
Technical notes and outcomes of robot-assisted and laparoscopic jejunostomy placement for tube feeding after esophagectomy
Introduction
Esophagectomy is the core of curative treatment for esophageal cancer, yielding a 5-year survival rate of 40–50% when preceded by neoadjuvant chemo(radio)therapy (1,2). A paradigm shift towards minimally invasive techniques occurred over the last years (3), as randomized trials found that both minimally invasive esophagectomy (MIE) and robot-assisted MIE (RAMIE) achieve good oncological results and offer benefits over an open approach in terms of blood loss, postoperative pain, pulmonary complications, and functional recovery (4-6). Nonetheless, MIE and RAMIE are still associated with an overall morbidity rate of approximately 60%, which is mainly explained by pulmonary complications and anastomotic leakage (7,8).
Aiming to minimize the risk of aspiration pneumonia and to protect the newly formed esophagogastric anastomosis, patients are often kept on a nil by mouth diet for the first few days after esophagectomy (9,10). Enteral tube feeding is mostly preferred during this period, which can be provided through a surgical jejunostomy or an endoscopically inserted naso-enteric tube. Whereas jejunostomy tubes may be associated with more serious complications requiring re-operations (e.g., intestinal torsions, intra-abdominal abscess), naso-enteric tubes might increase patient discomfort and dislocate in 20–35% of patients (11). Based on currently available literature, jejunostomy tubes seem preferable over nasoduodenal tube feeding for patients undergoing esophagectomy, as they are associated with better quality of life at 1 week after surgery, less tube dislocations (either intentional by the patient or otherwise), and may be used in combination with an early oral feeding protocol (12,13). These findings suggest that jejunostomy placement is still justified as a routine part of the esophagectomy procedure, warranting attempts to identify the technique that has the lowest morbidity.
Prior studies suggested that jejunostomy-related complications are common in patients undergoing esophagectomy (11). For example, a recent study found that intestinal torsions at the jejunostomy site occurred in 17% of patients after esophagectomy for cancer, which led the authors to question the appropriateness of this feeding strategy for routine care (14). However, the technique for jejunostomy placement seems to be unstandardized according to current literature. In a previous small case series that investigated the outcomes of a laparoscopic technique for jejunostomy tube placement with anti-rotation fixation to the abdominal wall, no severe complications occurred (15). Fixating the jejunum over a longer segment might mechanically decrease the rotational mobility at the jejunostomy site itself, reducing the incidence of this potentially severe complication. Comparable methods are now commonly used (e.g., the Stamm method for gastrostomy creation) and seem solid, but data on the outcomes of these techniques are largely lacking. Furthermore, the risk factors for jejunostomy-related complications are largely unclear.
The current study aimed to describe the technical elements and outcomes of a technique for jejunostomy tube placement with the essential step of anti-rotation fixation, which is facilitated by using an endoscope and (robot-assisted) laparoscopic instruments to get overview of the anterior abdominal wall and fixate the jejunal segment to it. The jejunostomy is created by placing a purse-string suture around the tube followed by anti-rotation fixation of the jejunal segment to the anterior abdominal wall. This technique might be beneficial in reducing the risk of intestinal torsion and avoids the need for an additional incision, which represents a step forward in minimally invasive surgery. This study reports the short-term outcomes of this technique and hypothesizes that it is safe and associated with low jejunostomy-related morbidity. In addition, this study intended to identify patient- and treatment characteristics that are associated with jejunostomy-related complications. We present the following article in accordance with the STROBE reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-4/rc).
Methods
Design and patient population
The institutional prospective databases of the University Medical Center Utrecht (Utrecht, The Netherlands) and Hospital Universitario Fundación Favaloro (Buenos Aires, Argentina) were used to select all patients who underwent (robot-assisted) MIE with minimally invasive jejunostomy tube placement for cancer between 2010 and 2019. No specific exclusion criteria were defined. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). The institutional reviews boards of the participating centers approved this study and the need for written informed consent was waived because of the retrospective study design and use of anonymized data.
Technique for jejunostomy tube placement
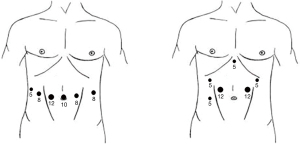
Robot-assisted or conventional laparoscopy was used to place a jejunostomy tube at the end of the abdominal phase during two-stage transthoracic esophagectomy. The trocar port positions are shown in Figure 1. In the robotic approach as performed in the UMC Utrecht, the 5 mm port is used for the liver retractor, the 8 mm ports are used for the robotic instruments, and the 12 mm port is used for the camera throughout the procedure. For jejunostomy tube placement, the instrument in the most lateral 8 mm trocar port on the left (from patient perspective) is removed. A Cadiere forceps and large robotic needle driver are introduced through the other 8 mm trocar ports while the camera remains in the 12 mm port. In the conventional laparoscopic approach as performed in Fundación Favaloro, the Dorsey grasper and laparoscopic needle driver are used.
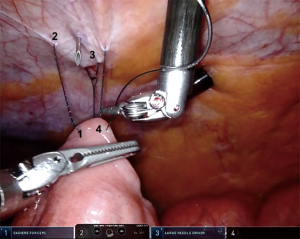
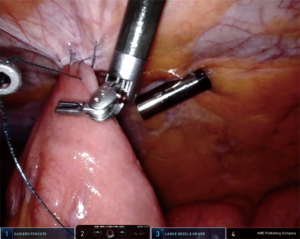
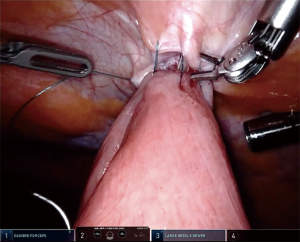
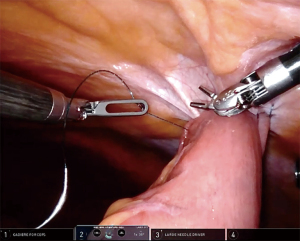
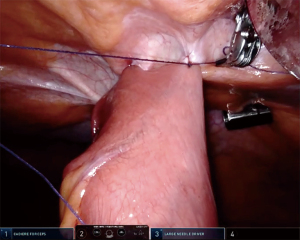
Key elements of the procedure involve a purse string suture around the tube and fixation of the jejunum to the anterior abdominal wall, which are somewhat similar to the laparoscopic Stamm technique for surgical placement of a gastrostomy tube. First, a suitable post-Treitz jejunal segment was selected. A needle was then introduced through the abdominal wall at the desired point of entrance for the jejunostomy tube (usually in the upper left abdominal quadrant), followed by attachment of the selected jejunal segment to the abdominal wall with an autoadjustable 3.0 suture (V-Loc, Medtronic, USA) (Figures 2,3). These steps were performed vice versa in the conventional laparoscopic cases. The needle was then pierced into the jejunum and the Seldinger technique was applied to insert a 9 to 14 French tube distally into the jejunum (Figure 4). Autoadjustable purse-string sutures were used to attach the jejunum to the abdominal wall around the tube (Figure 5). Finally, anti-rotation fixation of the jejunum was performed by one suture at the proximal side (Figure 6) and one suture at the distal side (Figure 7), or by a running suture over 3–4 centimeters in distal direction. The sutures were placed 1–2 centimeters from the tube. The jejunostomy tube was fixated to the skin by means of a suture and postoperatively kept in place until the patient could be fed adequately via the oral route or until complications necessitated prompt removal.
Outcome measures and data collection
The primary outcome was the rate of jejunostomy-related complications until the day of removing the jejunostomy tube, including infections, tube dislocations, or intestinal torsions. Other postoperative complications were scored until 30 days after surgery and defined according to the Esophagectomy Complications Consensus Group (ECCG) agreements (16). All complications were graded by means of the Clavien-Dindo classification system (17). The duration of jejunostomy tube feeding and the postoperative weight at 3 and 6 months after surgery were also evaluated. The prospective databases of the participating institutions were complemented by retrospective review of patient files to collect the required data. In case of missing follow-up data, the numbers of complete cases were reported and the outcomes were evaluated for this group.
Statistical analyses
All statistical analyses were performed by using SPSS 21.0 (Armonk, USA). Categorial data were shown as counts with percentages. Means with standard deviations or medians with ranges were calculated for continuous outcomes, depending on the distribution of data. To identify potential factors associated with jejunostomy-related complications, exploratory univariable analyses were performed comparing the characteristics of patient who developed jejunostomy-related complications were compared to those of patients who did not develop such complications. Chi-square tests (for categorical data), student’s t-tests (for normally distributed continuous data), or Mann-Whitney U tests (for non-normally distributed continuous data) were performed. A two-sided P<0.05 was considered to indicate a statistically significant difference. The limited sample size did not allow for a multivariable analysis to evaluate whether factors were predictive of jejunostomy-related complications.
Results
Patient population
A total of 93 patients (59 patients in hospital A and 34 patients in hospital B) were included. Patients were predominantly male (n=72, 77%) and the mean age was 62.9 years (±10.3 years). The median body mass index (BMI) was 26.5 kg/m2 [IQR, 24.0–29.2 kg/m2] and comorbidity was present in the majority of patients (n=70, 75%). The tumor was usually located in the distal esophagus (n=46, 50%) or esophagogastric junction (n=41, 44%). Neoadjuvant therapy was mostly provided (n=77, 83%), followed by esophagectomy by an Ivor-Lewis (n=73, 79%) or McKeown (n=20, 22%) approach. Jejunostomy tube placement was performed robotically (n=16, 17%) or by conventional laparoscopy (n=77, 83%). The overall morbidity and mortality rates were 67% and 1%, respectively, as is detailed in Table 1.
Table 1
| Parameters | N | (%) |
|---|---|---|
| Complications, yes | ||
| Any | 63 | −67 |
| Pulmonary (including pneumonia) | 34 | −37 |
| Anastomotic leakage | 30 | −32 |
| Chylothorax | 10 | −11 |
| Recurrent laryngeal nerve injury | 8 | −7 |
| Clavien-Dindo of the most severe complications | ||
| No complication | 31 | −33 |
| Clavien-Dindo 1 | 1 | −1 |
| Clavien-Dindo 2 | 23 | −25 |
| Clavien-Dindo 3a | 13 | −14 |
| Clavien-Dindo 3b | 11 | −12 |
| Clavien-Dindo 4 | 13 | −14 |
| Clavien-Dindo 5 | 1 | −1 |
| Length of hospital stay, days [IQR] | 12 | [8–20] |
| Re-admission <30 days after discharge | ||
| Yes | 12 | −13 |
| No | 79 | −87 |
| Unknown | 2 | |
| Mortality (in-hospital or <30 days after surgery) | 1 | −1 |
IQR, interquartile range. MIE, minimally invasive esophagectomy.
Jejunostomy-related complications
The data on postoperative jejunostomy-related complications were complete. Jejunostomy-related complications were observed in 13 cases (14%), which involved 12 skin infections and 1 abdominal wall abscess. No jejunostomy-related mortality occurred. The complications were classified as Clavien-Dindo 1 in 1 patient (1%), Clavien-Dindo 2 in 9 patients (10%), Clavien-Dindo 3a in 3 patients (3%), and Clavien-Dindo 3b in 1 patient (1%). The jejunostomy tube was removed because of infectious complications in 6 patients (7%), on median postoperative day 11 [IQR, 8–14]. One re-operation under general anesthesia (i.e., Clavien-Dindo 3b) was required to manage jejunostomy-related infection. During this re-operation at postoperative day 11, an abdominal wall abscess was found, likely caused by a defect in the jejunostomy tube. The jejunostomy tube was replaced and remained functional until it was removed in response to adequate oral intake on the 56th day after esophagectomy. The other re-interventions (i.e., Clavien-Dindo 3a) involved bed-side incision of a skin abscess in 2 cases and replacement of the jejunostomy tube under X-ray vision in 1 case.
Table 2 shows the characteristics of patients with jejunostomy-related complications versus patients without such complications. In patients with jejunostomy-related complications, a higher incidence of overall comorbidity (100% vs. 71%, P=0.033) and diabetes mellitus in particular (31% vs. 9%, P=0.044) were found when compared to patients without jejunostomy-related complications.
Table 2
| Parameters | Complication (n=13) | No complication (n=80) | P | |||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | |||
| Center | 0.439 | |||||
| A | 7 | −54 | 52 | −65 | ||
| B | 6 | −46 | 28 | −35 | ||
| Age in years, mean (± SD) | 62.2 | −10.5 | 66.9 | −8 | 0.129 | |
| Gender | ||||||
| Male | 10 | −77 | 62 | −77 | >0.999 | |
| Female | 3 | −23 | 18 | −23 | ||
| BMI in kg/m2, median [IQR] | 28 | [26.0–29.2] | 25.8 | [23.9–29.3] | 0.099 | |
| Comorbidity, yes | ||||||
| Any | 13 | −100 | 57 | −71 | 0.033 | |
| Cardiovascular | 7 | −54 | 34 | −43 | 0.445 | |
| Respiratory | 1 | −8 | 12 | −15 | 0.685 | |
| Diabetes | 4 | −31 | 7 | −9 | 0.044 | |
| Tumor location | ||||||
| Middle third | 1 | −8 | 5 | −6 | >0.999 | |
| Distal third or GE-junction | 13 | −92 | 75 | −94 | ||
| Tumor histology | 0.918 | |||||
| Adenocarcinoma | 10 | −77 | 60 | −75 | ||
| Squamous cell carcinoma | 3 | −23 | 19 | −24 | ||
| Other | 0 | 0 | 1 | −1 | ||
| Neoadjuvant therapy | 0.272 | |||||
| Chemoradiotherapy | 10 | −77 | 48 | −60 | ||
| Chemotherapy | 0 | 0 | 18 | −23 | ||
| Radiotherapy | 0 | 0 | 1 | −1 | ||
| None | 3 | −23 | 13 | −16 | ||
| Abdominal approach | ||||||
| Conventional laparoscopy | 11 | −85 | 66 | −83 | >0.999 | |
| Robot-assisted laparoscopy | 2 | −15 | 14 | −17 | ||
| Pathological T stage | 0.449 | |||||
| pT0 | 2 | −15 | 24 | −30 | ||
| pT1 | 5 | −39 | 18 | −23 | ||
| pT2 | 1 | −8 | 16 | −20 | ||
| pT3 | 5 | −39 | 21 | −26 | ||
| pT4 | 0 | 0 | 1 | −1 | ||
| Pathological N stage | 0.525 | |||||
| pN0 | 9 | −69 | 57 | −71 | ||
| pN1 | 3 | −23 | 10 | −13 | ||
| pN2 | 0 | 0 | 8 | −10 | ||
| pN3 | 1 | −8 | 5 | −6 | ||
| Completeness of resection | ||||||
| R0 | 13 | −100 | 76 | −95 | >0.999 | |
| R1-2 | 0 | 0 | 4 | −5 | ||
Pathological T and N staging were performed according to the American Joint Commission on Cander (AJCC) staging guidelines. BMI, body mass index; GE-junction, gastro-esophageal junction; IQR, interquartile range.
Duration of jejunostomy feeding and postoperative weight
Overall, patients had their jejunostomy tube in place for a median of 35 days [IQR, 27–71 days]. The jejunostomy tube was removed significantly earlier in patients with jejunostomy-related complications than in those who had an uneventful course regarding their jejunostomy [median day 21 (IQR, 11–61) vs. day 37 (IQR, 28–72), P=0.049]. Data regarding weight were available for 80 out of the 94 patients at 3 months postoperative follow-up (85%) and for 70 out of the 94 patients at 6 months postoperative follow-up (75%). Between surgery and 3 months postoperative follow-up, these patients lost a median of 5.9% [IQR, 2.8–11.8%] of their body weight. At 6 months postoperative follow-up, a median weight loss of 6.9% [IQR, 3.0–14.7%] was observed.
Discussion
Summary of findings
In this multicenter study that describes a technique for minimally invasive jejunostomy tube placement with additional anti-rotation fixation, jejunostomy-related complications occurred in 13 patients (14%). Pre-existent comorbidity (100% vs. 71%), specifically diabetes mellitus (31% vs. 9%), was observed significantly more frequently in patients who had jejunostomy-related complications when compared to patients without such complications. All jejunostomy-related complications involved infections and a re-operation was required in only 1 patient (1%). No intestinal obstructions due to torsion at the jejunostomy site were found in this study. Although the jejunostomy tube was removed significantly earlier in patients with jejunostomy-related complications than in patients without complications (median day 21 vs. day 37), no difference was observed regarding the change in body weight at 3- and 6-month postoperative follow-up.
Intestinal torsions
The current findings are contradictory to several recent reports of serious jejunostomy-associated morbidity and intestinal torsions occurring at the jejunostomy site. In one study, bowel obstructions due to intestinal torsion were found in 12% of patients who had a jejunostomy after esophagectomy (18). In another, the torsion rate was even as high as 17% (14). As intestinal torsions frequently require a re-operation, these findings may be interpreted as rationale to refrain from the routine use of jejunostomy tube feeding in the perioperative care of esophageal cancer patients. However, it should be noted that the authors of these studies used a technique that involved fixation of the jejunum only at the site of the jejunostomy (14,18). An older study already reported an intestinal torsion rate of 2% when placing a jejunostomy with extra anti-rotation fixation by an open Witzel approach (19). With the currently evaluated minimally invasive Seldinger technique using anti-rotation sutures, at least similar results were achieved in our study that evaluated anti-rotation fixation by means of two comparable methods (i.e., a torsion rate of 0%). These results demonstrate that the incidence of intestinal torsions can be very low after esophagectomy. Hence, concerns for intestinal torsions should probably not be the main reason to opt for alternative feeding routes (e.g., nasojejunal tube or total parenteral feeding) in patients undergoing MIE.
Jejunostomy site infections
In this study, infection of the jejunostomy was observed in 14% of all included patients, which is in line with available literature on laparoscopic jejunostomy tube placement (20). Notably, diabetic comorbidity was significantly more common in the group of patients with jejunostomy-related complications. Whereas one older study reported that infections at the jejunostomy site were seen in 40% of diabetic patients undergoing open bariatric surgery (21), the current study is the first to confirm a significant association between diabetic comorbidity and jejunostomy-related complications in patients undergoing MIE. Although the exact mechanisms are not well understood, diabetes is a known risk factor for surgical site infections (22). A previous systematic review showed that postoperative hyperglycemia might be the most important independent predictor for surgical site infections (23). However, while postoperative hyperglycemia could potentially represent a modifiable risk factor, a Cochrane review concluded that there currently is insufficient evidence to support the hypothesis that aggressive postoperative glucose management reduces surgical site infections (24). Considering this literature, it seems that physicians should basically aim at hygienic care for the jejunostomy and early detection of potential infections. One could also argue that jejunostomy tubes should be avoided in diabetic patients undergoing MIE. Feeding through a nasojejunal tube might be a suitable alternative for these patients, although it must be noted that tube dislocation is a common drawback of that strategy.
Infections at the jejunostomy site can be serious and require re-operation, as was the case in one patient in this study. In this particular case, an abdominal wall abscess developed after a robotic procedure, which turned out to be caused by a leak in the feeding tube. In all likelihood, the feeding tube was punctured at the level of the abdominal wall when attaching the jejunum to the abdominal wall with an autoadjustable suture, which remained unnoticed due to the absence of tactile feedback in robotic surgery. As a result, nutrition leaked into the abdominal wall, causing abscess formation. Whereas robotic assistance provides certain technical benefits in terms of camera stability and dexterity, which facilitates suturing towards the abdominal wall and probably reduces operative time for minimally invasive jejunostomy tube placement, the absence of tactile feedback should be bared in mind.
Strengths and limitations
This study derives strength from its multicenter study design and focus on a detailed description of the technique that is used for minimally invasive placement of a jejunostomy tube in two expert centers for esophageal surgery. However, the number of included patients was relatively small and the median duration of jejunostomy tube feeding was limited. Therefore, uncommon jejunostomy-related complications might have been missed, especially those that may occur with long-term jejunostomy tube feeding. Intestinal torsions around the jejunostomy site might occur long after cessation of jejunostomy tube feeding and such events could not be identified with the current follow-up. Larger studies are required to clarify the complete complication profile of the presented technique. Lastly, it must be mentioned that the currently reported anastomotic leakage rate was high in relation to recent benchmarks. This might be explained by the experienced learning curve of a hand-sewn intrathoracic anastomosis in RAMIE (25), as the anastomotic leakage rate is 14% in our most recent series.
Future studies
At present, there are no high-quality studies available to determine whether a laparoscopic approach should be preferred over an open approach to jejunostomy placement. However, a recent retrospective study suggested that laparoscopic jejunostomy placement has lower overall morbidity when compared to a mini-laparotomy approach (20.8% vs. 10.5%) (26). Furthermore, multiple laparoscopic techniques have been suggested. In a case series of 206 patients, a laparoscopic double semi purse-string suturing technique for jejunostomy placement was reported to have acceptable overall morbidity (16.5%) with only 1 event of intestinal torsion (0.5%) (27). Although their technique has similarities to our currently reported purse-string technique, no antirotation sutures were placed. Future studies are required to adequately compare laparoscopic versus open jejunostomy placement techniques and to further evaluate the clinical role of antirotation sutures.
Conclusions
Minimally invasive surgery, either robotically or laparoscopically, allows the surgeon a clear overview of both the jejunum and the anterior abdominal, which facilitates the placement of a jejunostomy that is additionally fixated to the anterior abdominal wall to reduce the risk of intestinal torsion. This study showed that the presented technique was safe for patients undergoing esophagectomy in this study. No short-term intestinal torsions at the jejunostomy site were found and only infectious complications occurred, which could be successfully managed without a re-operation in the majority of cases. Pre-existent comorbidity, and diabetes in particular, were significantly associated with the incidence of jejunostomy-related complications. Based on these results, the current technique may be advisable to establish the enteral feeding route in MIE.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-4/rc
Data Sharing Statement: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-4/dss
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-4/coif). The series “Anastomotic Techniques for Minimally Invasive Esophagectomy and Endoscopic Handling of Its Complications” was commissioned by the editorial office without any funding or sponsorship. AN served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from February 2020 to January 2022. RVH has received a clinical research grant from Intuitive Surgical Inc. and acts as a proctor for Intuitive Surgical Inc., outside the submitted work. JPR has received a clinical research grant from Intuitive Surgical Inc. and acts as a proctor for Intuitive Surgical Inc., outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). The institutional reviews boards of the participating centers approved this study and the need for written informed consent was waived because of the retrospective study design and use of anonymized data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol 2015;22:S1350-6. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Schmidt HM, Gisbertz SS, Moons J, et al. Defining Benchmarks for Transthoracic Esophagectomy: A Multicenter Analysis of Total Minimally Invasive Esophagectomy in Low Risk Patients. Ann Surg 2017;266:814-21. [Crossref] [PubMed]
- Berkelmans GHK, Kingma BF, Fransen LFC, et al. Feeding protocol deviation after esophagectomy: A retrospective multicenter study. Clin Nutr 2020;39:1258-63. [Crossref] [PubMed]
- Kingma BF, Steenhagen E, Ruurda JP, et al. Nutritional aspects of enhanced recovery after esophagectomy with gastric conduit reconstruction. J Surg Oncol 2017;116:623-9. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Routes for early enteral nutrition after esophagectomy. A systematic review. Clin Nutr 2015;34:1-6. [Crossref] [PubMed]
- Tao Z, Zhang Y, Zhu S, et al. A Prospective Randomized Trial Comparing Jejunostomy and Nasogastric Feeding in Minimally Invasive McKeown Esophagectomy. J Gastrointest Surg 2020;24:2187-96. [Crossref] [PubMed]
- Berkelmans GHK, Fransen LFC, Dolmans-Zwartjes ACP, et al. Direct Oral Feeding Following Minimally Invasive Esophagectomy (NUTRIENT II trial): An International, Multicenter, Open-label Randomized Controlled Trial. Ann Surg 2020;271:41-7. [Crossref] [PubMed]
- Kitagawa H, Namikawa T, Iwabu J, et al. Bowel obstruction associated with a feeding jejunostomy and its association to weight loss after thoracoscopic esophagectomy. BMC Gastroenterol 2019;19:104. [Crossref] [PubMed]
- Pili D, Ciotola F, Riganti JM, et al. Autoadjustable sutures and modified seldinger technique applied to laparoscopic jejunostomy. World J Surg 2015;39:325-7. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Koterazawa Y, Oshikiri T, Hasegawa H, et al. Routine placement of feeding jejunostomy tube during esophagectomy increases postoperative complications and does not improve postoperative malnutrition. Dis Esophagus 2020;33:doz021. [PubMed]
- Fenton JR, Bergeron EJ, Coello M, et al. Feeding jejunostomy tubes placed during esophagectomy: are they necessary? Ann Thorac Surg 2011;92:504-11; discussion 511. [Crossref] [PubMed]
- Shiraishi O, Kato H, Iwama M, et al. A simple, novel laparoscopic feeding jejunostomy technique to prevent bowel obstruction after esophagectomy: the "curtain method". Surg Endosc 2020;34:4967-74. [Crossref] [PubMed]
- Flanagan L. The Needle Catheter Jejunostomy: a useful and cost-effective adjunct in bariatric surgery. Obes Surg 1991;1:299-303. [Crossref] [PubMed]
- Martin ET, Kaye KS, Knott C, et al. Diabetes and Risk of Surgical Site Infection: A Systematic Review and Meta-analysis. Infect Control Hosp Epidemiol 2016;37:88-99. [Crossref] [PubMed]
- Ata A, Lee J, Bestle SL, et al. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg 2010;145:858-64. [Crossref] [PubMed]
- Kao LS, Meeks D, Moyer VA, et al. Peri-operative glycaemic control regimens for preventing surgical site infections in adults. Cochrane Database Syst Rev 2009;CD006806. [Crossref] [PubMed]
- de Groot EM, Möller T, Kingma BF, et al. Technical details of the hand-sewn and circular-stapled anastomosis in robot-assisted minimally invasive esophagectomy. Dis Esophagus 2020;33:doaa055.
- Davis CH, Ikoma N, Mansfield PF, et al. Comparison of laparoscopy versus mini-laparotomy for jejunostomy placement in patients with gastric adenocarcinoma. Surg Endosc 2021;35:6577-82. [Crossref] [PubMed]
- Peng X, Zhu X, Wu Z, et al. Laparoscopic needle catheter jejunostomy by using a double semipurse string suture method in minimally invasive Ivor Lewis esophagectomy. J Thorac Dis 2020;12:240-8. [Crossref] [PubMed]
Cite this article as: Kingma BF, Turchi MM, Lovera R, Ramirez M, Badaloni A, van Hillegersberg R, Ruurda JP, Nieponice A. Technical notes and outcomes of robot-assisted and laparoscopic jejunostomy placement for tube feeding after esophagectomy. Ann Esophagus 2022;5:21.



