref] [PubMed]
- Stefanidis D, Hope WW, Kohn GP, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010;24:2647-69. [Crossref] [PubMed]
Cite this article as: Shah S, Bhuta R, Malik Z. Management of Barrett’s esophagus: a narrative review. Ann Esophagus 2023;6:15.