
End to side anastomosis with a circular stapler for minimally invasive Ivor Lewis esophagectomy—how I do it
Introduction
The topic as to which esophago-gastric anastomosis (EGA) construction is the “best” is a debatable one. In real life, however, one will always require a “Plan A” EGA technique which delivers consistent and safe results for you, i.e., a low EGA leak (<5%) (1). This would be the EGA technique that is performed the most frequently, making one very familiar with the technique’s fine details. In addition, if a leak does occur using this EGA technique, the leak should be easy to diagnose and locate radiologically and endoscopically, and ideally, easily treated with an EndoVac. This paper aims to explain why the preferred technique is the end-to-side EGA with a circular stapler (28 or 29 mm diameter). This technique was demonstrated and taught to me by J. D. Luketich and has been proven to be safe and easy to execute with appropriate training and experience (2). We have replicated the technique in Norwich, UK with similar outcomes (3). There are other intrathoracic EGA techniques used in MIE such as the semi mechanical technique with a linear stapler, which produces good outcomes with low leak rates and stricture rates (4,5) but it requires a good level of thorocoscopic suturing skill, and this technique may be more suitable for robotic assisted minimally invasive esophagectomy. The other common intrathoracic EGA technique involve the use of an Orvil device with equally good outcome (6,7). The most important principle to appreciate is that intrathoracic EGA has a better outcome than cervical anastomosis (8) with a lower leak and stricture rate. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients.
The principles of any good esophago-gastric anastomotic technique
- Ease of use. The mechanical circular stapler, in particular the Echelon Circular Powered Stapler CDH29P (Ethicon, Johnson and Johnson Medical NV, Belgium) is easy to use, provided the main steps described below are followed meticulously. With a simple trigger by a finger, the staples are placed uniformly, with equal distribution of tension between the tissues in a circular configuration. In comparison, this is not easily replicated in a handsewn or robotic-sutured anastomosis. The stapler also allows for intraoperative adjustments to accommodate any variable tissue thickness with the use of variable staple heights that conform to the tissue thickness (the thickness of the oesophageal wall can vary, especially if there is esophageal obstruction). In addition, the ring of staples from the stapler comes with a 3D-stapling technology, which allows for a more even distribution of tension at the anastomosis.
- It must be easy to teach. The circular stapler technique requires some time to teach, but this pertains mainly to mastering how to manoeuver the anvil into the proximal esophagus and making a good purse-string suture. These would depend on the thoracoscopic skills of the surgeon. The next most important skill involves manoeuvring the stapler gun into the gastric conduit and docking the trocar with the anvil, which will be discussed in detail below.
- Should an EGA leak occur, it must be easy to diagnose and treat. Using this technique, any leak from the EGA is easy to diagnose and locate due to the ring of titanium staples applied. As the titanium staples are radio-opaque, they appear on a CT scan as a bright ring. Specifically, on a CT scan with intravenous contrast in the portal venous phase, leaks appear as small air bubbles that collect just outside the bright ring produced by the staple line. In the case of a suspected leak, both a CT scan and an upper endoscopy are recommended. Compared to a hand-sewn anastomosis, it is relatively easy to spot an EGA leak endoscopically using this technique, enabling swift treatment with an EndoVac (with or without sponge). Typical signs of a leak, or an impending leak or defect, at the EGA include the loss of continuity of the bright ring on CT and early shedding of the staples appearing as a defect in the circular staple line (seen on CT and endoscopically). There may also be a change in configuration or alignment of the C-shaped staples as seen endoscopically, which may indicate a leak. This is usually easily treated with an EndoVac. If the hole was too small to allow an EndoVac drain, the drain (with a small sponge at the tip) can be positioned within the lumen of the EGA and placed on −200 mmHg suction (intraluminal EndoVac). In addition, the leak is usually small and well contained within the omentum flap. Therefore, it is easily treated with an EndoVac inserted under intravenous sedation. This also obviates the need for another general anaesthetic, thoracotomy, or video-assisted thoracoscopic surgery.
The importance of the abdominal phase
For any EGA to heal properly i.e., without a leak or stricture, it is vital to appreciate that the abdominal phase of the MIE Ivor Lewis operation is equally as important as performing the anastomosis itself. The key is in the preservation of the submucosal plexus of the gastric conduit, which is vital for perfusion and the healing of the EGA. Hence, good tissue-handling is crucial.
Ideally, the stomach must be carefully mobilised without traumatising the submucosal plexus of the conduit. To do that, we perform a “no grab/no touch” technique to attain a non-traumatised and healthy gastric conduit at the end. In this technique, the stomach is mobilised without contact with the part of the stomach where the planned gastric conduit would reside. Instead, the lesser curve of the stomach or the omental fat on the greater curve is grabbed and used for retraction but avoiding the gastroepiploic arcade. The gastric conduit is also mobilised with an omental flap (which will be used to cover the EGA under a pleural tent at the end). The width of the constructed gastric conduit should be about 4 cm, constructed intracorporeally with an EndoGIA linear stapler. Purple cartridges are usually used but black cartridges are used in cases of morbidly obese patients, or other patients with thicker tissues in the gastric antrum. Which cartridge to use is a judgement call to be made intraoperatively.
The first fire of the EndoGIA stapler starts at the “crow’s foot” on the lesser curve, where the vessels resemble the foot of the crow and it leaves a gastric antral reservoir with the constructed conduit. When the conduit is completed with the EndoGIA stapler, ensure that the tip of the gastric conduit is fully detached from the gastric remnant (the cardia and lesser curve of stomach, which form the distal part of the esophago-gastric specimen). This step is important to allow time for the ischemic demarcation line to develop at the tip of the conduit, hence making it easier to identify this line later. When performing the EGA later, we use only the better perfused part of the conduit (Figure 1). To prevent narrowing of the conduit, do not oversew the staple line of the conduit. Any bleeding from the staple line (commonly near the antrum) is easily controlled with an endoclip (Endo Clip 10 mm, Medtronic, UK).

At the end of the abdominal phase, the gastric conduit must be correctly orientated with the staple line facing the patient’s right and sutured twice to the distal end of the gastric remnant. This allows the gastric conduit to be easily pulled up into the chest later via the hiatus. Apply two sutures to prevent the conduit from twisting when it is being pulled up into the chest. The omental flap is also sutured to the gastric remnant to make it easier to deliver the conduit and omental flap into the chest together (Figure 2).

End to side EGA with a circular stapler technique step-by-step (Video 1)
Step 1: At the end of the thoracic phase of the MIE-IL [with the patient placed in the left lateral decubitus position for the thoracic phase of the MIE (Figure 3)], the proximal esophagus is cut at the level of the azygos vein arch. Make the cut at the bottom edge of the staple line of the azygos vein stump, because the esophagus will retract up when cut. The scissors is inserted to the top 11 mm port near the axilla, and the esophagus is cut obliquely to allow for a bigger diameter lumen—this will help with the insertion of the anvil into the esophageal lumen later (Figure 4). Ensure that there is adequate mucosa covering the proximal esophageal lumen by cutting the mucosa a few millimetres distal to the muscle wall.

Step 2 Inserting the Anvil of the Circular Stapler (video 00:05): The anvil of an Echelon Circular Powered Stapler CDH29P (Ethicon, Johnson and Johnson Medical NV, Belgium) is inserted into the proximal esophagus. I recommend the use of a 29 mm anvil because there is a lower stricture rate post-op compared to a 25 mm. The Echelon stapler has an advantage as it can adjust the staple height according to the thickness of the tissues. To do this, the assistant holds the edge of the esophageal lumen at 3 o’clock, while the surgeon holds the 9 o’clock position. Grab both the muscle wall and the mucosa to ensure that the full thickness of the esophageal wall is stable while held open at that 3 o’clock and 9 o’clock positions. It is important to hold only the distal 5 mm edge of the esophageal wall to prevent traumatising the wall of the esophagus at the site where the EGA is to be formed (Figure 5). Use a grasper to hold the shaft of the anvil near the saucer-shaped circular top. The head of the anvil is inserted horizontally, like a flat flying saucer, into the esophageal lumen and then pushed in further, until only the anvil shaft remains visible outside. At the same time, the assistant and your grasper must move towards the middle to close the lumen and prevent the anvil from falling out.

Step 3 Inserting the Purse String Suture (video 00:40): A purse string is then inserted with an Endo stitch suturing device with a 20 cm long 2/0 Surgidac (both Medtronic, Watford, UK) starting at the 10 o’clock position of the esophageal lumen. The purse string should be inserted near the edge of the esophagus and incorporate the full thickness of the esophageal wall, i.e., both the mucosa and the muscular wall. To pull in any additional loose tissue, a second purse string suture (3/0 pds) is typically inserted at 6 or 7 o’clock position and tied on the opposite side of the anvil shaft. The aim is to create a small rose (Figure 6) and a “clean” esophageal wall. These steps are important to prevent any unnecessary tissue getting in the way of the staple line later. This also enables the gastric conduit and the esophageal muscle walls to be apposed without any intervening tissues getting in the way of the staples. Any piece of tissue on the esophageal wall, for example fat and nerves, that could get in the way of the staple line is removed to “tidy up” the area.

Step 4 Release the Adhesions Around the Esophagus (video 02:11): The proximal oesophagus is mobilised a little further to release the adhesions to the distal part of the esophagus, especially where the circular head of the anvil lay. Release the adhesions till just behind the anvil to allow for more mobility of the esophagus/anvil unit when performing the anastomosis later.
Step 5: Take a Break. At this point you have been staring at the screen/monitor with full concentration for the last two to three hours. Take a 10-minute recess for a non-alcoholic refreshment, toilet break etc. before resuming. This break will prove to be well spent as total focus and concentration will be required when performing the upcoming EGA.
Step 6: Start by carefully pulling up the gastric conduit into the thorax from the hiatus, aiming towards the apex of the chest. Only grab the distal tip of the conduit to avoid traumatising the conduit. This part will be excised later as part of the distal margin of the specimen. Ensure that the staple line is correctly orientated and facing upwards, towards the right side of the patient. Each of the above checkpoints is important to help prevent twisting of the gastric conduit.
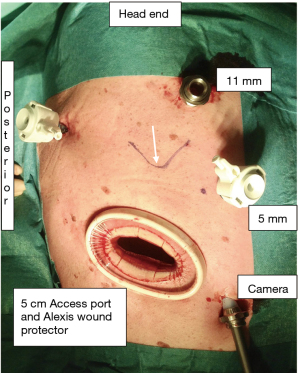
Step 7 Deliver the Conduit into the Chest and out through the Access port to Decompress (video 02:40): Hold the gastric conduit at its tip, ensuring that the gastric conduit is orientated with the staple line facing the right side of the patient. Pull the conduit out of the thoracic cavity via the 5 cm access wound (the access wound is protected by an Alexis wound protector) (Figure 1). The tip of the conduit is held with two Babcocks and opened from the tip along one side of the staple line with diathermy, and a Poole sucker is inserted into the conduit to remove all the gastric secretions. This is important to prevent any spillage of gastric contents and contamination in the chest/mediastinum when constructing the anastomosis later.
Step 8 Examine the conduit for the ischemic demarcation line (video 02:58): The mucosa of the gastric conduit is carefully examined with the naked eye under a bright light. The staple line of the conduit is opened further with a McIndoe scissors until healthy pink gastric mucosa is reached. This will be evident by healthy bleeding along the cut edges of the gastric conduit wall (ensure that the systolic blood pressure >100 mmHg). The ischemic demarcation line between the healthy gastric mucosa and the ischemic distal part is carefully noted, and then marked with a purple marker pen all around the gastric conduit wall (Figure 7). The purple mark serves as a clear guideline, which will be easily visible when constructing the anastomosis later, and also when stapling off the gastrostomy at the end with a linear stapler. At this point, one will have an idea where the site of the EGA will be. Remove any omental tissue on the gastric conduit that could get in the way of the EGA or linear stapler.

Step 9 Reinsert the conduit into the Chest with a Grasper holding the Left side of the Gastrostomy (video 03:28): Again, check that the gastric conduit is correctly orientated by looking at the staple line all the way to the hiatus. Use good grasping forceps to hold on to the left side of the gastrostomy at 9 o’clock position and reinsert the conduit into the chest via the access wound. The assistant then holds on to the right side of the gastrostomy (at 3 o’clock position) within the thorax via the middle 5 mm port, to keep the gastrostomy open.
Step 10 Insert the Head of the Circular Stapler (video 03:41): The black head of the circular stapler is lubricated with KY jelly. Check that the sharp trocar of the stapler is fully withdrawn before inserting into the thoracic cavity through the 5 cm access wound. Insert the circular stapler parallel to the long axis of the intercostal space. Note that the lower you make this 5 cm access wound, the wider the intercostal space. The wider space allows for an easier insertion of the head of the circular stapler or the removal of the specimen. The plastic cover of the wound protector also allows the lubricated stapler to slide in smoothly (Figure 8).

Step 11 Stapler held Upside-down and Inserted into the Conduit (video 03:51): With the stapler in the thorax, the stapler is held in an upside-down position to allow the angled black head of the stapler to slide down into the gastric conduit via the gastrostomy, like a “foot in sock” motion. Aim the stapler towards the hiatus. This action is performed near the access wound (but not directly under it) to allow the angled head of the stapler to slide into the conduit towards the hiatus. The assistant’s grasper (which is still holding onto the right side of the gastrostomy) will pull the conduit up on the black head of the stapler (staying directly on the black plastic of the stapler). In synchrony with the assistant, the surgeon’s grasper will pull the left side of the gastrostomy up the stapler, for the same distance as the assistant.
Step 12 Surgeon Holds the Right side of the Gastrostomy (video 03:59): Next, the surgeon inserts another good grasper via the 5 cm access port to grab the right side of the gastrostomy from the assistant. From henceforth, the surgeon will be in full control of the whole unit, i.e., the stapler in the conduit, as well as the two good graspers which are firmly holding both sides of the gastrostomy.
Step 13 Ensure the Whole Black Head of the Stapler is below the Purple line (video 04:06): Check to ensure that the head of the stapler has passed at least 2 cm below the purple mark on the conduit. This is visibly seen as the margin between the black and the silver stem of the stapler at the gastrostomy opening.
Step 14 Turn the Circular Stapler into an Upright Position (video 04:20): Keep the two graspers firmly on the edges of the gastrostomy and the conduit under some tension, over the head of the stapler. Now carefully turn the stapler into an upright position.
Step 15 Check the Orientation of the Conduit Again (video 04:40): Check again that the gastric conduit staple line is still orientated correctly, and there is no twist in the conduit. At this point, move the camera up to the port near the axilla to look back on the gastric conduit, especially over the site where the trocar pin will emerge from the stapler. The aim is for the trocar to emerge through the greater curve of the gastric conduit. The camera is then moved back to the original camera port to check that the linear staple line of the conduit is still orientated correctly. If it is not orientated correctly, rotate and adjust until it is.
Step 16 Advance the Trocar Pin of the Stapler while keeping tension (video 04:55): While still holding onto the two graspers to keep tension on the wall of the conduit laying over the head of the stapler, the trocar pin of the stapler is carefully advanced to come through the greater curvature of the conduit (this is opposite to the site of the linear staple line). Continue until the orange band of the trocar is fully visible then insert a curved laparoscopic Petelin grasper (via the 12 mm port near the axilla) to push on the tented wall of the conduit to allow the full extent of the trocar to emerge. This step is always done under full vision.
Step 17 Docking the Trocar to the Anvil (video 05:15): Under vision, move the stapler and conduit towards the anvil, then dock the trocar with the anvil shaft. Ensure that there is no unwanted tissue or omental flap intervening between the anvil and the stapler/conduit.
Step 18 Close the Stapler while moving it towards the Anvil (video 05:25): The assistant lifts the pleural tent while the surgeon closes the stapler. To close the stapler, turn the black adjusting knob clockwise until the marker lays within the green zone while simultaneously moving the stapler towards the anvil. It is important to keep the stem of the stapler as parallel as possible to the long axis of the proximal esophagus. When the tissues are fully apposed and reasonably tight (do not overt-tighten), the marker will be in the green zone, typically within the top half (Figure 9).

Step 19 Wait 5 minutes (video 05:43): Wait for at least 5 minutes to allow the tissue edema to dissipate prior to firing the stapler. While the instruction manual states to wait 15 seconds, past experience has shown that waiting several minutes more is time well spent. Make use of these five minutes to remove the graspers from the gastrostomy and to once again check that the gastric conduit is correctly orientated. Use two hands to keep the stapler still and stable, and keep the long axis of the stem of the stapler parallel to the long axis of the proximal esophagus before firing (Figure 10). Use two hands to hold the stapler when firing will minimises any wayward movement of the anvil tip. A green tick mark will light up on the indicator of the stapler, once the staples are fully formed.

Step 20 Removing the Stapler (video 05:46): Before removing the stapler, turn the adjusting knob two full 360 degrees in an anti-clockwise direction. When pulling the head of the staple gun out of the gastrostomy of the conduit, make sure that the long axis of the stapler is as parallel as possible to the long axis of the proximal esophagus. Gently rotate the stapler back and forth, clockwise and anti-clockwise, to loosen and release any tissue that may still be adherent to the stapler before pulling.
Step 21: Check that the proximal donut of the esophagus is complete with a complete ring of mucosa and muscle, and the donut contains the two intact purse string sutures (Figure 11).

Step 22 Close the Gastrostomy with a Linear Stapler (video 06:03): Gently lift the left side and right side of the gastrostomy without applying tension to the newly formed EGA anastomosis. The gastrostomy is now closed with the EndoGIA stapler (purple cartridge), applied along or just below the purple marker line (I use two 45 mm staplers to complete this). Ensure that there is a minimum of 1 to 2 cm distance from the linear staple line to the EGA anastomosis.
Step 23 Examine the Anastomosis from the Outside (video 07:01): The anastomosis is carefully examined with the camera in the port near the axilla. Then two, or more, horizontal mattress sutures (3/0 pds) are placed to invert the staple line at the EGA anastomosis, especially at the 3 o-clock and 9 o’clock positions, if the staples are visible. This removes the tension at the staple line and inverts the staple line. It also takes some weight of the conduit off the EGA. Also insert sutures at the sites where the staple line is visibly under tension, e.g., a visible or distorted staple at the EGA.
Step 24 Pull the Omental flap out of the Mediastinum (video 07:38): The omental flap/fat is pulled out of the mediastinum from under the gastric conduit. Pull it towards the right ribs so that the gastric conduit now resides within the native oesophageal bed. The staple line of the conduit will tend to point towards the pericardium when this is done.
Step 25 Cover the Anastomosis with an Omental Flap under the Pleural Tent (video 07:52): The omental flap covers the right side of the EGA anastomosis under the pleural tent. Two 3/0 PDS sutures holds the omental flap in place under the pleural tent with sutures placed on the edges of the pleural tent.
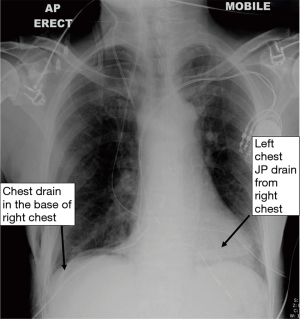
Step 26 Insert the Drains (video 08:13): Never insert any suction drain near the anastomosis because it will result in a leak. A JP drain is inserted into the left chest base from the right chest wall. The route of the JP drain lays just behind the gastric conduit, in front of the aorta, and finally in the base of the left chest. Then a 28Ch right-angled chest drain is inserted into the base of the right chest near the hiatus (Figure 12). This chest drain will later be removed post-op on Day 1 or 2, and the JP drain is usually removed on Day 4 or 5.
Step 27: Finally, an upper endoscopy (esophago-gastroscopy) is performed to check the integrity of the anastomosis, to look at the healthy conduit mucosa, and finally to guide a large (16/18 Ch) nasogastric tube (NGT) into the conduit under direct vision (the NGT is removed post-op on Day 2 or 3). This is typically 42 cm at the nose, and serves to decompress the conduit, thus reducing the pressure within the EGA anastomosis. The upper endoscopy at the end also serves to remove the secretions in the oropharynx to prevent aspiration upon extubation later.
The multimodal post-operative analgesia used are spinal analgesia (administered while the patient is positioned in the left lateral decubitus position), patient-controlled analgesia and a right-sided paravertebral catheter (inserted under vision at the end). The paravertebral catheter infusion rate is typically at 15 mL/hr of 0.125% levobupivacaine. No epidural is used to prevent any post-operative hypotension which tend to lead to a sequalae of excess intravenous fluid infusion and vasoconstrictors given. Also, intercostal nerve block (20 mL 0.25% Chirocaine) is given at two or three levels prior to opening the 5 cm access wound during the thoracic phase.
The principles behind any good anastomosis technique are universal regardless of choice of technique: The anastomosed ends of the gut must have a good blood supply, be under no tension, and be anastomosed with a meticulous technique. It is crucial to pay attention to the fine details and never be sloppy when constructing the anastomosis. Take a short break to recharge and refresh before starting on the most important part of the operation. One will require full focus and concentration at this critical stage. It is also vital to handle the tissue with utmost care and precision.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alejandro Nieponice) for the series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” published in Annals of Esophagus. The article has undergone external peer review.
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-35/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-35/coif). The series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kuppusamy MK, Low DEInternational Esodata Study Group. Evaluation of International Contemporary Operative Outcomes and Management Trends Associated With Esophagectomy: A 4-year study with >6000 patients using ECCG definitions and the online Esodata database. Ann Surg 2022;275:515-25. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Cheong E. How minimally invasive esophagectomy was implemented at the Norfolk and Norwich University Hospital. J Thorac Dis 2017;9:S879-85. [Crossref] [PubMed]
- Irino T, Tsai JA, Ericson J, et al. Thoracoscopic side-to-side esophagogastrostomy by use of linear stapler-a simplified technique facilitating a minimally invasive Ivor-Lewis operation. Langenbecks Arch Surg 2016;401:315-22. [Crossref] [PubMed]
- Kukar M, Ben-David K, Peng JS, et al. Minimally Invasive Ivor Lewis Esophagectomy with Linear Stapled Anastomosis Associated with Low Leak and Stricture Rates. J Gastrointest Surg 2020;24:1729-35. [Crossref] [PubMed]
- Laxa BU, Harold KL, Jaroszewski DE. Minimally Invasive Esophagectomy: Esophagogastric Anastomosis Using the Transoral Orvil for the End-to-Side Ivor-Lewis Technique. Innovations (Phila) 2009;4:319-25. [Crossref] [PubMed]
- Foley DM, Emanuwa EJE, Knight WRC, et al. Analysis of outcomes of a transoral circular stapled anastomosis following major upper gastrointestinal cancer resection. Dis Esophagus 2021;34:doab004.
- van Workum F, Verstegen MHP, Klarenbeek BR, et al. Intrathoracic vs Cervical Anastomosis After Totally or Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer: A Randomized Clinical Trial. JAMA Surg 2021;156:601-10. [Crossref] [PubMed]
Cite this article as: Cheong E, Luketich JD. End to side anastomosis with a circular stapler for minimally invasive Ivor Lewis esophagectomy—how I do it. Ann Esophagus 2022;5:12.


