Minimally invasive surgery: hiatal hernia repair—a narrative review
Introduction
Hiatus hernias occur in more than 16% of the population (1). The majority of these are sliding hiatus hernias and being associated with gastroesophageal reflux and having a low rate of other complications, are often managed according to the severity of the symptoms of gastroesophageal reflux. These are addressed in a previous manuscript of this series in this volume of the Journal. The less common sub-type of hiatal hernias, that of paraesophageal hernia is a more common cause of obstructive upper gastrointestinal symptoms, is more likely to cause complications and necessitate emergency hospital admission and forms the focus of this manuscript. The etiology, pathophysiology, and indications for repair for paraesophageal hernias, are reviewed and the required preoperative investigations and surgical technique of paraesophageal hernia repair are discussed. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-12/rc).
Methods
An extensive literature review up to 2011 has previously been performed by the senior author and others to inform a previously published systematic review (2). More recent literature was identified by repeat search of the PubMed database from January 2011 to January 2021, targeting any areas of controversy identified by the authors, namely the utility of reinforced crural repair, the role of fundoplication and the management of the short esophagus.
Discussion
Anatomy
Esophagus and esophageal hiatus
The esophagus is a muscular tube of 25 centimeters commencing from the cricoid cartilage and extending to the cardia of the stomach. The intra-abdominal portion is approximately 2 cm in length, though this varies depending on the tone of the esophageal muscle and degree of stomach distension (3). The esophagus enters into the abdominal cavity via the esophageal hiatus of the diaphragm, located one centimeter to the left of the midline at the level of the tenth thoracic vertebra (4). The anterior and lateral borders of the hiatus are formed by the diaphragmatic crura, and its posterior angle by the medial arcuate ligament (4). The posterior hiatus, formed by the right crus is invariably muscular, and the anterior hiatus more tendinous (5). The hiatus border is also stronger anteriorly due to the presence of endothoracic fascia, endoabdominal fascia, and the central tendon of the diaphragm (4).
Phrenoesophageal membrane and ligament
The phreno-esophageal membrane fills the potential space between the esophagus and the hiatal musculature and maintains the lower esophageal sphincter (LES) to be below the hiatus (6). The phrenoesophageal ligament is formed by an extension of endoabdominal fascia and mediastinal fascia at the hiatus to envelope the esophagus. The abdominal fibers pass through the hiatus, insert into the adventitia of the esophagus muscle fibers 1–2 cm above hiatus. The thoracic fibers descend from above the hiatus to insert into the adventitia of the anterior abdominal esophagus 1–2 cm below the hiatus (4). The phrenoesophageal ligament allows for movement of the esophagus to be independent from the diaphragm during respiration and swallow (6). The hiatal aperture is not fixed and narrows with increased intra-abdominal pressure. The abdominal portion of the esophagus is anchored at the level of the gastroesophageal junction (GEJ) by the phrenoesophageal ligament, usually preventing the stomach to be displaced through the hiatus (4).
Anatomic antireflux mechanisms
Hiatal hernias in which the GEJ has migrated into the chest may experience gastroesophageal reflux as a result of disruption of the usual antireflux barrier. This barrier is formed by multiple components (7,8):
- The intrinsic LES is formed by the muscles of the distal esophagus and proximal stomach, and sling fibers of the cardia. This results in a pressure gradient between the esophagus and stomach. The LES is a high-pressure zone on manometry.
- A “pinchcock” effect is created from the diaphragmatic crura contraction to squeeze the distal esophagus during deep inspiration or when abrupt rises in intra-abdominal pressure occur.
- The phrenoesophageal ligament holds the left side of the esophagus at an acute angle as it passes through the hiatus. This angle is maintained between the esophagus and stomach to prevent reflux of gastric contents back to the esophagus.
- The angle of His is the acute angle formed by the esophagus and cardia of the stomach, which exhibits a valve-like function to prevent reflux.
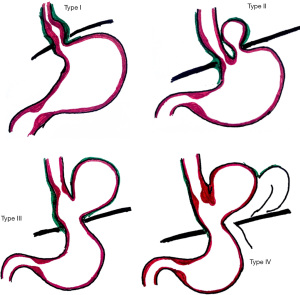
Classification of hiatus hernia
Most hiatal hernias are acquired, very few are congenital, and there are reports of familial cases (2,8-11). Hiatus hernias can be anatomically classified into four types (2). Type I hernias are the most common (85–95%) (2,12), and are also known as sliding hernias. Types II, III and IV are paraesophageal hernias, and are true hernias with a hernia sac. The gastric fundus is displaced through the phrenoesophageal membrane defect and lies adjacent to the esophagus.
Classification (Figure 1)
Type I “sliding” hiatus hernia
Sliding hernias result from the widening of the hiatal aperture (8). The GEJ is displaced above the diaphragm, with herniation of the stomach cardia. The stomach fundus remains below the diaphragm and is in its usual longitudinal alignment.
As complication directly related to Type I hernias a quite rare, management strategies for these hernias simply aim to address any symptoms of gastro-esophageal reflux disease (GERD). These are no discussed further below and this review will address the management of paraesophageal hernias only, that is hiatal hernias of types II–IV.
Paraesophageal hernias
Type II hiatus hernia
Type II hernias result from a defect in the phreno-esophageal membrane. The GEJ remains in its native position, being fixed to the posterior fascia and arcuate ligament (2,8). The gastric fundus is the lead herniation point through the hiatus and lies next to the esophagus. Thus the gastric fundus is located above the hiatus, and the GEJ remains at or below the diaphragm (13).
Type III hiatus hernia
Type III hernias are a combination of types I & II hiatus hernias. Both the GEJ and gastric fundus herniate through the hiatus.
Type IV hiatus hernia
Type IV hernias are usually a large defect in the phrenoesophageal membrane, characterized by the involvement of organs other than the stomach herniating through, such as the pancreas or transverse colon.
Etiology & pathophysiology of a hiatal hernia
The initiating factor for hiatal hernia development is uncertain. It has been proposed that chronic esophagitis from acid reflux predisposes to hiatus hernias (14). Acid induced contraction of the esophageal muscles may cause shortening and proximal migration of the esophagus. The resultant fibrosis of the longitudinal muscles, along with loss of tissue elasticity with age, may predispose to a hiatus hernia. Furthermore, the hiatus is enlarged overtime by the hernia itself, and impairs the effectiveness of the diaphragmatic sphincter. The combination of a hiatus hernia, incompetent diaphragmatic sphincter and acid induced esophagitis may establish a feedback loop to further increase the hernia size. Hiatal hernia development from other causes may be the initiating factor for reflux (15).
Advanced age is known to be risk factors for hiatus hernias (16). The degeneration of the phrenoesophageal membrane over time loosens the attachment of the GEJ to the diaphragmatic crus. The laxity predisposes to the formation of a hiatal hernia.
The increase in intraabdominal pressure seen in obesity and pregnancy is a risk factor for hiatal hernia formation (13), though whether this is a result of increased pressure per se, or increased intragastric pressure, or simply increased abdominal girth is unclear (17). The relative risk increases in parallel with increased body mass index (12,15) and it is certainly plausible that the intra-abdominal pressure rise is the culprit.
Other risk factors include previous GEJ surgery or trauma, anatomical distortion of the diaphragm, such as from scoliosis, kyphosis and pectus excavatum, or a disease of the extracellular matrix, such as altered collagen metabolism (15).
A hiatal hernia causes a number of altered physiological effects. The segment of LES exposed to positive intra-abdominal pressure is inversely proportional to the size of the hernia. The size of the hernia results in spatial separation of the LES and esophageal compression. The GEJ cross-section area is also increased. This reduces the pressure applied to the LES and lower pressures are required to open the GEJ, allowing for a higher volumes of reflux, even during deglutitive relaxation (18). The impaired diaphragmatic sphincter action results in abnormal acid exposure. The reservoir results in increased acid exposure time, with longer acid clearance, and more prolonged reflux episodes.
History and examination
Many patients with hiatus hernia are asymptomatic and there is no direct correlation between the size of a hernia and the severity of symptoms. Symptoms tend to be vague and nonspecific. With the disruption of the anti-reflux barrier and impaired esophageal acid clearance, patients can present with heartburn and regurgitation (12). Other symptoms include chest or epigastric pain, dysphagia, postprandial fullness, and nausea, from a herniated stomach compressing against the esophagus. An iron deficiency anemia can result from Cameron erosions or hemorrhagic esophagitis.
Large hiatus hernias can present with progressive intolerance to solids and liquid foods, regurgitation, nausea, vomiting, or related to space occupancy in the mediastinum, such as shortness of breath (19). A paraesophageal hiatus hernia can cause acute mechanical problems, such as gastric outlet obstruction, gastric volvulus, incarceration, or strangulation (19). An ischemic strangulated stomach may perforate resulting in gastric contents in the mediastinum, and is the main cause of mortality of large paraesophageal hernias (20).
Diagnostic investigations
Since hiatus hernias maybe symptomatic or asymptomatic, the investigations performed should be guided by the patient’s presentation. Hiatus hernias can be difficult to diagnose due to the mobile nature of the GEJ during swallow, respiration, and movement (8,21).
Radiographs (2)
Plain chest radiographs can show a soft tissue opacity in the posterior mediastinum. A retrocardiac air-fluid level confirms a paraesophageal hernia.
Contrast study
Contrast swallow studies can provide information about the size, reducibility and type (2) of hiatus hernia (Figure 2). The orientation (13), location of the GEJ, and relation of the hiatus can be identified under vision (15,21). A common definition of a hiatus hernia is one where at least 2 cm for stomach is identified above the level of the hiatus (8,13). Less than 2 cm of herniation can be difficult to detect, and is generally clinically insignificant (13).
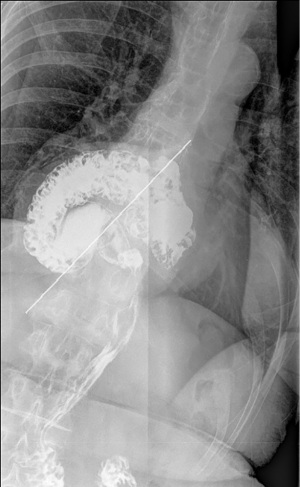
In a paraesophageal hernia, the stomach fundus is seen to herniate along the esophagus (13). Real time swallow allows for the examination of bolus transit (13), motility dysfunction, stenosis, stricture (21), and a short esophagus. These are useful preoperative information to plan operative approach.
Computed tomography
Computed tomography is not a requirement for the diagnosis of a hiatus hernia. However, images can provide information about the site, dimension of the hernia contents, direction, and the involved herniated organs. It is useful for the identification of hiatus hernia complications such as obstruction, strangulation, or gastric volvulus (2,21).
Gastroscopy
Indications for a gastroscopy include GERD symptoms refractory to treatment or symptoms, such as weight loss, dysphagia, or anemia (12). Benefits of a gastroscopy allows the visual assessment of mucosa, with the option of biopsy. It is useful to diagnose hiatus hernia complications such as esophagitis and Cameron erosions (13).
The size a of hernia can be identified on gastroscopy (2,21) though the diaphragmatic landmark is often difficult to determine in a large paraesophageal hernia. The endoscopist should be aware that excess insufflation can exaggerate the size of the hernia (12) and that there is currently, there is no standardization for the degree of air insufflation, or timing of the respiration phrase when a hernia measurement is made during gastroscopy (8).
Esophageal physiology assessment
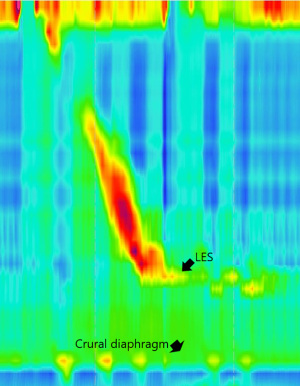
Manometry studies also allow for classification of the hiatus into subtypes based on the separation between the LES and the crural diaphragm (CD) (22,23). Type I is characterized by no separation between the LES & CD. The CD is superimposed on the LES. Type II shows minimal axial separation <2 cm between LES & CD. Type III demonstrates ≥2 cm of separation, allowing for independent assessment of the LES & CD, and is characteristic of a hiatus hernia.
It may be prudent to identify an esophageal motility defect by manometry prior to hiatal hernia repair surgery (7), particularly in patients with dysphagia. Nonetheless, it should be noted that some esophageal body motility issues can improve after repair of the hernia (24), and outflow obstruction frequently resolves. Hiatal hernia repair is usually accompanied by fundoplication, the degree of which is often tailored according to preoperative manometry results despite very little data existing to support this common practice.
Manometry can also provide anatomical information, and demonstrate level of the diaphragmatic crura, the respiratory inversion point, and location and length of the LES, as well as the possibility of encountering a short esophagus at operation (22) (Figure 3). The relationship of the respiratory inversion point to the LES and the CD may be useful in defining subtypes of esophagogastric junction pressure topology and predict reflux (25). Manometry testing is highly accurate the diagnosis of a hiatus hernia and can assist in preoperative workup (26-29).
pH testing assists to correlate the pH level with the patient’s complaints of reflux, and be helpful for the quantitative analysis of reflux episodes (21). Though this provides very important information in the diagnosis and management of GERD and type I hiatal hernias, it is not considered an essential investigation for the diagnosis of a paraesophageal hernia where management is usually targeted towards relief of obstructive symptoms.
Indications for surgery
Patients with paraesophageal hiatal hernia (types II–IV) hernia usually have symptoms attributable to the hernia. Many patients who might appear to be asymptomatic on casual questioning may be found on more detailed history to have symptoms which have been increasing in a slow and relatively unnoticed manner of the preceding years. Truly asymptomatic hernias certainly do occur, though may be less frequent than appreciated by many physicians.
Obstructive symptoms range from mild nausea, bloating or postprandial fullness to acute distress with dysphagia, chest pain and vomiting (2). There is a paucity of published data documenting the natural history of untreated hiatal hernias, thought the risk of progression from asymptomatic to symptomatic is said to be approximately 14% per year (30,31). Once symptomatic, complication risk is higher (32) and, with the possibility of major morbidity or mortality resulting from these complications, particularly from gastric necrosis, elective repair has long been advocated (33,34). However, with more modern series suggesting that the need for emergency hiatal hernia surgery is <2% per year, and with mortality rates for emergency repair averaging around 17%, this recommendation has recently been re-examined (35,36). Decision analysis modeling has suggested that prophylactic surgery to prevent future complications of an asymptomatic paraesophageal hernia should be avoided, particularly in patients over 65 years of age (30). This model, based on analysis of five studies, suggested that surgery be reserved for those patients with symptoms of gastric outlet obstruction as well as those with complications of gastroesophageal reflux disease.
Choice of operative approach
Laparoscopic repair is at least as effective as open repair, with reduced perioperative morbidity and shorter hospital lengths of stay (37,38), and has become the standard of care in most institutions.
Fundoplication
The extensive dissection required during hiatal hernia repair may disrupt the antireflux mechanism. Indeed, the antireflux barrier is frequently incompetent in the presence of a hiatal hernia with much of the tone lacking in the absence of the diaphragmatic pinch (39). The addition of a fundoplication to a hiatal hernia is thought to buttress the repair to decrease recurrence rates and to decrease postoperative gastroesophageal reflux, though it is not universally practiced. Hiatal hernia recurrence rates in the absence of fundoplication are not adequately addressed in the current body of surgical literature (2), with no high level evidence to support its use. Nonetheless, expert opinion supports routine fundoplication when performing hiatal hernia repair (2).
Adequate esophageal length
Hiatal hernia recurrence rates can be reduced by extensive mediastinal mobilization of the esophagus, with the aim of achieving 2–3 cm of intraabdominal esophageal length prior to crural approximation. Measurement should be performed without artificially increasing esophageal length by tension or excessive pneumoperitoneum pressures which result in elevating the diaphragm (40,41). Some authors propose purposeful entering of the left pleural space prior to crural closure to flatten the diaphragm and ease tension (42). We have not found this necessary and there is a risk that hypercarbia may be precipitated leading to possible ventilatory difficulties.
With achievement of adequate intra-abdominal esophageal length, low hernia recurrence rates are to be expected and as well as better postoperative reflux control (43). Rarely, despite extensive mobilization which can be carried as high as the inferior pulmonary veins if needed, adequate length cannot be achieved (44); in these circumstances, an esophageal lengthening procedure should be performed, such as a stapled wedge fundectomy or Collis gastroplasty over a large caliber bougie. The gastric neoesophagus is non-motile and dysphagia is a potential problem, though the rate of dysphagia varies widely between reports (45-47).
Role of mesh hernioplasty
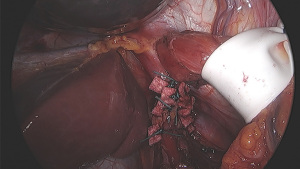
Recurrence rates of hiatal hernia after initial repair are very high and approximate 50% by 5 years in some series (48,49). This has prompted many surgeons to advocate that the crural repair be reinforced. Reinforcement techniques include the use of native tissue such as the ligamentum teres or left lobe of liver, but most reinforced repairs use some type of mesh. A variation is to use pledgets of various materials to buttress the primary sutured crural closure (2). Mesh can be synthetic or biologic, permanent or absorbable. Mesh can be placed over the crural repair posteriorly, or can encircle the esophagus wherein there lies concern, with very limited supporting data, that it may increase the risk of mesh erosion into the esophagus (Figure 4). At least four randomized controlled trials have examined whether mesh repair is beneficia (48,50-52). Short-term results of these trials support the use of reinforcement by mesh, though benefits have not been demonstrated in the long-term. Outcomes examined have included hernia recurrence, symptom control, dysphagia and quality of life, among others. Mesh repair of hiatal hernias, by any type of mesh, is therefore not supported by evidence. It is acknowledged that not all types of mesh and not all locations and orientations of placement have been studies and that new materials are constantly being developed. Though these factors have been considered and addressed to a certain degree by the available literature (52), this will likely be an area further examined in the future.
Surgical technique of laparoscopic paraesophageal hernia repair
The aims of hiatal hernias repair are to return the stomach (and other herniated organs) to the abdominal cavity, to decrease the size of the hiatal defect and to prevent recurrence, all while minimizing morbidity.
The patient is placed in a steep reverse-Trendelenburg position, with legs separated. The surgeon stands between the patient’s legs, and the assistant to the patient’s left side. The instrument nurse stands to the patient’s right; 10 mm ports are placed in the left rectus sheath and the left midclavicular line; 5 mm ports are placed in the left flank and right midclavicular line. We prefer a Nathanson hook liver retractor for liver retraction, placed via an epigastric incision. Pneumoperitoneum of 15 mmHg is established. Reduction of herniated contents is performed upon initial assessment of the hiatus, being careful to avoid trauma by excessive traction to the stomach and other organs (Video 1).
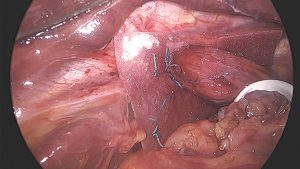
Starting on the front of the left crus, the hernia sac is dissected towards the patient’s right. Surgical repair of a paraesophageal hernia should be an operation of the hernia sac rather than of the stomach. By dissection of the sac, and by avoiding direct traction on the stomach, visceral injury is minimized, blood loss is decreased, and outcomes optimized. The assistant retracts the sac allowing for complete reduction of the hernia (Figure 5). There will be minimal bleeding if the dissection plane remains directly outside the sac, and small vessels are easily controlled with ultrasonic shears. Care must be taken when dissection extends to the right as it is not uncommon to find large vessels crossing the right crus, arising from the left gastric artery. If an accessory left hepatic artery is encountered, it can often be preserved with some extra dissection. Alternatively, it can usually be divided to improve access to the hiatus with no sequalae, though if it is large it should first be temporarily clipped and the effect on liver perfusion assessed. Dissection of the sac posteriorly is often a little challenging, and the esophagus and crura must be clearly identified to as not to cause injury.
Once the sac has been mobilized, a retroesophageal window is developed for introduction of a soft silicone drain. This is passed around the esophagus, secured by Endoloop and allows for caudad retraction of the GEJ. This is a definitive step in the operation; once retraction is achieved, the operation is greatly facilitated.
The sac is bivalved with the ultrasonic shears and then completely excised. Excision ensures disconnection from the crura, allowing for complete reduction of the hernia without tension. Sac excision also decreases the bulk of tissue around which a fundoplication will subsequently be wrapped. Short gastric vessels are divided from the inferior pole of the spleen to the left crus. The esophagus is widely mobilized high into the mediastinum, identifying and protecting both vagus nerves. The aim is to achieve adequate intra-abdominal esophageal length without tension. If adequate intraabdominal length is achieved, the GEJ will easily remain below the diaphragm, decreasing recurrence risk.
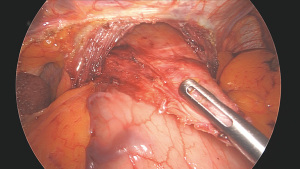
Pneumoperitoneum pressure is then decreased to 10 mmHg to ease tension on subsequent crural repair sutures. We perform posterior crural repair without placing a bougie, using nonabsorbable sutures reinforced with polytetrafluoroethylene pledgets. To further minimize the risk of sutures cutting through the muscle, the needle is passed through the crural muscles as well as the peritoneum overlying the crura which should be left intact during the previous dissection. To ensure no contact between the pledgets and the esophagus, the last stitch or two on the esophageal side are left unpledged (Figure 6).
Various options exist if the hernia defect cannot be closed adequately to achieve a snug fit around the esophagus. Further anterior sutures can be placed, either plicating the anterior border or else approximating the anterior border to the right crus. Care should be taken to avoid passing the needle too deep into the heart or pericardium. Diaphragmatic relaxing incisions are useful too, with right-sided incisions between the right crus and the inferior vena cava being utilized more frequently than left-sided incisions. These incisions allow the ipsilateral crus to slide centrally, and the newly created lateral defect should be covered by mesh. Using mesh to bridge an unclosed defect is contraindicated both due to high recurrence rates and the risk of mesh erosion.
A fundoplication is always performed after the hernia repair. We prefer a posterior 270-degree fundoplication, with nonabsorbable sutures placed between the stomach and esophagus. We do not affix the fundoplication to the diaphragm which is therefore permitted to move independently of the GEJ. If a fundectomy has been performed, an anterior partial fundoplication is performed to cover the staple line.
The patient is started on free fluids postoperatively. Nausea is treated aggressively, and proton pump inhibitors and other acid suppressant medications are not required. If fluids are well tolerated and pain well controlled, then the majority of patients will be suitable for discharge home on the first postoperative day. Lifting of more than 5 kg is to be avoided for 3 months.
Conclusions
Paraesophageal hernias are often symptomatic and treatment is directed towards relief of obstructive symptoms and prevention of complications. Asymptomatic patients may not warrant hernia repair. The various preoperative investigations often employed have individualized rationales for their use. There are several key steps to minimally invasive paraesophageal hernia repair: (I) sac reduction and excision, (II) preservation of the vagus nerves, (III) wide mediastinal mobilization of the esophagus, (IV) recognition and management of the short esophagus, (V) tension-free crural repair and (VI) performance of a fundoplication. Laparoscopic repair is preferable to open repair, and symptom control is excellent (53).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-12/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-21-12/coif). The series “Minimally Invasive Procedures for Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. GPK served as the Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ténaiová J, Tůma L, Hrubant K, et al. Incidence of hiatal hernias in the current endoscopic praxis. Cas Lek Cesk 2007;146:74-6. [PubMed]
- Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc 2013;27:4409-28. [Crossref] [PubMed]
- Last RJ. Last's Anatomy, Regional and Applied. Ninth Edition ed. Churchill Livingston; 1998.
- Kuster GG, Innocenti FA. Laparoscopic anatomy of the region of the esophageal hiatus. Surg Endosc 1997;11:883-93. [Crossref] [PubMed]
- Bowden RE, el-Ramli HA. The anatomy of the oesophageal hiatus. Br J Surg 1967;54:983-9. [Crossref] [PubMed]
- Gryglewski A, Pena IZ, Tomaszewski KA, et al. Unsolved questions regarding the role of esophageal hiatus anatomy in the development of esophageal hiatal hernias. Adv Clin Exp Med 2014;23:639-44. [Crossref] [PubMed]
- Petrov RV, Su S, Bakhos CT, et al. Surgical Anatomy of Paraesophageal Hernias. Thorac Surg Clin 2019;29:359-68. [Crossref] [PubMed]
- Gordon C, Kang JY, Neild PJ, et al. The role of the hiatus hernia in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2004;20:719-32. [Crossref] [PubMed]
- Carré IJ, Johnston BT, Thomas PS, et al. Familial hiatal hernia in a large five generation family confirming true autosomal dominant inheritance. Gut 1999;45:649-52. [Crossref] [PubMed]
- Baglaj SM, Noblett HR. Paraoesophageal hernia in children: familial occurrence and review of the literature. Pediatr Surg Int 1999;15:85-7. [Crossref] [PubMed]
- Chana J, Crabbe DC, Spitz L. Familial hiatus hernia and gastro-oesophageal reflux. Eur J Pediatr Surg 1996;6:175-6. [Crossref] [PubMed]
- Roman S, Kahrilas PJ. The diagnosis and management of hiatus hernia. BMJ 2014;349:g6154. [Crossref] [PubMed]
- Siegal SR, Dolan JP, Hunter JG. Modern diagnosis and treatment of hiatal hernias. Langenbecks Arch Surg 2017;402:1145-51. [Crossref] [PubMed]
- Mittal RK. Hiatal hernia: myth or reality? Am J Med 1997;103:33S-39S. [Crossref] [PubMed]
- Yu HX, Han CS, Xue JR, et al. Esophageal hiatal hernia: risk, diagnosis and management. Expert Rev Gastroenterol Hepatol 2018;12:319-29. [Crossref] [PubMed]
- Menon S, Trudgill N. Risk factors in the aetiology of hiatus hernia: a meta-analysis. Eur J Gastroenterol Hepatol 2011;23:133-8. [Crossref] [PubMed]
- Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology 2006;130:639-49. [Crossref] [PubMed]
- Pandolfino JE, Shi G, Trueworthy B, et al. Esophagogastric junction opening during relaxation distinguishes nonhernia reflux patients, hernia patients, and normal subjects. Gastroenterology 2003;125:1018-24. [Crossref] [PubMed]
- Oleynikov D, Jolley JM. Paraesophageal hernia. Surg Clin North Am 2015;95:555-65. [Crossref] [PubMed]
- Dellaportas D, Papaconstantinou I, Nastos C, et al. Large Paraesophageal Hiatus Hernia: Is Surgery Mandatory? Chirurgia (Bucur) 2018;113:765-71. [Crossref] [PubMed]
- Sfara A, Dumitrascu DL. The management of hiatal hernia: an update on diagnosis and treatment. Med Pharm Rep 2019;92:321-5. [Crossref] [PubMed]
- Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol 2008;22:601-16. [Crossref] [PubMed]
- Kahrilas PJ, Peters JH. Evaluation of the esophagogastric junction using high resolution manometry and esophageal pressure topography. Neurogastroenterol Motil 2012;24:11-9. [Crossref] [PubMed]
- Swanstrom LL, Jobe BA, Kinzie LR, et al. Esophageal motility and outcomes following laparoscopic paraesophageal hernia repair and fundoplication. Am J Surg 1999;177:359-63. [Crossref] [PubMed]
- Kahrilas PJ, Mittal RK, Bor S, et al. Chicago Classification update (v4.0): Technical review of high‐resolution manometry metrics for EGJ barrier function. Neurogastroenterol Motil 2021;33:e14113. [Crossref] [PubMed]
- Pandolfino JE, Leslie E, Luger D, et al. The contractile deceleration point: an important physiologic landmark on oesophageal pressure topography. Neurogastroenterol Motil 2010;22:395-400.e90. [Crossref] [PubMed]
- Tolone S, Savarino E, Zaninotto G, et al. High-resolution manometry is superior to endoscopy and radiology in assessing and grading sliding hiatal hernia: A comparison with surgical in vivo evaluation. United European Gastroenterol J 2018;6:981-9. [Crossref] [PubMed]
- Li L, Gao H, Zhang C, et al. Diagnostic value of X-ray, endoscopy, and high-resolution manometry for hiatal hernia: A systematic review and meta-analysis. J Gastroenterol Hepatol 2020;35:13-8. [Crossref] [PubMed]
- Weijenborg PW, van Hoeij FB, Smout AJ, et al. Accuracy of hiatal hernia detection with esophageal high-resolution manometry. Neurogastroenterol Motil 2015;27:293-9. [Crossref] [PubMed]
- Stylopoulos N, Gazelle GS, Rattner DW. Paraesophageal hernias: operation or observation? Ann Surg 2002;236:492-500; discussion 500-1. [Crossref] [PubMed]
- Treacy PJ, Jamieson GG. An approach to the management of para-oesophageal hiatus hernias. Aust N Z J Surg 1987;57:813-7. [Crossref] [PubMed]
- Harriss DR, Graham TR, Galea M, et al. Paraoesophageal hiatal hernias: when to operate. J R Coll Surg Edinb 1992;37:97-8. [PubMed]
- Skinner DB, Belsey RH. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg 1967;53:33-54. [Crossref] [PubMed]
- Hill LD. Incarcerated paraesophageal hernia. A surgical emergency. Am J Surg 1973;126:286-91. [Crossref] [PubMed]
- Gantert WA, Patti MG, Arcerito M, et al. Laparoscopic repair of paraesophageal hiatal hernias. J Am Coll Surg 1998;186:428-32; discussion 432-3. [Crossref] [PubMed]
- Allen MS, Trastek VF, Deschamps C, et al. Intrathoracic stomach. Presentation and results of operation. J Thorac Cardiovasc Surg 1993;105:253-8; discussion 258-9. [Crossref] [PubMed]
- Diez Tabernilla M, Ruiz-Tovar J, Grajal Marino R, et al. Paraesophageal hiatal hernia. Open vs. laparoscopic surgery. Rev Esp Enferm Dig 2009;101:706-11. [Crossref] [PubMed]
- Zehetner J, Demeester SR, Ayazi S, et al. Laparoscopic versus open repair of paraesophageal hernia: the second decade. J Am Coll Surg 2011;212:813-20. [Crossref] [PubMed]
- Mittal RK. Current concepts of the antireflux barrier. Gastroenterol Clin North Am 1990;19:501-16. [Crossref] [PubMed]
- Awais O, Luketich JD. Management of giant paraesophageal hernia. Minerva Chir 2009;64:159-68. [PubMed]
- Mittal SK, Awad ZT, Tasset M, et al. The preoperative predictability of the short esophagus in patients with stricture or paraesophageal hernia. Surg Endosc 2000;14:464-8. [Crossref] [PubMed]
- Schaheen LW, Christie I, Luketich JD. Chapter 26 - Laparoscopic Paraesophageal Hernia Repair: Technique, Outcomes, and Management of Complications. In: Yeo CJ, editor. Shackelford's Surgery of the Alimentary Tract, 2 Volume Set (Eighth Edition). Philadelphia: Elsevier; 2019:284-90.
- O'Sullivan GC, DeMeester TR, Joelsson BE, et al. Interaction of lower esophageal sphincter pressure and length of sphincter in the abdomen as determinants of gastroesophageal competence. Am J Surg 1982;143:40-7. [Crossref] [PubMed]
- Watson DI, Davies N, Devitt PG, et al. Importance of dissection of the hernial sac in laparoscopic surgery for large hiatal hernias. Arch Surg 1999;134:1069-73. [Crossref] [PubMed]
- Garg N, Yano F, Filipi CJ, et al. Long-term symptomatic outcomes after Collis gastroplasty with fundoplication. Dis Esophagus 2009;22:532-8. [Crossref] [PubMed]
- Gastal OL, Hagen JA, Peters JH, et al. Short esophagus: analysis of predictors and clinical implications. Arch Surg 1999;134:633-6; discussion 637-8. [Crossref] [PubMed]
- Johnson AB, Oddsdottir M, Hunter JG. Laparoscopic Collis gastroplasty and Nissen fundoplication. A new technique for the management of esophageal foreshortening. Surg Endosc 1998;12:1055-60. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 2011;213:461-8. [Crossref] [PubMed]
- Hashemi M, Peters JH, DeMeester TR, et al. Laparoscopic repair of large type III hiatal hernia: objective followup reveals high recurrence rate. J Am Coll Surg 2000;190:553-60; discussion 560-1. [Crossref] [PubMed]
- Granderath FA, Schweiger UM, Kamolz T, et al. Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg 2005;140:40-8. [Crossref] [PubMed]
- Frantzides CT, Madan AK, Carlson MA, et al. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg 2002;137:649-52. [Crossref] [PubMed]
- Watson DI, Thompson SK, Devitt PG, et al. Five Year Follow-up of a Randomized Controlled Trial of Laparoscopic Repair of Very Large Hiatus Hernia With Sutures Versus Absorbable Versus Nonabsorbable Mesh. Ann Surg 2020;272:241-7. [Crossref] [PubMed]
- Lidor AO, Steele KE, Stem M, et al. Long-term quality of life and risk factors for recurrence after laparoscopic repair of paraesophageal hernia. JAMA Surg 2015;150:424-31. [Crossref] [PubMed]
Cite this article as: Hua L, Kohn GP. Minimally invasive surgery: hiatal hernia repair—a narrative review. Ann Esophagus 2022;5:38.