Cricopharyngeal myotomy and toxin botulinum injection for the treatment of upper esophageal sphincter disorders: a narrative review
Introduction
The upper esophageal sphincter (UES) is mostly formed by the cricopharyngeal muscle (CM) which at rest, keeps a continuous tone with the underneath esophageal lumen closed, allowing automatic relaxations when swallowing (1).
Normal swallowing involves the relaxation of the CM, thereby sphincter relaxation failure or cricopharyngeal dysfunction leads to dysphagia and obstruction of food passage. The UES disorders can provoke overwhelming consequences such as bronchopulmonary aspiration, malnutrition, impaired quality of life or even death. Patients with cricopharyngeal dysfunction may refer upper esophageal reflux, coughing, halitosis (especially if associated with a Zenker’s diverticulum) or progressive swallowing weakness in neurological disorders. Several studies can be used to reach the diagnosis, such as flexible endoscopy, manometry, videofluoroscopy, and manofluorography. In patients with UES abnormalities, several treatments have been described being the surgical myotomy and the toxin botulinum injection (TBI) the most widely accepted (2).
Since its introduction in 1951 for the treatment of post-poliomyelitis dysphagia, surgical cricopharyngeal myotomy (CPM) has been considered the treatment of choice for patients with deficient CM relaxation (3). Since 1994, endoscopic laser-assisted transmucosal myotomy has been increasingly replacing the open approach (4). On the other hand, TBI into the CM has also been described with promising results (5). However, indications techniques and outcomes have varied among different studies (6-8).
We performed a review of the literature to assess the indications, safety and outcomes of CPM and TBI for the treatment of UES disorders. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-8/rc).
Methods
A literature search using the Medline database was performed to identify articles evaluating surgical myotomy and TBI for the treatment of UES disorders. Electronic searches in PubMed and Cochrane Central Register of Controlled Trials were performed using the following Medical Subject Headings (MeSH): “Upper esophageal sphincter disorders”, “Cricopharyngeal myotomy”, “Surgical myotomy”, “Toxin botulinum injection”, “Surgical myotomy vs. Toxin botulinum injection”. Each set of keywords was used to obtain the maximal number of articles. The search was limited to the adult population and to the English language.
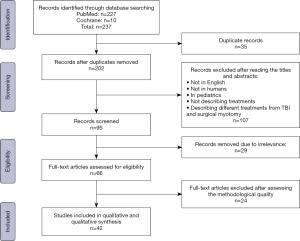
All articles between 1990 and 2020 describing CPM, TBI, or those comparing both techniques were analyzed. A total of 237 articles were initially screened; after removing duplicates and excluding titles and abstracts that did not meet the inclusion criteria, 66 articles were revised based on the methodological quality of the publications. Finally, 42 articles were included in the review. The inclusion flowchart is displayed in Figure 1.
Treatment indications, safety and outcomes of surgical myotomy and TBI were evaluated as primary endpoints. Quality of life improvement was evaluated as a secondary endpoint.
Surgical CPM
Several treatment modalities for UES disorders have been described, being the CPM the most frequently used (9). Historically, CPM has been performed by an open approach through a left-sided cervical incision and has been recognized as a definitive treatment option for UES swallowing disorders. It was first described in 1951 by Kaplan et al. for the treatment of a patient with post-poliomyelitis deglutition paralysis with substantial symptoms improvement (10).
CPM has also been used to treat oropharyngeal dysphagia in neurogenic, idiopathic, structural, and/or myogenic disorders. It is also indicated in patients with moderate or severe pharyngeal dysphagia associated with a defective UES opening with a normal swallowing, in patients with manometric, electromiographic, and/or radiological abnormalities, in those with severe complications due to progressive dysphagia (pneumonia, weight loss, significant impairment of quality of life), or in patients with associated comorbidities (3,10,11).
Surgical technique
The open technique for the CPM is usually performed through a left cervical incision as the preferred approach. After separating the sternocleidomastoid muscle and dividing the omohyoid muscle, the posterior portion of the pharyngo-esophagus is exposed by rotating and retracting the carotid sheath posteriorly and the larynx anteriorly. The extramucosal myotomy of the CM is performed extending the incision 3–4 cm above the esophagus. A proper division of the muscle fibers is crucial for success (11,12).
Outcomes
Since Kaplan et al. described the surgical technique in 1951 (10), several studies have been published analyzing CPM results. In 1988, Duranceau and colleagues reported a total of 188 patients undergoing CPM for UES disorders of different etiologies, with a success rate of 73% (13). Similarly, Buchholz reported 79% of success in 244 patients undergoing CPM for neurological UES disorders (14).
A previous study analyzed 28 patients who underwent CPM for dysphagia or aspiration secondary to UES abnormalities. Every patient had a pre- and postoperative video-fluoroscopy and manometry to evaluate postoperative results. Treatment success was defined as complete symptoms resolution (dysphagia and/or aspiration), and results showed a success rate of 75% (21/28) (4).
Even when cricopharyngeal disorders are associated with a Zenker’s diverticulum, surgical myotomy results are encouraging. A prospective study was conducted comparing the results between the surgical myotomy in patients with and without Zenker’s diverticulectomy and a normal control group, evaluating the impact of the myotomy in the UES opening. Twenty patients were included (12/20 had a Zenker’s diverticulum), and after the myotomy, UES opening was comparable with control healthy patients (15).
Although most studies analyzed post-myotomy outcomes, only a few included objective measurement instruments. Interestingly, Jiang et al. conducted a retrospective study measuring reflux symptoms and dysphagia with the Reflux Symptom Index and the Eating Assessment Tool 10, respectively, after an open CPM. Twenty patients underwent transcervical CPM, with or without Zenker’s diverticulectomy, with statistically significant improvement of reflux and dysphagia symptoms [21.8 to 8.9 and 19.1 to 5 pre- and post-operatively, respectively (P<0.001)] (16). Overall, data suggest that surgical CPM is safe, with encouraging postoperative results in terms of symptoms relief and quality of life improvement. Studies describing surgical CPM are summarized in Table 1.
Table 1
| Author | Year | Number of patients | Surgical or endoscopic myotomy | Complications | Success rate (%) | Follow-up (months) |
|---|---|---|---|---|---|---|
| St Guily et al. (17) | 1994 | 11 | Surgical | None | 72 | 5–53 |
| Herberhold et al. (18) | 1995 | 32 | Endoscopic | Mediastinitis, supraglotic edema | 97 | >84 |
| Lim et al. (19) | 1995 | 40 | Endoscopic | Esophageal perforation | 90 | 2–22 |
| Poirier et al. (20) | 1997 | 40 | Surgical | Retropharyngeal hematoma | 72.5 | 1–255 |
| Ali et al. (21) | 1997 | 8 | Surgical | Not available | 75 | 1.5 |
| Halvorson et al. (22) | 1998 | 18 | Endoscopic | Not available | 78 | NA |
| Mason et al. (23) | 1998 | 31 | Surgical | Pneumonia, neck hematoma, pulmonary edema | 77 | 2–48 |
| Lawson et al. (24) | 2003 | 29 | Endoscopic | None | 88 | 1–36 |
| Zaninotto et al. (1) | 2004 | 11 | Surgical | None | 73 | 6–31 |
| Takes et al. (25) | 2005 | 10 | Endoscopic | None | 60 | 2–24 |
| Dauer et al. (26) | 2006 | 22 | Surgical and endoscopic | Chest pain, fever, pharyngocutaneous fistula, tracheotomy | 58 | NA |
| Munoz et al. (27) | 2007 | 14 | Surgical | Not available | 25 | 6–10 |
| Lawson et al. (28) | 2008 | 31 | Endoscopic | None | 64.5 | 12–23 |
| Kos et al. (4) | 2010 | 28 | Surgical | Aspirative pneumonia, mucosal fistula, fever | 79 | 2.5–203 |
| Ozgursoy et al. (29) | 2010 | 14 | Endoscopic | None | 100 | 6 |
| Bachy et al. (30) | 2013 | 32 | Endoscopic | Bleeding | 84 | 6–99 |
| Jiang et al. (16) | 2017 | 41 | Surgical and endoscopic | Not available | 97.5 | 5.4 |
| Shibata et al. (9) | 2020 | 14 | Surgical | Pneumonia, fever, nerve paralysis | 100 | 66 |
Endoscopic CPM
Based on the promising results published by Dohlman treating Zenker’s diverticulum endoscopically, Halvorson in 1994 using the KTP laser, and Herberhold in 1995 using the CO2 laser, described the endoscopic laser-assisted myotomy (18,31). However, controversy arises regarding safety and efficacy of this highly demanding technique (24,25).
Technique
A laryngoscope is positioned at the post-cricoid place. A vertical midline incision over the mucosa covering the CM is then performed with the laser, exposing the muscle. After that, a submucosal resection of the CM is performed with the laser as extensively as possible. Finally, the incised area is covered with the incised mucosa (32).
Outcomes
Originally, CPM has been performed using potassium-titanyl-phosphate lasers. over the years, CO2 lasers have gained popularity due to their reduced postoperative morbidity (33).
Gilheaney et al. conducted a systematic review analyzing effectiveness of endoscopic CPM in patients with neurological disorders. Two studies were included, both of them using CO2 laser for CPM. Reduction of food aspiration and laryngeal penetration with no adverse events were reported. However, evidence is still scarce to strongly recommend the endoscopic approach for CPM in patients with neurological UES disorders (33).
A retrospective study was published in 2000, including 17 patients with UES disorders related to different etiologies. All the patients but one, improved swallowing characteristics and quality of life after the procedure, and no complications were reported, suggesting that the endoscopic CPM was a safe approach (34). Another retrospective review of patients undergoing CPM was conducted in the Mayo Clinic of Jacksonville in 2006, comparing the open myotomy versus the endoscopic approach. Eight patients underwent an open surgical myotomy, whereas 14 underwent an endoscopic approach. Improvements in swallowing function were similar between groups (objectively evaluated by the Functional Outcome Swallowing Scale), and there were 3 minor complications in the endoscopic group and 3 complications (1 minor and 2 major) in the open group (26). According to the published data, the endoscopic approach seems to be safe and effective. Studies describing endoscopic CPM are summarized in Table 1.
TBI
TBI has emerged as a less invasive treatment modality for patients with UES disorders (35). Toxin botulinum inhibits the release of acetylcholine, blocking the neuromuscular transmission and inhibiting the active contraction of the muscles and their tonicity; helping patients with hypertonicity of the UES (35).
TBI is often used for patients with transitory CM dysfunction and/or for those who are not candidates for a procedure under general anesthesia. TBI might also be helpful to select patients who could benefit from a definitive surgical myotomy (1,2).
TBI technique
Administered doses of toxin botulinum vary among authors, from 2.5 to 100 Botox units, and the injection technique is also diverse. Some authors prefer injecting botulinum neurotoxin under direct vision with an endoscopic approach, and others perform a percutaneous technique. TBI can be performed under electromyographic, fluoroscopic, and/or imaging guidance (36).
Outcomes
Zaninotto and colleagues, conducted one the largest series of patients with oropharyngeal dysphagia undergoing TBI. Twenty-one patients were included in the analysis, and 43% showed an improvement of the dysphagia. One patient died due to bronchopulmonary aspiration, and 12 failed to improve dysphagia symptoms [11 of these patients underwent a surgical myotomy of the CM, with a swallowing improvement of 72.7% (8/11)] (1).
Another study included 34 patients with symptomatic dysphagia, and analyzed cricopharyngeal electrophysiological characteristics to predict the efficacy of the cricopharyngeal TBI. Improvement of dysphagia two months after TBI was observed in 50% of the patients, with no complications reported in this series (37).
Restivo et al. published the results of 14 patients with UES disorders associated with multiple sclerosis, who underwent percutaneous TBI guided by electromyographic control. After two years follow-up, all patients (100%) showed significant improvement of the swallowing mechanism with no complications related to the procedure (38).
Finally, Kelly and colleagues published the outcomes of the largest series of patients undergoing TBI for UES disorders: 65% of patients showed symptom’s improvement after the treatment (32/49 patients) (3). Studies describing TBI are summarized in Table 2.
Table 2
| Author | Year | Number of patients | Cause of UES dysfunction | Complications | Symptom’s improvement (%) | Follow-up (months) |
|---|---|---|---|---|---|---|
| Schneider et al. (5) | 1994 | 7 | Heterogeneous | None | 71 | 2.5 |
| Atkinson and Rees (39) | 1997 | 5 | Heterogeneous | Left vocal paralysis and pneumonia secondary to aspiration when injection effect ended | 80 | N/A |
| Brant et al. (40) | 1999 | 1 | Stroke | None | 100 | 12 |
| Alberty et al. (7) | 2000 | 10 | Heterogeneous | None | 100 | N/A |
| Shaw and Searl (41) | 2001 | 12 | Heterogeneous | Worsening dysphagia and pharyngeal laceration | 83 | N/A |
| Haapaniemi et al. (42) | 2001 | 4 | Heterogeneous | None | 75 | N/A |
| Moerman et al. (43) | 2002 | 4 | Heterogeneous | None | 100 | N/A |
| Parameswaran and Soliman (8) | 2002 | 12 | Heterogeneous | Neck cellulitis | 92 | N/A |
| Zaninotto et al. (1) | 2004 | 21 | Heterogeneous | Death secondary to a bronchopulmonary aspiration | 43 | 17 |
| Liu et al. (44) | 2004 | 2 | Inclusion body myositis | None | 100 | 23 |
| Chiu et al. (45) | 2004 | 1 | Stroke | None | 100 | 12 |
| Murry et al. (35) | 2005 | 13 | Heterogeneous | None | 85 | 5–9 |
| Kim et al. (46) | 2006 | 8 | Stroke | None | 62.5 | 1–3 |
| Masiero et al. (12) | 2006 | 2 | Stroke | None | 100 | 24 |
| Restivo et al. (47) | 2006 | 12 | Diabetic neuropathy | None | 100 | 6 |
| Suzukia et al. (48) | 2007 | 1 | Type 2 spinal muscular atrophy | Temporary worsening of dysphagia | 100 | 1 |
| Krause et al. (49) | 2008 | 1 | Subarachnoid hemorrhage spasticity | None | 100 | N/A |
| Alfonsi et al. (37) | 2010 | 34 | Heterogeneous | None | 50 | 2 |
| Restivo et al. (38) | 2011 | 14 | Multiple sclerosis | None | 100 | 6 |
| Woisard-Bassols et al. (50) | 2013 | 11 | Heterogeneous | Worsening dysphagia and GERD | 45 | 12 |
| Kelly et al. (3) | 2000 | 49 | Heterogeneous | Worsening dysphagia | 65 | N/A |
| Terré et al. (51) | 2013 | 23 | Stroke | None | 82 | 12 |
| Kim et al. (52) | 2017 | 10 | Heterogeneous | Unilateral vocal fold paralysis | 63.9 | 6 |
| Wei et al. (53) | 2019 | 1 | Stroke | None | 100 | 6 |
UES, upper esophageal sphincter; GERD, gastroesophageal reflux disease.
TBI for the treatment of CM dysfunction seems to be safe and effective, with success rates comparable to surgical myotomy. Although one of the most important disadvantages is its temporary effect, it is a valid and less invasive alternative to surgical myotomy. In addition, in case of failure, a surgical myotomy can always be attempted.
Expert commentary
We intended to analyze all the available literature regarding CPM and TBI for the treatment of UES disorders. With all the existing data, it is hard to give an evidence-based recommendation for this complex and poorly understood entity. However, we were able to draw some conclusions.
The TBI for the treatment of CM dysfunction is safe and effective. However, it offers only a temporary resolution of the symptoms. On the other hand, the CPM offers more durable results of the cricopharyngeal dysfunction. We often use the TBI as well as the cricopharyngeal dilation to select which patients might be benefited from surgical treatment. Overall, we prefer a myotomy as definitive treatment.
Promising results are reported regarding novel techniques such as the Z-POEM, which consists of an endoscopic myotomy through a submucosal tunnel for the treatment of Zenker’s diverticulum. Although we do not have experience with this treatment modality, we strongly believe that this kind of novel procedures should be explored in the future.
Overall, further studies analyzing diverse treatment modalities for UES disorders with objective assessments of their outcomes are still needed.
Conclusions
Current data are heterogeneous and show that both CPM and TBI are safe and effective treatment modalities for UES disorders. Better long-lasting effects, however, seem to be achieved after surgical or endoscopic myotomy, as compared to TBI.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Upper Esophageal Sphincter”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-8/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-8/coif). The series “Upper Esophageal Sphincter” was commissioned by the editorial office without any funding or sponsorship. FAMH served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from September 2020 to August 2022. FS serves as an unpaid editorial board member of Annals of Esophagus from August 2020 to July 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zaninotto G, Marchese Ragona R, Briani C, et al. The role of botulinum toxin injection and upper esophageal sphincter myotomy in treating oropharyngeal dysphagia. J Gastrointest Surg 2004;8:997-1006. [Crossref] [PubMed]
- Kelly EA, Koszewski IJ, Jaradeh SS, et al. Botulinum toxin injection for the treatment of upper esophageal sphincter dysfunction. Ann Otol Rhinol Laryngol 2013;122:100-8. [Crossref] [PubMed]
- Kelly JH. Management of upper esophageal sphincter disorders: indications and complications of myotomy. Am J Med 2000;108:43S-46S. [Crossref] [PubMed]
- Kos MP, David EF, Klinkenberg-Knol EC, et al. Long-term results of external upper esophageal sphincter myotomy for oropharyngeal Dysphagia. Dysphagia 2010;25:169-76. [Crossref] [PubMed]
- Schneider I, Thumfart WF, Pototschnig C, et al. Treatment of dysfunction of the cricopharyngeal muscle with botulinum A toxin: introduction of a new, noninvasive method. Ann Otol Rhinol Laryngol 1994;103:31-5. [Crossref] [PubMed]
- Crary MA, Glowasky AL. Using botulinum toxin A to improve speech and swallowing function following total laryngectomy. Arch Otolaryngol Head Neck Surg 1996;122:760-3. [Crossref] [PubMed]
- Alberty J, Oelerich M, Ludwig K, et al. Efficacy of botulinum toxin A for treatment of upper esophageal sphincter dysfunction. Laryngoscope 2000;110:1151-6. [Crossref] [PubMed]
- Parameswaran MS, Soliman AM. Endoscopic botulinum toxin injection for cricopharyngeal dysphagia. Ann Otol Rhinol Laryngol 2002;111:871-4. [Crossref] [PubMed]
- Shibata S, Kagaya H, Ozeki Y, et al. Effect of Laryngeal Suspension and Upper Esophageal Sphincter Myotomy for Severe Dysphagia Due to Brainstem Disease. Ann Otol Rhinol Laryngol 2020;129:689-94. [Crossref] [PubMed]
- Kaplan S. Paralysis of deglutition, a post-poliomyelitis complication treated by section of the cricopharyngeus muscle. Ann Surg 1951;133:572-3. [Crossref] [PubMed]
- Lawson G, Remacle M. Endoscopic cricopharyngeal myotomy: indications and technique. Curr Opin Otolaryngol Head Neck Surg 2006;14:437-41. [Crossref] [PubMed]
- Masiero S, Briani C, Marchese-Ragona R, et al. Successful treatment of long-standing post-stroke dysphagia with botulinum toxin and rehabilitation. J Rehabil Med 2006;38:201-3. [Crossref] [PubMed]
- Duranceau AC, Lafontaine E, Taillefer R. Oropharyngeal dysphagia. In: Jamieson GG, ed. Oropharyngeal Dysphagia in Surgery of the Oesophagus. New York: Churchill Livingstone, 1988:413-33.
- Buchholz DW. Cricopharyngeal myotomy may be effective treatment for selected patients with neurogenic oropharyngeal dysphagia. Dysphagia 1995;10:255-8. [Crossref] [PubMed]
- Yip HT, Leonard R, Kendall KA. Cricopharyngeal myotomy normalizes the opening size of the upper esophageal sphincter in cricopharyngeal dysfunction. Laryngoscope 2006;116:93-6. [Crossref] [PubMed]
- Jiang N, Sung CK, Damrose EJ. Improvement in the Reflux Symptom Index Following Surgery for Cricopharyngeal Dysfunction. J Voice 2017;31:86-9. [Crossref] [PubMed]
- St Guily JL, Perie S, Willig TN, et al. Swallowing disorders in muscular diseases: functional assessment and indications of cricopharyngeal myotomy. Ear Nose Throat J 1994;73:34-40. [Crossref] [PubMed]
- Herberhold C, Walther EK. Endoscopic laser myotomy in cricopharyngeal achalasia. Adv Otorhinolaryngol 1995;49:144-7. [Crossref] [PubMed]
- Lim RY. Endoscopic CO2 laser cricopharyngeal myotomy. J Clin Laser Med Surg 1995;13:241-7. [Crossref] [PubMed]
- Poirier NC, Bonavina L, Taillefer R, et al. Cricopharyngeal myotomy for neurogenic oropharyngeal dysphagia. J Thorac Cardiovasc Surg 1997;113:233-40; discussion 240-1. [Crossref] [PubMed]
- Ali GN, Wallace KL, Laundl TM, et al. Predictors of outcome following cricopharyngeal disruption for pharyngeal dysphagia. Dysphagia 1997;12:133-9. [Crossref] [PubMed]
- Halvorson DJ. The treatment of cricopharyngeal dysmotility with a transmucosal cricopharyngeal myotomy using the potassium-titanyl-phosphate (KTP) laser. Endoscopy 1998;30:46-50. [Crossref] [PubMed]
- Mason RJ, Bremner CG, DeMeester TR, et al. Pharyngeal swallowing disorders: selection for and outcome after myotomy. Ann Surg 1998;228:598-608. [Crossref] [PubMed]
- Lawson G, Remacle M, Jamart J, et al. Endoscopic CO2 laser-assisted surgery for cricopharyngeal dysfunction. Eur Arch Otorhinolaryngol 2003;260:475-80. [Crossref] [PubMed]
- Takes RP, van den Hoogen FJ, Marres HA. Endoscopic myotomy of the cricopharyngeal muscle with CO2 laser surgery. Head Neck 2005;27:703-9. [Crossref] [PubMed]
- Dauer E, Salassa J, Iuga L, et al. Endoscopic laser vs open approach for cricopharyngeal myotomy. Otolaryngol Head Neck Surg 2006;134:830-5. [Crossref] [PubMed]
- Muñoz AA, Shapiro J, Cuddy LD, et al. Videofluoroscopic findings in dysphagic patients with cricopharyngeal dysfunction: before and after open cricopharyngeal myotomy. Ann Otol Rhinol Laryngol 2007;116:49-56. [Crossref] [PubMed]
- Lawson G, Remacle M. Ins and outs of myotomy of the upper esophageal sphincter in swallowing disorders. B-ENT 2008;10:83-9.
- Ozgursoy OB, Salassa JR. Manofluorographic and functional outcomes after endoscopic laser cricopharyngeal myotomy for cricopharyngeal bar. Otolaryngol Head Neck Surg 2010;142:735-40. [Crossref] [PubMed]
- Bachy V, Matar N, Remacle M, et al. Long-term functional results after endoscopic cricopharyngeal myotomy with CO2 laser: a retrospective study of 32 cases. Eur Arch Otorhinolaryngol 2013;270:965-8. [Crossref] [PubMed]
- Halvorson DJ, Kuhn FA. Transmucosal cricopharyngeal myotomy with the potassium-titanyl-phosphate laser in the treatment of cricopharyngeal dysmotility. Ann Otol Rhinol Laryngol 1994;103:173-7. [Crossref] [PubMed]
- Chitose S, Sato K, Hamakawa S, et al. A new paradigm of endoscopic cricopharyngeal myotomy with CO2 laser. Laryngoscope 2011;121:567-70. [Crossref] [PubMed]
- Gilheaney Ó, Kerr P, Béchet S, et al. Effectiveness of endoscopic cricopharyngeal myotomy in adults with neurological disease: systematic review. J Laryngol Otol 2016;130:1077-85. [Crossref] [PubMed]
- Brøndbo K. Treatment of cricopharyngeal dysfunction by endoscopic laser myotomy. Acta Otolaryngol Suppl 2000;543:222-4. [Crossref] [PubMed]
- Murry T, Wasserman T, Carrau RL, et al. Injection of botulinum toxin A for the treatment of dysfunction of the upper esophageal sphincter. Am J Otolaryngol 2005;26:157-62. [Crossref] [PubMed]
- Moerman MB. Cricopharyngeal Botox injection: indications and technique. Curr Opin Otolaryngol Head Neck Surg 2006;14:431-6. [Crossref] [PubMed]
- Alfonsi E, Merlo IM, Ponzio M, et al. An electrophysiological approach to the diagnosis of neurogenic dysphagia: implications for botulinum toxin treatment. J Neurol Neurosurg Psychiatry 2010;81:54-60. [Crossref] [PubMed]
- Restivo DA, Marchese-Ragona R, Patti F, et al. Botulinum toxin improves dysphagia associated with multiple sclerosis. Eur J Neurol 2011;18:486-90. [Crossref] [PubMed]
- Atkinson SI, Rees J. Botulinum toxin for cricopharyngeal dysphagia: case reports of CT-guided injection. J Otolaryngol 1997;26:273-6. [PubMed]
- Brant CQ, Siqueira ES, Ferrari AP Jr. Botulinum toxin for oropharyngeal dysphagia: case report of flexible endoscopeguided injection. Dis Esophagus 1999;12:68-73. [Crossref] [PubMed]
- Shaw GY, Searl JP. Botulinum toxin treatment for cricopharyngeal dysfunction. Dysphagia 2001;16:161-7. [Crossref] [PubMed]
- Haapaniemi JJ, Laurikainen EA, Pulkkinen J, et al. Botulinum toxin in the treatment of cricopharyngeal dysphagia. Dysphagia 2001;16:171-5. [Crossref] [PubMed]
- Moerman M, Callier Y, Dick C, et al. Botulinum toxin for dysphagia due to cricopharyngeal dysfunction. Eur Arch Otorhinolaryngol 2002;259:1-3. [Crossref] [PubMed]
- Liu LWC, Tarnopolsky M, Armstrong D. Injection of botulinum toxin A to the upper esophageal sphincter for oropharyngeal dysphagia in two patients with inclusion body myositis. Can J Gastroenterol 2004;18:397-9. [Crossref] [PubMed]
- Chiu MJ, Chang YC, Hsiao TY. Prolonged effect of botulinum toxin injection in the treatment of cricopharyngeal dysphagia: case report and literature review. Dysphagia 2004;19:52-7. [Crossref] [PubMed]
- Kim DY, Park CI, Ohn SH, et al. Botulinum toxin type A for poststroke cricopharyngeal muscle dysfunction. Arch Phys Med Rehabil 2006;87:1346-51. [Crossref] [PubMed]
- Restivo DA, Marchese-Ragona R, Lauria G, et al. Botulinum toxin treatment for oropharyngeal dysphagia associated with diabetic neuropathy. Diabetes Care 2006;29:2650-3. [Crossref] [PubMed]
- Suzukia Y, Sano N, Shinonaga C, et al. Successful botulinum toxin treatment of dysphagia in a spinal muscular atrophy type 2 patient. Brain Dev 2007;29:662-5. [Crossref] [PubMed]
- Krause E, Schirra J, Gürkov R. Botulinum toxin A treatment of cricopharyngeal dysphagia after subarachnoid hemorrhage. Dysphagia 2008;23:406-10. [Crossref] [PubMed]
- Woisard-Bassols V, Alshehri S, Simonetta-Moreau M. The effects of botulinum toxin injections into the cricopharyngeus muscle of patients with cricopharyngeus dysfunction associated with pharyngo-laryngeal weakness. Eur Arch Otorhinolaryngol 2013;270:805-15. [Crossref] [PubMed]
- Terré R, Panadés A, Mearin F. Botulinum toxin treatment for oropharyngeal dysphagia in patients with stroke. Neurogastroenterol Motil 2013;25:896-e702. [Crossref] [PubMed]
- Kim MS, Kim GW, Rho YS, et al. Office-based Electromyography-guided Botulinum Toxin Injection to the Cricopharyngeus Muscle: Optimal Patient Selection and Technique. Ann Otol Rhinol Laryngol 2017;126:349-56. [Crossref] [PubMed]
- Wei P, Xu Y, Zhang Z, et al. Treatment for upper esophageal sphincter dysfunction in a patient with poststroke dysphagia: A case report. Medicine (Baltimore) 2019;98:e14988. [Crossref] [PubMed]
Cite this article as: Laxague F, Herbella FAM, Schlottmann F. Cricopharyngeal myotomy and toxin botulinum injection for the treatment of upper esophageal sphincter disorders: a narrative review. Ann Esophagus 2022;5:27.