
The rheological properties of an alginate satiety formulation in a physiologically relevant human model gut system
Introduction
Satiety is a sensation of fullness which can result in a reduced drive to eat. This is brought about by various mechanisms following consumption of a meal with gastric satiation being volumetric and intestinal satiation being nutritive (1). In the stomach when it is distended the satiating feeling is triggered through afferent vagal signals (2,3) with stimulus from intraganglionic laminar endings (IGLEs), intramuscular arrays (IMAs) and mucosal afferents (4). The food structure and viscosity of the gastric contents also play a role modulating the time a meal is retained in the stomach.
The manipulation of these mechanisms is a potential method to reduce energy intake and aid weight management. The inducement of satiety may be preferable mode of weight management as many other forms of weight management often increase the feelings of hunger such as reduced portion size, reduction in calories diets, or increasing physical activity. Inducing satiety is an effective way to reduce appetite and reduce energy intake with a meal (2,5) however it is of great importance to the effectiveness of the product for a sustained feeling of satiety to ensure that the deficit in food/energy intake is maintained without next meal compensation (2).
Dietary fibres are by definition not digested in the upper digestive tract but the removal of dietary fibres from foods or meals has shown to decrease the time it takes for feelings of hunger to return (6) and increase the rate of gastric emptying (7-9). Many soluble fibres have the ability to increase viscosity (10) and some can gel at low pH inducing feelings of satiety (11), helping control appetite (12). The addition of these components to foods or through supplements has been a targeted mechanism to induce satiety by diet food producers over many years (2,13,14).
The consumption of high viscosity products is often unappetising so the ability to significantly increase viscosity or form gels after consumption is important for consumer acceptance and enjoyment (10,12,15). The speed and strength of the gelation would be important to maximise the mechanism of satiety triggered. Rapid gelation in the stomach could potentially increase the steric hindrance for digestive enzymes to break down chyme in the stomach, maintaining food structure and reducing gastric emptying (11). Also forming a strong and robust gel would distend the stomach triggering gastric stretch receptors (3,11,16-18).
One fibre that has been investigated to this end is alginate, a dietary fibre often included into food products as a thickener, emulsifier or stabiliser (E401-405) (19) but has been shown to have a more functional role in the gastrointestinal (GI) tract (20). Alginates can form both acid and ionic gels but have been shown to also reduce digestive enzyme activity (21-24) and potentially reduce circulating cholesterol, triacylglycerol and glucose (22,25-27).
The increase in viscosity or gel formation after the consumption of a product with alginate increases the likelihood of an appealing and acceptable product for consumers (15).
Alginates have been shown to reduce postprandial energy intake (28-31) but the optimisation of a formulation containing alginate to not only consistently gel rapidly and robustly regardless of buffering capacity of a mixed meal but also entrap and retain gas generated by the formulation to increase the volume of the gel would have potential to aid in appetite suppression, and weight management.
The aim of this study was to quantify the viscosity of a proprietary alginate formulation and its relevant rheological and viscoelastic parameters under physiological conditions as it passes through a model of the human GI tract. Quantifying the gelation and breakdown achieved and whether sufficient to stimulate satiety through gastric distension and be broken down and emptied from the stomach under normal gastric forces so as not to cause issues with retention. These parameters have been collected in the presence and absence of a mixed meal.
We present the following article in accordance with the MDAR reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-89/rc).
Methods
Materials
The proprietary alginate formulation, alginate formulation to induce satiety (AFIS) (composition in Table 1) was a gift from Technostics (Hull, UK) and was stored at room temperature until required.
Table 1
| Component | Amount (g) per 12.81 g dose |
|---|---|
| Sodium alginate Manugel GMB | 1.50 |
| Calcium carbonate | 0.70 |
| Sodium bicarbonate | 0.50 |
| Glucono-delta-lactone | 2.80 |
| Malic acid | 0.05 |
| Isomaltose | 7.00 |
| Sucralose | 0.02 |
| Vanilla flavour | 0.24 |
| Total | 12.81 |
The mixed meal was a ‘Double Sausage and Egg McMuffin’ with a regular (300 mL) black coffee (McDonalds, Newcastle, UK), 34 g fat, 28 g carbohydrate, 2.5 g fibre, 2.7 g salt, and 36 g protein, as previously used as standard meal in alginate studies (32).
All reagents for the model gut systems were purchased from Sigma (Poole, Dorset, UK) with the exception of the enzymes pepsin (Affimetrix, High Wycombe, UK), gastric like lipase (Amano Enzyme Inc., Nishiki, Japan) and bile (of porcine origin) which was collected fresh from 30 animals at a local abattoir, pooled and frozen in aliquots until required. The composition of the model gut solutions are described in detail by Houghton et al. [2014] (33).
As this is an in vitro study, there were no patients or human tissue involved in this study and thus the requirement of ethical approval and informed consent were waived.
Study methods
Model gut system
The methodology of Houghton et al. [2014] (33) was followed with some minor adjustments, described as follows. AFIS was added to 100 mL of vortexing (300 rpm) deionised water and allowed to mix for 1 minute before the synthetic saliva was added and in turn added to resting gastric juices. The gastric phase of the model was performed in a bag mixer (400S, Interscience, Saint-Nom-la-Bretèche, France) generating the same forces as gastric contractions, as observed in the stomach in vivo by Koziolek [2015] et al. and Cassilly et al. [2008] (34,35).
The gastric secretions (5 mL) were added every 10 minutes over the 60 minutes of the gastric phase. At the end of the gastric phase the contents were added to pre incubated bile (37 ℃) and the pancreatic secretions continuously added over the two hours of the small intestinal phase of the digestion model as described by Houghton et al. [2014] (33). The same protocol was followed with and without AFIS present.
Mixed meal model gut system
The same model gut system procedure was followed as for the model gut system however a mixed meal of ‘Double Sausage and Egg McMuffin’ with a regular (300 mL) black coffee was homogenised (Cookworks handheld stick blender, Argos, Newcastle, UK) for 30 seconds before the AFIS was added. The same protocol was followed with and without AFIS present.
Pressure measurements during gastric phase of the model gut system
A digital pressure meter (MH3111 Sika Chipping Norton, UK) was attached to the paddle of the bag mixer and data logged using EBS 20 M software (Greisinger, Regenstauf, Germany). The measurements were made for the midpoint (30 minutes) and full (60 minutes) through the gastric phase of the model, to account for the volume change caused by the gastric secretions.
Viscoelastic properties
Samples of the digesta (3 mL) from the model gut system were taken at 0, 30, 60, 61, 120 and 180 minutes in the model. The linear viscoelastic region (LVER), breakdown point (the transition from gel to viscous liquid, δ>45°) and subsequent breakdown points (transition from gel to viscous liquid after the gel has reformed after the force was removed) were measured at 37 ℃ using a Kinexus Pro Rheometer (Malvern Panalytical, Malvern, UK) using 40 mm serrated parallel plates with a 1 mm gap.
Viscosity
Samples of the digesta (1 mL) from the model gut system were taken at 0, 30, 60, 61, 120 and 180 minutes in the model. Increasing shear rates were applied (table of shear rates) and measured at 37 ℃ using a Kinexus Pro Rheometer (Malvern Panalytical, Malvern, UK) using a 60 mm 1° cone plate. The shear ranged from 0.1 to 100 s−1. The pH of the gastric phase of the model gut system as adjusted to pH 6.5 to assess the effect on the sample viscosity. The viscosity (consistency) constant K was calculated using the power law equation.
Statistical analysis
The comparison of viscosity between the standard pH and the higher pH used in the gastric phase of the model gut system was performed by one-way ANOVA. The comparison between LVER, breakdown shear stress and viscosity of AFIS in the ‘model gut system (MGS)’ with and without food was also performed by a one-way ANOVA. All values are shown as mean ± standard deviation, unless otherwise stated. All experiments were repeated 3 times.
Results
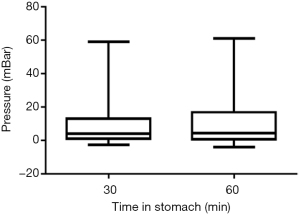
The mean pressure measured in the gastric phase of the model with the appropriate volume at 30 minutes was 10±13 and 11±15 mBar at 60 minutes. The maximum pressures were 59 and 62 mBar and the median were both 4 mBar for 30 and 60 minutes duration in the gastric phase (Figure 1).
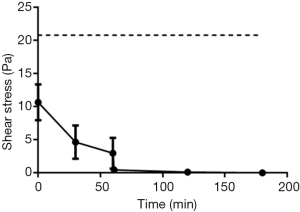
Throughout the gastric phase of the model gut system, AFIS retains a LVER greater than 3±2 Pa, however once the conditions change to small intestinal like environment the LVER is greatly reduced (Figure 2). AFIS that has not been added to the model gut system has a LVER twice that of the initial measurement in the MGS, 10±3 vs. 21±18 Pa, but the model gut system had 50% additional volume.
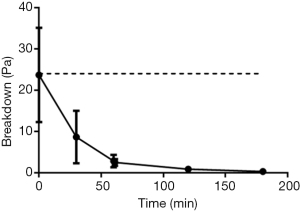
The yield stress required to disrupt the AFIS gel is unaffected by the addition of saliva and resting gastric juice, 24±14 vs. 24±11 Pa without additional 50% volume (Figure 3). It is only with repeat breakdown of the gel structure through application and removal of force as well as the addition of gastric secretions that the breakdown point is lowered. This continues through the gastric and the small intestinal phases of the model. Although the samples remain a gel at all timepoints through the model but only at a shear stress below 3±2 Pa in the gastric phase and 0.4±0.3 Pa in the small intestinal phase.
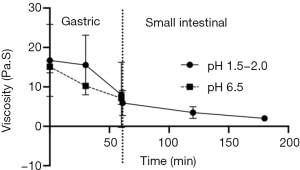
As shown with the rheology of AFIS as is passes through the MSG, the viscosity is also reduced as it progresses (Figure 4). From the initial measurement in the gastric phase, a reduction of 9±7 (SEM) Pa·S over the hour of the gastric phase. Increasing the pH of the gastric phase did not significantly alter the initial or final viscosity of AFIS in the gastric phase and there was no statistical difference measured halfway through the gastric phase at 30 minutes.
When AFIS was tested with a mixed meal there was a larger initial LVER (accounting for the effect of food) at the start of the gastric phase than when the AFIS alone was digested through the MGS 34±31 (SEM) Pa vs. 116±1 (SEM) Pa (Figure 5). In comparison with the food alone through the MGS the AFIS and food through the gastric phase has a greater LVER, although once into the small intestinal phase of the model there is little difference between presence and absence of food.
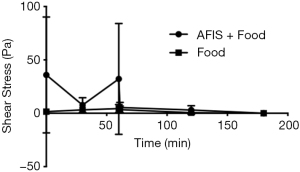
The shear stress required to disrupt the gel and make it flow is much greater in the gastric phase for AFIS with food than it is with the food alone (Figure 6). Although during the small intestinal phase of the model there is no statistical difference seen between AFIS and food combined (146±124 Pa) compared to the food alone (37±28 Pa). It would be expected that due to the similarity between the food with and without AFIS in required breakdown force to make the gastric contents flow, the AFIS would pass through the small intestine as the meal would.
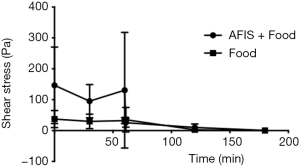
The viscosity of AFIS taken in combination with food also decreases through the MGS (Figure 7). The initial viscosity of AFIS with food and the food alone is very similar, however, the viscosity of the AFIS and food together remain higher than the food alone at 30, 60, 120 and 180 minutes.
Discussion
The forces generated by the stomacher mimic the forces observed in the stomach in vivo by Koziolek [2015] et al. and Cassilly et al. [2008] (34,35). The forces, as described by Koziolek, are highly variable between volunteers but with very few measurements above 100 mbar. The maximum measured pressure in the 20 volunteers tested was 496 mbar but mean maximum of 293±109 mBar. Cassilly et al. (35) similarly describe the pressures generated within the stomach with both smart pill and fixed pressure catheter showing the majority of the first two hours in the stomach being greater than 10 mmHg (over 1,300 Pa, 13 mBar).
As the gastric phase of digestion is where volumetric satiety would be induced (1), the force required to breakdown the gel and cause it to flow would be a major factor in determining the satiety effect (3). The same shear strain was required to breakdown the AFIS gel alone as was required for the AFIS gel at the initial stages of the gastric phase which would have had oral and gastric secretions equivalent to an additional 50%. This highlights the ability of the AFIS to retain its gelled strength in the stomach and the potential satiating affect.
The additional volume of oral and gastric secretions does lower the LVER, the range of shear strain that does not affect gel properties, but more importantly the force required to break down the gel remained unchanged.
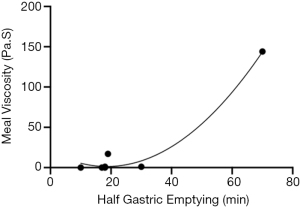
Repeated breakdown of the gel in the gastric phase does weaken the gel by reducing the force required to disrupt the gel but the gel does reform each time. Gastric emptying rates could be inferred from data taken for humans and dogs by Marciani et al. [2000] and Ehrlein et al. [1987] (36,37). Figure 8 demonstrates the relationship between viscosity and time it takes to empty half the gastric contents. Both Marciani et al. [2000] and Ehrlein et al. [1987] measured the viscosity of a meal before it was consumed, and the half gastric emptying times in the stomach. Using the viscosity of AFIS at the initial phase of the model gut system a time to half gastric emptying can be estimated at 32 minutes. The addition of a mixed meal increased the viscosity 30-fold but is unlikely to increase the half gastric emptying time by a similar amount, as it is hypothesised that an increased meal viscosity would be partially compensated for with increased gastric secretions, however the retention time would still be greatly increased (38).
The repeated disruption of the gel and the addition of gastric secretions reduce the gel breakdown point as it moves through the model gut system. At the end of the gastric phase (60 min) the breakdown point is only 28% of what it was initially and when at the end of the small intestinal phase (effectively the ileum) the viscosity matches that measured by Ehrlein et al. in dog of 1.3–46 Pa·S (36).
Increasing pH of the gastric phase did reduce the viscosity of AFIS in the midpoint of the gastric phase but at the end of the model the viscosity was the same for both the higher pH gastric phase as well as the standard pH for the gastric phase (pH1.5). This indicates that although the acidity of the stomach may be beneficial for a high viscosity of the formulation it is the formulation itself that creates the optimum gelling condition for satiety, independent of physiologically relevant pH.
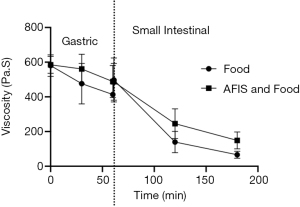
The inclusion of a mixed meal into the model gut system greatly increases the viscosity throughout the whole digestive tract model. At the mid-point and end of the gastric phase the AFIS increases the viscosity by more than just the viscosity of the AFIS alone. The increase in viscosity indicates an interaction between the AFIS and the food to generate the increased viscosity above that of the AFIS alone. Potentially the alginate in the formulation can interact with protein (39) but also the gelation of the formulation in the gastric phase of the model could prevent the breakdown of the food structure (40). Similarly, the LVER and the breakdown shear stress were also increased when combined with the mixed meal and were greater again with AFIS. Highlighting again, that AFIS can interact with components from the meal, increasing the force required to disrupt the gel.
The increase in rheological properties and viscosity with AFIS are more pronounced in the gastric phase of the model. The half retention time of the mixed meal with AFIS would be difficult to predict but would be significantly greater than that of mixed meal alone. However, the rheological properties and viscosity are comparable to that of the mixed meal alone during the small intestinal phase of the model.
These data suggest that the AFIS causes a strong gel in the gastric phase which would be retained in the stomach for longer than food alone and potentially when included with a mixed meal this may increase further. The forces of the stomach and the secretion of further gastric juice reduce the gel strength over time, which would allow the stomach to eventually empty the contents into the small intestines. This was estimated, and without a mixed meal the AFIS would take 32 minutes to be half emptied and the time greatly increased with a mixed meal.
Acknowledgments
Funding: This work was supported by Technostics Ltd.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Epidemiology, Biomarkers and Modelling of Gastroesophageal Reflux Disease”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-89/rc
Data Sharing Statement: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-89/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-89/coif). The series “Epidemiology, Biomarkers and Modelling of Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. PWD served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from March 2020 to February 2022. JPP served as the unpaid Guest Editor of the series. MDW reports grants from Technostics Ltd., during the conduct of the study; other from Aelius Biotech, outside the submitted work; in addition, MDW has a patent WO2015075467A3 issued. PIC reports other from Aelius Biotech, outside the submitted work; in addition, PIC has a patent EP3074769A2 issued. KJS reports grants from Technostics, during the conduct of the study. ADW reports other from Technostics, outside the submitted work. PWD reports other from Technostics, outside the submitted work; in addition, PWD has a patent EP1931222B1 issued. JPP reports grants from Technostics Ltd., during the conduct of the study; other from Aelius Biotech, outside the submitted work; in addition, JPP has a patent WO2015075467A3 issued. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. As this is an
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Powley TL, Phillips RJ. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol Behav 2004;82:69-74. [Crossref] [PubMed]
- Halford JC, Harrold JA. Satiety-enhancing products for appetite control: science and regulation of functional foods for weight management. Proc Nutr Soc 2012;71:350-62. [Crossref] [PubMed]
- Paintal AS. A study of gastric stretch receptors; their role in the peripheral mechanism of satiation of hunger and thirst. J Physiol 1954;126:255-70. [Crossref] [PubMed]
- Kentish SJ, Page AJ. Plasticity of gastro-intestinal vagal afferent endings. Physiol Behav 2014;136:170-8. [Crossref] [PubMed]
- López-Nicolás R, Marzorati M, Scarabottolo L, et al. Satiety Innovations: Food Products to Assist Consumers with Weight Loss, Evidence on the Role of Satiety in Healthy Eating: Overview and In Vitro Approximation. Curr Obes Rep 2016;5:97-105. [Crossref] [PubMed]
- Benini L, Castellani G, Brighenti F, et al. Gastric emptying of a solid meal is accelerated by the removal of dietary fibre naturally present in food. Gut 1995;36:825-30. [Crossref] [PubMed]
- Wolever TMS, Tosh SM, Spruill SE, et al. Increasing oat beta-glucan viscosity in a breakfast meal slows gastric emptying and reduces glycemic and insulinemic responses but has no effect on appetite, food intake, or plasma ghrelin and PYY responses in healthy humans: a randomized, placebo-controlled, crossover trial. Am J Clin Nutr 2020;111:319-28. [Crossref] [PubMed]
- Wanders AJ, Feskens EJM, Jonathan MC, et al. Pectin is not pectin: A randomized trial on the effect of different physicochemical properties of dietary fiber on appetite and energy intake. Physiol Behav 2014;128:212-9. [Crossref] [PubMed]
- McIntyre A, Vincent RM, Perkins AC, et al. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: The role of physical factors. Gut 1997;40:223-7. [Crossref] [PubMed]
- Ho IH, Matia-Merino L, Huffman LM. Use of viscous fibres in beverages for appetite control: a review of studies. Int J Food Sci Nutr 2015;66:479-90. [Crossref] [PubMed]
- Hoad CL, Rayment P, Spiller RC, et al. In vivo imaging of intragastric gelation and its effect on satiety in humans. J Nutr 2004;134:2293-300. [Crossref] [PubMed]
- Wanders AJ, Jonathan MC, van den Borne JJ, et al. The effects of bulking, viscous and gel-forming dietary fibres on satiation. Br J Nutr 2013;109:1330-7. [Crossref] [PubMed]
- Salleh SN, Fairus AAH, Zahary MN, et al. Unravelling the Effects of Soluble Dietary Fibre Supplementation on Energy Intake and Perceived Satiety in Healthy Adults: Evidence from Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Foods 2019;8:22. [Crossref] [PubMed]
- Fiszman S, Varela P. The role of gums in satiety/satiation. A review. Food Hydrocoll 2013;32:147-54. [Crossref]
- Wilde PJ. Eating for life: designing foods for appetite control. J Diabetes Sci Technol 2009;3:366-70. [Crossref] [PubMed]
- Maljaars PW, Peters HP, Mela DJ, et al. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav 2008;95:271-81. [Crossref] [PubMed]
- Osterholt KM, Roe LS, Rolls BJ. Incorporation of air into a snack food reduces energy intake. Appetite 2007;48:351-8. [Crossref] [PubMed]
- Oesch S, Ruegg C, Fischer B, et al. Effect of gastric distension prior to eating on food intake and feelings of satiety in humans. Physiol Behav 2006;87:903-10. [Crossref] [PubMed]
- Brownlee IA, Allen A, Pearson JP, et al. Alginate as a source of dietary fiber. Crit Rev Food Sci Nutr 2005;45:497-510. [Crossref] [PubMed]
- Chater PI, Wilcox MD, Houghton D, et al. The role of seaweed bioactives in the control of digestion: implications for obesity treatments. Food Funct 2015;6:3420-7. [Crossref] [PubMed]
- Chater PI, Wilcox MD, Brownlee LA, et al. Alginate as a protease inhibitor in vitro and in a model gut system; selective inhibition of pepsin but not trypsin. Carbohydr Polym 2015;131:142-51. [Crossref] [PubMed]
- Houghton D, Wilcox MD, Brownlee IA, et al. Acceptability of alginate enriched bread and its effect on fat digestion in humans. Food Hydrocoll 2019;93:395-401. [Crossref] [PubMed]
- Houghton D, Wilcox MD, Chater PI, et al. Biological activity of alginate and its effect on pancreatic lipase inhibition as a potential treatment for obesity. Food Hydrocoll 2015;49:18-24. [Crossref] [PubMed]
- Wilcox MD, Brownlee IA, Richardson JC, et al. The modulation of pancreatic lipase activity by alginates. Food Chem 2014;146:479-84. [Crossref] [PubMed]
- Seal CJ, Mathers JC. Comparative gastrointestinal and plasma cholesterol responses of rats fed on cholesterol-free diets supplemented with guar gum and sodium alginate. Br J Nutr 2001;85:317-24. [Crossref] [PubMed]
- Harden CJ, Richardson JC, Dettmar PW, et al. An ionic-gelling alginate drink attenuates postprandial glycaemia in males. J Funct Foods 2012;4:122-8. [Crossref]
- Torsdottir I, Alpsten M, Holm G, et al. A small dose of soluble alginate-fiber affects postprandial glycemia and gastric-emptying in humans with diabetes. J Nutr 1991;121:795-9. [Crossref] [PubMed]
- Georg Jensen M, Kristensen M, Belza A, et al. Acute effect of alginate-based preload on satiety feelings, energy intake, and gastric emptying rate in healthy subjects. Obesity (Silver Spring) 2012;20:1851-8. [Crossref] [PubMed]
- Georg Jensen M, Pedersen C, Kristensen M, et al. Review: efficacy of alginate supplementation in relation to appetite regulation and metabolic risk factors: evidence from animal and human studies. Obes Rev 2013;14:129-44. [Crossref] [PubMed]
- Odunsi ST, Vazquez-Roque MI, Camilleri M, et al. Effect of alginate on satiation, appetite, gastric function, and selected gut satiety hormones in overweight and obesity. Obesity (Silver Spring) 2010;18:1579-84. [Crossref] [PubMed]
- El Khoury D, Goff HD, Anderson GH. The role of alginates in regulation of food intake and glycemia: a gastroenterological perspective. Crit Rev Food Sci Nutr 2015;55:1406-24. [Crossref] [PubMed]
- Sweis R, Kaufman E, Anggiansah A, et al. Post-prandial reflux suppression by a raft-forming alginate (Gaviscon Advance) compared to a simple antacid documented by magnetic resonance imaging and pH-impedance monitoring: mechanistic assessment in healthy volunteers and randomised, controlled, double-blind study in reflux patients. Aliment Pharmacol Ther 2013;37:1093-102. [Crossref] [PubMed]
- Houghton D, Wilcox MD, Brownlee LA, et al. Method for quantifying alginate and determining release from a food vehicle in gastrointestinal digesta. Food Chem 2014;151:352-7. [Crossref] [PubMed]
- Koziolek M, Schneider F, Grimm M, et al. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J Control Release 2015;220:71-8. [Crossref] [PubMed]
- Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 2008;20:311-9. [Crossref] [PubMed]
- Ehrlein HJ, Buhner S, Thoma G, et al. Gastric emptying after Roux-Y and Billroth-I gastrectomy depends on viscosity of meal and contractile patterns of small intestine in dogs. Dig Dis Sci 1987;32:529-37. [Crossref] [PubMed]
- Marciani L, Gowland PA, Spiller RC, et al. Gastric response to increased meal viscosity assessed by echo-planar magnetic resonance imaging in humans. J Nutr 2000;130:122-7. [Crossref] [PubMed]
- Dikeman CL, Murphy MR, Fahey GC Jr. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J Nutr 2006;136:913-9. [Crossref] [PubMed]
- Taylor C, Draget KI, Pearson JP, et al. Mucous Systems Show a Novel Mechanical Response to Applied Deformation. Biomacromolecules 2005;6:1524-30. [Crossref] [PubMed]
- Spyropoulos F, Norton IT. Self-structuring foods based on acid-sensitive mixed biopolymer to impact on satiety. Procedia Food Sci 2011;1:1487-93. [Crossref]
Cite this article as: Wilcox MD, Chater PI, Stanforth KJ, Woodcock AD, Dettmar PW, Pearson JP. The rheological properties of an alginate satiety formulation in a physiologically relevant human model gut system. Ann Esophagus 2022;5:3.


