Minimally invasive fundoplication for gastroesophageal reflux disease
Introduction
Gastroesophageal reflux disease (GERD), defined by the Montreal consensus as “a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications”, affects roughly 20% of Americans weekly (1). The incidence of GERD is increasing in developed countries, likely driven by an increase in obesity since treatment of obesity results in improvement in GERD (2). There is a large spectrum of reported symptoms including gastrointestinal symptoms such as heartburn, regurgitation, and dysphagia, as well as non-gastrointestinal symptoms such as chronic cough, recurrent pneumonia, and hoarseness. Careful workup can elucidate whether these symptoms are likely due to GERD, as alternative etiologies producing similar symptoms will have different treatment algorithms. The Lyon consensus has attempted to more objectively define pathologic GERD as either the presence of LA grade C or D esophagitis, or a distal acid exposure time of greater than 6% on ambulatory pH monitoring (3).
While lifestyle modifications and oral medications, typically proton pump inhibitors (PPIs), will control symptoms in the overwhelming majority of patients with GERD (4), there remains a group of patients who either fail to have adequate symptom relief, or who develop complications of GERD such as persistent esophagitis, peptic stricture, or Barrett’s esophagus. Furthermore, while PPIs are normally well tolerated, there are growing concerns regarding the long-term effects of PPIs. These potential effects include, but are not limited to, decreased bone mineral density (5), increased infections such as pneumonia (6) and Clostridium difficile diarrhea (7), and an association with dementia in elderly patients (8), although the latter finding is quite controversial (9). These findings, while limited by study heterogeneity, have led to practitioners questioning the complete benignity of long-term PPI. Patients themselves may opt for surgical treatment of GERD to potentially obviate the need for long-term PPI use. Furthermore, certain non-acid reflux conditions, such as high-volume regurgitation, respond poorly to PPIs, and may be better managed with surgical treatment (10).
While the detailed preoperative workup is beyond the scope of this review paper, the following studies are recommended in a 2013 consensus panel: All patients require endoscopic evaluation of the esophagus and stomach to evaluate for complications of GERD, including dysplastic Barrett’s esophagus and adenocarcinoma, which require treatment before fundoplication. Furthermore, concomitant pathology such as eosinophilic esophagitis, can be evaluated by biopsy if suspected. Ambulatory pH monitoring is required in all patients without either endoscopically documented erosive esophagitis or a large hiatal hernia. If there is suspicion for non-acid or bile reflux, impedance testing can be added to increase the sensitivity for pH testing. High resolution esophageal manometry is able to confirm the presence of a hypotensive lower esophageal sphincter, as well as exclude diagnoses that can mimic GERD such as achalasia and can help tailor the choice of fundoplication. Barium esophagram can be helpful in the evaluation of a concomitant hiatal hernia, as well as in identifying strictures, diverticula, and the extent of reflux with provocation (11). The following manuscript describes our method for performance of laparoscopic complete and partial fundoplication in the treatment of GERD.
Technical aspects
Patient preparation and port placement
The patient is placed in the supine position with the legs abducted. The surgeon stands between the patient’s legs to facilitate triangulation at the hiatus. The first assistant stands to the patient’s left. Pneumoperitoneum is established with a Veress needle inserted in the left upper quadrant at Palmer’s point. We place a 12 mm camera port 15 cm inferior to the xyphoid process to the left of midline. A 12 mm port is placed in the left upper quadrant, typically at the Veress insertion site, for the surgeon’s right hand. A 5 mm port is placed laterally beneath the left costal margin for the first assistant. A flexible liver retractor is placed through a 5 mm port in the right mid anterior axillary line and secured to a table-mounted post to retract the left lobe of the liver. Finally, a 5 mm port is placed roughly 6 cm inferior to the right mid costal margin for the surgeons left hand, ideally directed from right-to-left through the falciform ligament. The patient is placed in the reverse Trendelenburg position to assist with caudad retraction of the stomach and omentum.
Gastroesophageal junction mobilization
Dissection is performed primarily with a laparoscopic bipolar vessel sealing device. The pars flaccida is divided. If present, small accessory left hepatic arteries can typically be divided, but if a large replaced left hepatic artery is present, preservation should be attempted. The gastrohepatic ligament is further divided to the anterior aspect of the right crus. The GE junction is retracted laterally and inferiorly by the first assistant, and a plane between the esophagus and right crus is bluntly developed to access the mediastinum. Blunt dissection is continued anteriorly, protecting the esophagus and anterior vagus nerve, and the phrenoesophageal membrane is divided from right to left. Peritoneal attachments to the Angle of His and the left crus are divided.
Fundic mobilization
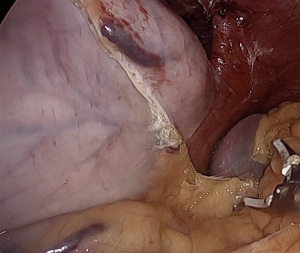
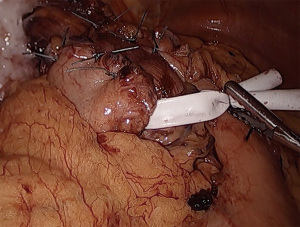
To mobilize the fundus, we divide the omentum off of the greater curvature beginning at the proximal one-third of the stomach. Short gastric vessels are coagulated and divided. As the fundus is freed from the gastrosplenic ligament, the assistant’s retraction switches from inferolateral traction on the omentum to the posterior wall of the stomach. The surgeon elevates the fundus anteriorly, the assistant pushes the posterior wall of the stomach towards the right lower quadrant. This provides a fan-like exposure of the proximal posterior fundus and allows more medial posterior short gastric vessels to be visualized and divided (Figure 1). Dissection is carried to the base of the left crus, and the prior plane from the angle of His dissection is met. It is critical to divide all fundic attachments both laterally and posteriorly, or else there will be tension when attempting to create the fundoplication.
Mediastinal dissection
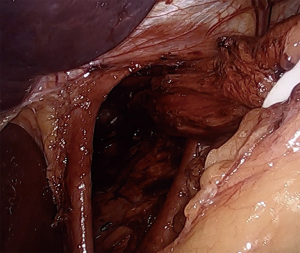
Returning to the right crus, the medial border is fully dissected to the confluence of the crura. The left crus is identified from the right side, staying posterior enough to ensure the esophagus and posterior vagus nerve are swept anteriorly away from the crural junction. At this point a retroesophageal window is bluntly created lateral to the left crus. A quarter-inch Penrose drain is passed to encompass the esophagus and both vagus nerves. The legs of the Penrose are secured together with either endoscopic clips or a ligating loop. Using a locking grasper the assistant then provides inferior retraction of the esophagus. It is very useful to ensure that all GE junction fat and any associated hernia sac are maintained distal to the Penrose, or else tissue will interfere with esophageal retraction. The esophagus is then dissected circumferentially proximally into the mediastinum, dissecting the plane between the esophageal wall and the lymphoadipose tissue of the mediastinum. By dissecting along the esophagus, inadvertent injury to the parietal pleura can be avoided. If the pleura is entered, anesthesia should be notified, and larger tidal volumes should be provided to overcome the capnothorax. There are typically no postoperative issues with pleural entry as the capnothorax dissipates quickly. Dissection is continued until at least 3cm of intraabdominal esophagus is established without tension placed on the Penrose drain (Figure 2). Downward traction on the esophagus, plus elevation of the diaphragm due to pneumoperitoneum, can lead to the surgeon overestimating the amount of intraabdominal esophageal length, and must be considered intraoperatively. Dissection can be carried cephalad to the level of the inferior pulmonary veins, if necessary.
Hiatal closure
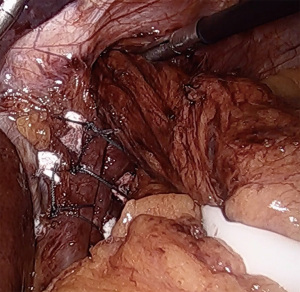
A posterior cruroplasty is then performed. While techniques vary, our practice is to use non-absorbable, multifilament, interrupted sutures with polytetrafluoroethylene (PTFE) pledget reinforcement (Figure 3). We find intracorporeal sliding knots very useful to maintain apposition and minimize tearing of frail crura. The completed cruroplasty should permit the tip of a 5 mm instrument to pass without resistance. It is critical to examine the hiatus with the esophagus in a neutral position, that is, without downward retraction on the Penrose in order to calibrate a proper closure. Rarely, anterior cruroplasty sutures are placed if there is excessive laxity due to the divided phrenoesophageal membrane. In the setting of a large paraesophageal hernia, consideration may be given to mesh reinforcement of the cruroplasty, although recent data do not support a decrease in long term hernia recurrence rates (12).
Fundoplication
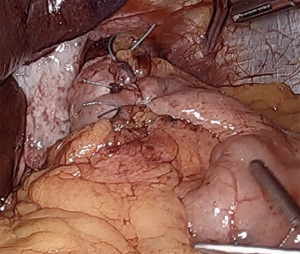
If esophageal motility is preserved, especially in the setting of GERD complications such as high-grade erosive esophagitis or Barrett’s esophagus, we typically perform a complete, floppy, fundoplication. While the use of a large (56 to 60 French) bougie has been shown in some studies to reduce in the incidence of postoperative dysphagia (13), we tend to forego its use, due to the inherent risk of esophageal perforation. This practice has been proven acceptable by experienced surgeons (14). The medial aspect of the fundus is tucked from left to right into the previously created retroesophageal window. The fundus is then grasped from the right, pulling from the divided short gastric vessels in order to maintain proper orientation without folding. The fundus should sit comfortably on the right side of the esophagus and should not spontaneously retract to the left when released. A “shoe-shine” maneuver is performed by grasping both sides of the fundus and rocking back and forth. If the fundus does not sit comfortably on release, or the esophagus twists with movement, there are likely additional posterior or lateral fundic attachments that must be divided. A 360-degree fundoplication is fashioned by approximating the greater curvature of the right sided fundus to a similarly distanced point on the left anterior fundus. The wrap should be seated above the GE junction, and not around the gastric cardia. Using 3 interrupted non-absorbable sutures, the sides of the fundoplication are approximated, ensuring that no significant fundus is left proximal to the wrap, which will reduce wrap slippage. The proximal 2 sutures incorporate the anterior esophageal wall, again to reduce slippage, while protecting the anterior vagus nerve. The completed fundoplication should be short (less than 2 cm), floppy, symmetrical, and without twist (Figure 4). Completion endoscopy can be performed to both confirm easy passage of an endoscope through the hiatal closure and wrap, as well as to confirm wrap symmetry and proper valve creation.
In patients without preoperative manometry, or patients with underlying esophageal motility disorders, elderly patients, and those with prominent preoperative dysphagia, we typically perform a posterior partial 270-degree (Toupet) fundoplication. On manometry, we typically require at least 70% peristaltic swallows, with a distal esophageal amplitude greater than 30 mmHg. To fashion a partial fundoplication, the fundic limbs are sutured directly to the ipsilateral anterolateral esophageal wall, again, ensuring that the proximal fundoplication includes the most cephalad aspect of the fundic limbs. Three interrupted sutures are placed bilaterally (Figure 5).
Outcomes
Surgical treatment, typically in the form of laparoscopic fundoplication and repair of associated hiatal hernia, is safe and effective in well selected patients. Mortality and need for early reoperation (<90 days) are low, less than 0.1%, and less than 1% respectively (15). A 2015 Cochrane review showed improved short-term and medium-term heartburn improvement with surgical management compared to medical management, although surgical patients had higher rates of dysphagia (16). The presence of typical GERD symptoms, objective documentation of GERD by endoscopy or pH testing, and a positive response to PPIs are associated with improved outcomes with fundoplication (17). Dassinger and colleagues’ 5-year follow up of 52 patients having undergone laparoscopic fundoplication showed a 92% satisfaction with the procedure, with over 80% of patients maintained off antisecretory medications (18). Compared to open anti-reflux surgery, minimally invasive techniques are associated with shorter hospital stays, less time off from work, and decreased wound complications and incisional hernia rates, with comparable efficacy in GERD control (19). The use of robotic-assisted laparoscopic procedures are increasing, with similar efficacy to standard laparoscopy, albeit with increased cost (20).
Randomized clinical trials have compared the efficacy and adverse effect profile of complete (Nissen) versus partial (Toupet) fundoplication. Meta-analyses have shown similar efficacy, although the Toupet fundoplication has been associated with less gas-bloat, dysphagia, need for dilation, and need for reoperation (21). We reserve complete fundoplication for younger patients with preserved esophageal function on manometry, especially in the presence of grade C or D esophagitis or Barrett’s esophagus.
Conclusions
Laparoscopic antireflux surgery is a safe and effective treatment of GERD. With proper patient selection, a comprehensive preoperative workup, and attention to technical detail, patients can receive a durable result.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-100/coif). The series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. TMF served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from April 2020 to March 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871-80. [Crossref] [PubMed]
- Ness-Jensen E, Hveem K, El-Serag H, et al. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2016;14:175-82.e1-3.
- Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: The Lyon consensus. Gut 2018;67:1351-62. [Crossref] [PubMed]
- Khan M, Santana J, Donnellan C, et al. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev 2007;CD003244. [PubMed]
- Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006;296:2947-53. [Crossref] [PubMed]
- Eom CS, Jeon CY, Lim JW, et al. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ 2011;183:310-9. [Crossref] [PubMed]
- Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol 2017;23:6500-15. [Crossref] [PubMed]
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: A pharmacoepidemiological claims data analysis. JAMA Neurol 2016;73:410-6. [Crossref] [PubMed]
- Goldstein FC, Steenland K, Zhao L, et al. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc 2017;65:1969-74. [Crossref] [PubMed]
- Patti MG. An evidence-based approach to the treatment of gastroesophageal reflux disease. JAMA Surg 2016;151:73-8. [Crossref] [PubMed]
- Jobe BA, Richter JE, Hoppo T, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg 2013;217:586-97. [Crossref] [PubMed]
- Watson DI, Thompson SK, Devitt PG, et al. Five year follow-up of a randomized controlled trial of laparoscopic repair of very large hiatus hernia with sutures versus absorbable versus nonabsorbable mesh. Ann Surg 2020;272:241-7. [Crossref] [PubMed]
- Patterson EJ. Effect of an esophageal bougie on the incidence of dysphagia following Nissen fundoplication: A prospective, blinded, randomized clinical trial. Arch Surg 2000;135:1055. [Crossref] [PubMed]
- Bochkarev V, Iqbal A, Lee YK, et al. One hundred consecutive laparoscopic Nissen’s without the use of a bougie. Am J Surg 2007;194:866-70; discussion 870-1. [Crossref] [PubMed]
- Maret-Ouda J, Yanes M, Konings P, et al. Mortality from laparoscopic antireflux surgery in a nationwide cohort of the working-age population: Mortality from primary laparoscopic antireflux surgery in the working-age population. Br J Surg 2016;103:863-70. [Crossref] [PubMed]
- Garg SK, Gurusamy KS. Laparoscopic fundoplication surgery versus medical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Libr [Internet] 2015; Available online:
10.1002/14651858.CD003243.pub3 10.1002/14651858.CD003243.pub3 - Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg. 1999;3:292-300. [Crossref] [PubMed]
- Dassinger MS, Torquati A, Houston HL, et al. Laparoscopic fundoplication: 5-year follow-up. Am Surg 2004;70:691-4; discussion 694-5. [PubMed]
- Chrysos E, Tsiaoussis J, Athanasakis E, et al. Laparoscopic vs open approach for Nissen fundoplication. Surg Endosc 2002;16:1679-84. [Crossref] [PubMed]
- Morino M, Pellegrino L, Giaccone C, et al. Randomized clinical trial of robot-assisted versus laparoscopic Nissen fundoplication. Br J Surg 2006;93:553-8. [Crossref] [PubMed]
- Broeders JA, Mauritz FA, Ahmed Ali U, et al. Systematic review and meta-analysis of laparoscopic Nissen (posterior total) versus Toupet (posterior partial) fundoplication for gastro-oesophageal reflux disease. Br J Surg 2010;97:1318-30. [Crossref] [PubMed]
Cite this article as: Lipman JN, Farrell TM. Minimally invasive fundoplication for gastroesophageal reflux disease. Ann Esophagus 2022;5:37.