Endoscopic ultrasonography in esophageal carcinoma: a narrative review
Introduction
Endoscopic ultrasound (EUS) was first introduced in the 1980s as a diagnostic technique, utilizing the hypothesis that superior ultrasound resolution and images could be obtained by directly visualizing the gastrointestinal lumen in close proximity, avoiding intervening tissues. It was first utilized in the United States in the early 1990s to image the pancreas. Initial studies were performed to assess EUS as a diagnostic tool in pancreatic cancer. The field of EUS blossomed over time to assume an integral role in the diagnosis and staging of numerous intraluminal and extraluminal diseases of the gastrointestinal tract and surrounding structures. EUS was first used in the staging of esophageal cancer as a supplement to computed tomography (CT) scans, as the ultrasound images provided a superior modality for differentiating layers of the esophageal wall, and by extension, enabling more accurate staging of the disease. In addition to visualization, EUS-guided fine needle aspiration (FNA) with cytologic analysis has also become a commonplace method for tissue acquisition (1). The ability to obtain tissue has proven to be instrumental, increasing the specificity of EUS for diagnosing malignant lymph nodes and adjacent structures. On the whole, EUS has become an integral component in the diagnosis and staging of esophageal carcinoma. The role of EUS in the staging and diagnosis of esophageal carcinoma is described herein. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-25/rc).
Methods
The information used to write this review was gathered from available resources and literature sourced in validated databases, most notably PubMed. Articles included in this review include original articles, meta-analyses, and systematic reviews of the literature. All articles used in the composition of this narrative review are peer reviewed and include information that is central to the objectives of this article.
Discussion
Staging of esophageal cancer
The ‘TNM’ staging system, as described and developed by the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC), is the universally adopted method of staging esophageal cancer (2). The primary tumor (T) is staged 0–4, with an exception TX used to denote tumors that cannot be properly assessed due to imaging limitations or specific aspects of the tumor. T0 is the absence of invasive cancer. Tis refers to high-grade dysplasia in the tissue specimen, but without frank tumor. T1 refers to mucosal tumor and is divided into T1a and T1b, with the former invading the lamina propria and/or muscularis mucosae and the latter invading submucosa. T2 tumors invade but do not penetrate through the muscularis propria. T3 tumors invade through the muscularis propria into the adventitia. T4 tumors are the most advanced, marked by invasion of adjacent structures. The most updated guidelines differentiate between T4 tumors, with T4a denoting resectable tumors invading pleura, pericardium, or diaphragm, and T4b referring to unresectable tumor in tissues such as the aorta, vertebrae, or trachea). There are no differences in the T-staging of squamous cell carcinoma (SCC) of the esophagus and adenocarcinoma/esophago-gastric junction (EGJ) tumors.
Regional lymph nodes (N) are staged from N0 to N3, again including NX for lymph nodes that cannot be assessed. N0 refers to no lymph node metastasis. N1 denotes metastases to 1–2 regional lymph nodes. N2 designates metastases to 3–6 regional lymph nodes. N3 refers to metastasis to 7 or more regional lymph nodes. Squamous cell and adenocarcinoma/EGJ tumors are N-staged similarly.
The M category is binary, with M0 referring to no known distant metastasis and M1 referring to distant metastasis, either to non-regional lymph nodes or distant organs. An overview of the TNM staging system can be seen in Figure 1.
Using the combination of the TNM stage and histologic grade of the tumor, an overall clinical stage is assigned to the tumor, ranging from T1a to 4b. Tumor stage is critical in determining the optimal therapy such as primary surgery, neoadjuvant chemoradiation followed by surgery, definitive chemoradiation, or palliative chemotherapy. Staging is also a strong determinant of prognosis. A further discussion of the details of this staging system and treatment implications are beyond the scope of this discussion. Nonetheless, it is this overall stage that subtly differentiates between SCC and adenocarcinoma/EGJ tumors. In this system, proximal and mid-esophagus SCCs are considered to be more aggressive than distal esophageal SCC in the stage I and II categories; conversely the location of adenocarcinomas/EGJ tumors along the length of the esophagus is not included in their prognostic staging. The specifics of staging of esophageal carcinoma are detailed in the text and summarized in Table 1 below.
Table 1
| Category | Description |
|---|---|
| T | |
| TX | Tumor cannot be assessed |
| T0 | No evidence of tumor in esophagus |
| Tis | High-grade dysplasia, no extension beyond basement membrane |
| T1 | Tumor invading the lamina propria, muscularis mucosae, or submucosa |
| T1a | Tumor invading the lamina propria or muscularis mucosae |
| T1b | Tumor invading the submucosa |
| T2 | Tumor invading the muscularis propria |
| T3 | Tumor invading the adventitia |
| T4 | Tumor invading adjacent structures |
| T4a | Tissue involved: pleura, pericardium, azygous vein, diaphragm, peritoneum |
| T4b | Tissue involved: aorta, vertebral body, trachea, other |
| N | |
| NX | Regional nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis identified in 1–2 regional lymph nodes |
| N2 | Metastasis identified in 3–6 regional lymph nodes |
| N3 | Metastasis identified in 7 or more regional lymph nodes |
| M | |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
Staging of esophageal cancer generally requires a multimodal approach, including imaging and invasive procedures (3). The initial diagnosis of esophageal cancer is usually made by upper endoscopy, with imaging following upper endoscopy with mucosal biopsies confirming the diagnosis of cancer. The available diagnostic studies include CT scan, full body integrated fluorodeoxyglucose positron emission tomography (FDG-PET) CT, EUS, and diagnostic laparoscopy and mediastinoscopy. Although CT is easily obtained and noninvasive, it lacks sensitivity and accuracy for identifying tumor stage in esophageal carcinoma (4,5). With regard to diagnosis of early esophageal cancer (T2 disease and below), EUS has been considered the gold standard with sensitivity of 80%, significantly outperforming CT (6). Despite the added benefits of FDG-PET, EUS outperforms that modality as well in detection of locoregional spread of primary tumor and regional lymph node metastasis and N3 describes more than 7 lymph nodes involvement (7). Numerous studies have shown superior accuracy of EUS in the staging of esophageal cancer. One significant meta-analysis of 44 studies reported overall T-stage accuracy of 79%; T1a stage sensitivity and specificity of 84% and 91%, and T1b stage pooled sensitivity and specificity of 83% and 89% (8). FDG-PET and CT scan provide obvious benefits in the diagnosis of distant metastatic disease, as well as staging early cancers which are potentially curable (9). A few factors contribute to the superiority of EUS over these modalities for the primary tumor and regional lymph nodes. First, EUS enables differentiation of esophageal wall layers that classify the T stage of the tumors. With regard to locoregional extension and lymph node involvement, FDG-PET/CT has shown significantly high rate of false positivity (10). This, combined with the improved sensitivity and specificity of EUS solidifies the importance of EUS in diagnostic staging. When FDG-PET/CT is used as the first modality, findings should be confirmed by EUS. EUS is the most accurate modality for locoregional staging of esophageal cancer.
Based on the above findings and those confirmed by a large meta-analysis, EUS, CT, and FDG-PET are complementary components of the diagnostic approach to esophageal cancer (11). Whereas EUS is the most sensitive modality, CT and FDG-PET are more specific for the diagnosis of lymph node metastases. EUS-FNA (as addressed below) can supplement CT and FDG-PET in confirming a diagnosis of malignant lymphadenopathy whereas EUS alone is useful in ruling out metastatic disease. CT and FDG-PET are invaluable for diagnosing distant metastases, that are beyond the reach of EUS. Several studies have compared CT and FDG-PET for this purpose, but this is beyond the scope of this discussion.
EUS technology
EUS employs radial or curved linear array echoendoscopes to relay signal that projects an image of the esophagus and surrounding tissues. In the USA, the radial echoendoscope is the most commonly used for a diagnostic exam, whereas curved linear echoendoscopes have wider use in Europe. In radial echoendoscopes the image produced is a 360-degree transverse plane perpendicular to the endoscope. Only linear echoendoscopes have capability for FNA. Frequencies of the sonography range from 5–20 MHz with higher frequency transducers (12–30 MHz) that can be introduced into small catheters that can be passed through the scope. The product is a high-resolution image of the esophageal wall (12).
EUS capabilities
Depending on the type of endoscope, echoendoscopes can visualize the esophageal wall as either a five- or a nine-layered structure. Generally, tissue with high content of connective tissue appears hyperechoic, while tissue with high water content (muscle) appears hypoechoic on the EUS image. Endoscopes operating at 7.5–12 MHz can visualize a five-layered esophagus as follows:
- First, hyperechoic layer, superficial mucosa;
- Second, hypoechoic layer, deep mucosa;
- Third, hyperechoic layer, submucosa;
- Fourth, hypoechoic layer, muscularis propria;
- Fifth, hyperechoic layer, adventitia.
Endoscopes can employ high frequency mini-probes (20+ MHz) can be inserted through the working channel of the echoendoscopes and provide more detailed 9-layer wall as follows:
- First, hyperechoic, superficial mucosa;
- Second, hypoechoic, deeper superficial mucosa;
- Third, hyperechoic, lamina propria;
- Fourth, hypoechoic, muscularis mucosa;
- Fifth, hyperechoic, submucosa;
- Sixth, hypoechoic, inner circular muscle [myenteric plexus (MP)];
- Seventh, hyperechoic, intermuscular connective tissue;
- Eighth, hypoechoic, outer longitudinal muscle (MP);
- Ninth, hyperechoic, adventitia.
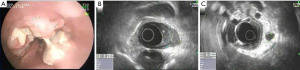
Although the high frequency mini-probes are available, they are not as widely used in practice. Even using the more available lower frequency echoendoscopes, the degree of detail in imaging the esophageal wall has significant implications in the realm of T-staging tumors. The very specific layer that a tumor invades has dictates the modality of therapy, whether ablative, endoscopic resection, or surgical approach is appropriate. For example, if EUS detects a tumor with only mucosal disease, endoscopic mucosal resection is the treatment of choice rather than a surgical approach. This is based on robust data reporting excellent outcomes in patients who underwent removal of T1a cancers with endoscopic mucosal resection (13,14). Ell et al. reported 99% cure rate in these patients (13). Conversely, if EUS detects a T3 lesion, the surgical approach is the preferred modality and a neoadjuvant chemotherapeutic regimen may be considered. T1b lesions and lesions with indeterminate invasion present situations that are best dealt with in an interdisciplinary fashion with consideration of local expertise and patient preferences and comorbidities. Figure 2, below, includes a direct endoscopic view and an EUS depiction of T1b esophageal carcinoma. Generally speaking, a combination of surgery and chemoradiation is the standard of care for most lesions beyond T1a stage (15-17).
EUS can also identify lymph nodes, both regional and those located at the celiac axis, which can aid in N-staging the tumor. Malignant lymph nodes have a typical appearance that can be detected on ultrasound. Regional lymph nodes cannot be visualized by standard upper endoscopy. The characteristics that are evaluated on EUS include size, shape, border appearance, and echogenicity of the node (18,19). The number of lymph nodes present combined with the features of the lymph nodes evaluated will increase or decrease the yield of EUS in the diagnostic staging of esophageal cancer. The nodal sites that can be evaluated during an EUS include cervical paraesophageal, right recurrent laryngeal, left paratracheal, upper paraesophageal, lower paraesophageal, infra-aortic, infracarinal, lower posterior mediastinal, and perigastric. As previously noted, the presence of malignant lymph nodes and resultant upstaging of the clinical tumor stage carries significant implications for prognosis. Numerous studies have demonstrated that both the number and the location of regional lymph nodes affect prognosis (20,21).
Aside from identifying the lymph node groups noted above, celiac lymph nodes are of particular interest. Celiac nodes, when identified, increase the likelihood of malignancy independent of the characteristics of the lymph node. In one study, 90% of celiac lymph nodes identified by EUS were found to be malignant (22). Although traditional CT and PET-CT could be used to identify celiac lymph nodes, studies have compared EUS and CT/PET-CT, supporting the superiority of EUS (4,23,24).
Concerning endosonographic features of a lymph node that increase their likelihood of being malignant include diameter greater than 1 cm, round appearance, smooth edges, and poor echogenicity. Small, irregular, elongated, more echogenic lymph nodes lower the likelihood of malignancy. Without the inclusion of number of lymph nodes, endosonographic characteristics support an 80% to 100% likelihood of malignancy when one of the four concerning features is present (18,19). No studies have been done since 2010, when the decision was made by AJCC/UICC to include lymph node number in staging criteria; but it stands to reason that including the number of lymph nodes in assessment of risk would increase the yield of EUS in N staging of the tumor.
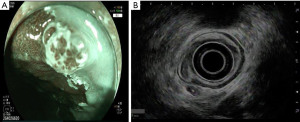
Regardless of the appearance of the lymph node(s) in question, EUS has the added benefit of the ability to obtain tissue using FNA. Accuracy of EUS nodal staging of esophageal cancer increases when combined with FNA, from 74% to 90% (25). Studies using EUS-FNA have compared EUS guided FNA with surgical specimens or cytology as the gold standard and EUS has exceeded 85% in sensitivity, specificity, and accuracy. A prospective study performed in 2003 showed that EUS-FNA improved lymph node staging of esophageal carcinoma over EUS alone or CT-guided FNA (25). EUS FNA has been studied in comparison with surgical resection/cytology as the gold standard and has yielded robust results (26-28). Figure 3 depicts a FNA of a malignant regional lymph node.
Pitfalls of EUS
EUS requires an advanced skill set that is not available to all providers and in all settings. Training for EUS is generally provided during a fourth year of advanced endoscopy training after completing a standard gastroenterology fellowship. Even among expert gastroenterologists, there are significant rates of inter-observer variability in staging esophageal cancers (22). More specifically, tumors in the T2 category are subject to the greatest variability, and extension to T3 is not always obvious. It may be difficult to differentiate between T2 and T3 lesions even for expert providers. This challenge may not diminish the value of EUS in this context, as there are no current differences in management between T2 and T3 lesions.
Another conundrum encountered when performing EUS is the use of EUS following neoadjuvant chemotherapy or radiation. As noted above, T-staging of esophageal cancer is important in that it has implications for preoperative therapeutic interventions. In patients who received these therapies, EUS may be performed to monitor response to therapy and restage the tumor. Unfortunately, the accuracy of EUS in the initial assessment of esophageal cancer and lymph nodes is not matched after neoadjuvant chemoradiation. Certainly, residual lymphadenopathy predicts a more complicated post-surgical course and worse post-operative survival (29). However, T-stage accuracy by EUS is low after neoadjuvant chemoradiation. In the largest meta-analysis available, analyzing 16 studies that compared EUS assessments to surgical pathology, sensitivity and specificity of T-staging varied widely depending on the T-stage assigned to the tumor. T1 and T4 tumors had the highest specificity (95% and 96%, 93–97% and 94–97%), whereas T3 tumors had higher sensitivity (81%, 72–88%) (30). Regardless of the number, the more outstanding finding is that there was significant variance, lowering the overall accuracy, and possibly utility, of EUS in this setting. The cause of the inaccuracies are theoretical, but include tissue destruction and inflammation following chemoradiation as well as observer bias as no studies have blinded endoscopists to the patient’s pre-EUS history. Still, despite the inaccuracies, EUS in conjunction with upper endoscopy remains the best modality for reassessing disease following chemoradiation. Here again, EUS-FNA has a role in providing information about remaining lymph nodes after therapy, increasing the value of visual inspection alone.
One final limitation of EUS is that it depends upon the ability of the echoendoscope to traverse the tumor. Unfortunately, many patients diagnosed with esophageal cancer present with advanced disease and tight malignant esophageal stenosis (31). Echoendoscopes are approximately 12 mm in diameter, and stiffer than diagnostic gastroscopes, thereby limiting the ability to traverse firm, narrow stenoses with the echoendoscope. Dilations can be utilized to transiently expand the esophageal luminal diameter, but this intervention carries significant risk (32,33). The risk of dilation may not be worth any potential diagnostic benefit. Even in such cases, EUS retains some accuracy in staging the tumor (34). However, the diagnostic approach relies more heavily on imaging or direct surgical visualization for staging.
Summary
In the last 30 years, EUS has come to assume a central role in the diagnosis and staging of esophageal carcinoma. It is not highly invasive, and is extremely accurate in providing T and N staging. The technique of EUS FNA is an accurate nonsurgical modality of tissue acquisition for confirmation of metastatic disease from adjacent lymph nodes and other nearby structures such as liver. Despite its limitations in obstructing tumors and reassessment of disease following neoadjuvant therapy, EUS is likely to remain a cornerstone in the care of our patients with esophageal cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Abbas E. Abbas and Roman V. Petrov) for the series “New Technologies in Esophageal Surgery and Endoscopy” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-25/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-25/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-25/coif). The series “New Technologies in Esophageal Surgery and Endoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc 1992;38:172-3. [Crossref] [PubMed]
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119-30.
- Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis 2014;6:S289-97. [PubMed]
- Romagnuolo J, Scott J, Hawes RH, et al. Helical CT versus EUS with fine needle aspiration for celiac nodal assessment in patients with esophageal cancer. Gastrointest Endosc 2002;55:648-54. [Crossref] [PubMed]
- Sultan R, Haider Z, Chawla TU. Diagnostic accuracy of CT scan in staging resectable esophageal cancer. J Pak Med Assoc 2016;66:90-2. [PubMed]
- Singhal S, Roy S. cT2N0 esophageal adenocarcinoma: predictors of lymph nodal involvement and clinical significance. J Thorac Dis 2019;11:S453-6. [Crossref] [PubMed]
- Pfau PR, Perlman SB, Stanko P, et al. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc 2007;65:377-84. [Crossref] [PubMed]
- Luo LN, He LJ, Gao XY, et al. Endoscopic ultrasound for preoperative esophageal squamous cell carcinoma: a meta-analysis. PLoS One 2016;11:e0158373. [Crossref] [PubMed]
- van Westreenen HL, Westerterp M, Bossuyt PM, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 2004;22:3805-12. [Crossref] [PubMed]
- van Westreenen HL, Heeren PA, Jager PL, et al. Pitfalls of positive findings in staging esophageal cancer with F-18-fluorodeoxyglucose positron emission tomography. Ann Surg Oncol 2003;10:1100-5. [Crossref] [PubMed]
- van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 2008;98:547-57. [Crossref] [PubMed]
- ASGE Technology Committee. Echoendoscopes. Gastrointest Endosc 2015;82:189-202. [Crossref] [PubMed]
- Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc 2007;65:3-10. [Crossref] [PubMed]
- Balmadrid B, Hwang JH. Endoscopic resection of gastric and esophageal cancer. Gastroenterol Rep (Oxf) 2015;3:330-8. [Crossref] [PubMed]
- Gockel I, Hoffmeister A. Endoscopic or surgical resection for gastro-esophageal cancer. Dtsch Arztebl Int 2018;115:513-9. [Crossref] [PubMed]
- Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today 2020;50:12-20. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol 2013;19:1424-37. [Crossref] [PubMed]
- Catalano MF, Sivak MV Jr, Rice T, et al. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc 1994;40:442-6. [Crossref] [PubMed]
- Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc 1997;45:474-9. [Crossref] [PubMed]
- Rizk N, Venkatraman E, Park B, et al. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 2006;132:1374-81. [Crossref] [PubMed]
- Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. [Crossref] [PubMed]
- Eloubeidi MA, Wallace MB, Reed CE, et al. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: a single-center experience. Gastrointest Endosc 2001;54:714-9. [Crossref] [PubMed]
- Catalano MF, Alcocer E, Chak A, et al. Evaluation of metastatic celiac axis lymph nodes in patients with esophageal carcinoma: accuracy of EUS. Gastrointest Endosc 1999;50:352-6. [Crossref] [PubMed]
- Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol 2005;23:4483-9. [Crossref] [PubMed]
- Vazquez-Sequeiros E, Wiersema MJ, Clain JE, et al. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology 2003;125:1626-35. [Crossref] [PubMed]
- Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087-95. [Crossref] [PubMed]
- Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy 1995;27:171-7. [Crossref] [PubMed]
- van Vliet EP, Eijkemans MJ, Poley JW, et al. Staging of esophageal carcinoma in a low-volume EUS center compared with reported results from high-volume centers. Gastrointest Endosc 2006;63:938-47. [Crossref] [PubMed]
- Agarwal B, Swisher S, Ajani J, et al. Endoscopic ultrasound after preoperative chemoradiation can help identify patients who benefit maximally after surgical esophageal resection. Am J Gastroenterol 2004;99:1258-66. [Crossref] [PubMed]
- Sun F, Chen T, Han J, et al. Staging accuracy of endoscopic ultrasound for esophageal cancer after neoadjuvant chemotherapy: a meta-analysis and systematic review. Dis Esophagus 2015;28:757-71. [Crossref] [PubMed]
- Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535-9. [Crossref] [PubMed]
- Pfau PR, Ginsberg GG, Lew RJ, et al. Esophageal dilation for endosonographic evaluation of malignant esophageal strictures is safe and effective. Am J Gastroenterol 2000;95:2813-5. [Crossref] [PubMed]
- Hernandez LV, Jacobson JW, Harris MS. Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures. Gastrointest Endosc 2000;51:460-2. Erratum in: Gastrointest Endosc 2003;58:642. [Crossref] [PubMed]
- Shimpi RA, George J, Jowell P, et al. Staging of esophageal cancer by EUS: staging accuracy revisited. Gastrointest Endosc 2007;66:475-82. [Crossref] [PubMed]
Cite this article as: Daitch ZE, Heller SJ. Endoscopic ultrasonography in esophageal carcinoma: a narrative review. Ann Esophagus 2023;6:14.