
The future of therapy of Barrett’s esophagus and related cancer: a narrative review
Introduction
Barrett’s esophagus (BE) is an acquired condition in response to chronic gastro-esophageal reflux where a portion of the squamous epithelial lining of the esophagus is replaced by a metaplastic columnar epithelium with intestinal-like differentiation (1). Since the association between BE and esophageal adenocarcinoma (EAC) was established approximately 50 years ago (2), endoscopy has played a key role in the management of this condition, initially as a procedure to diagnose and survey, and more recently as therapeutic intervention to deliver curative treatments. The in-depth characterisation of the stepwise progression sequence from BE to EAC through intermediate histopathologic stages, i.e., (I) low-grade dysplasia (LGD); (II) high-grade dysplasia (HGD); and (III) intramucosal EAC has offered a window of opportunity to intervene before progression to invasive cancer (3-5). A dramatic progress has been made in the last two decades in endoscopic techniques to cure BE-related neoplasia, which has transformed the management of HGD and early EAC from surgical, with associated morbidity, into endoscopic and minimally invasive. Currently, all major gastroenterology societies recommend endoscopic treatment for patients with dysplastic BE and intramucosal EAC. This generally includes endoscopic resection of superficial neoplastic lesions and/or endoscopic ablation of BE with flat inconspicuous dysplasia. A body of evidence, including randomized controlled trials (RCTs), has shown the effectiveness of endoscopy therapy in achieving complete remission of intestinal metaplasia (CR-IM) and dysplasia (CR-D) (6-8), with a better safety profile when compared with esophagectomy (9). On the other hand, given the overall low risk associated to non-dysplastic BE and the non-negligible risk of complication related to endoscopic therapy, routine ablation of BE in the absence of histopathological dysplasia is not recommended (10-12). Despite the treatment algorithm for BE is now well defined, many questions remain open and one can imagine that further progress in the medical diagnostic and therapeutic technologies can provide novel evidence to address the outstanding issues. In this article we will discuss how improved knowledge about the molecular background of the disease and emerging technologies can help reshape the current treatment pathway of BE. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-93/rc).
Halting the progression of BE to cancer
The progression of BE to cancer occurs due to the progressive accumulation of genomic aberrations that drive cellular transformation. These include somatic mutations of known tumor suppressor genes (such as TP53 and ARID1A), copy number alterations affecting genes coding for proteins involved in signal transduction, particularly receptor tyrosine kinase, and genomic catastrophes leading to gross genomic rearrangements (13). Even in the absence of dysplasia, it is possible to detect mutations in key driver genes commonly mutated in EAC, such as SMARCA4, ARID1A, and CNTNAP5, suggesting that these mutational events can occur very early in cancer development (14). There is evidence that the inflammation induced by chronic acid reflux create a mutagenic environment and activates cellular pathways implicated in the carcinogenetic process (15-17). It is therefore intuitive that pharmacological control of reflux through proton pump inhibitors (PPI), that suppress gastric acid production, may reduce the risk of cancer (18,19). A meta-analysis correlated current PPI treatment with a 71% cancer risk reduction (20). However, in clinical practice the majority of patients with BE are treated with PPI, yet a significant proportion of them will progress to cancer. The question arises as to whether other pharmacological interventions could help preventing cancer progression.
In keeping with the primary role of inflammation in the etiopathogenesis of BE related neoplasia, use of aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) has been shown to reduce the incidence of gross DNA abnormalities as well as point mutation linked to cancer development (21). Nevertheless, a recent large UK-based randomised controlled trial (AspECT) investigating the chemopreventive properties of aspirin in patients with BE failed to produce convincing evidence that aspirin reduces the risk of cancer (22). Interestingly the combination of high dose esomeprazole (80 mg/daily) and aspirin demonstrated some protective effect in the composite endpoint including all cause of mortality as well as development of HGD/EAC (time ratio 1.59, 95% CI: 1.14–2.23). However, this is likely to be related to the protective effects of aspirin against cardiovascular events, which in fact represent the single commonest cause of death in patients with BE (23).
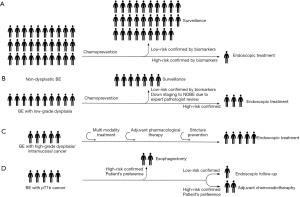
Statins represent another interesting class of drug which might interfere with the cancer development in BE. In vitro experiments demonstrate that statins reduce cell proliferation and induce apoptosis via inhibition of Ras farnesylation and ERK/AKT pathways (24). A recent meta-analysis showed that statin use is associated to an approximately 2-fold reduction in the risk of cancer progression (odds ratio 0.48; 95% CI: 0.31–0.73) (25). Considering that BE is associated with obesity and metabolic syndrome, it is possible to envisage that in the future a combination of PPI with statins and aspirin, which also reduces the cardiovascular risk, might be the preferred pharmacological treatment of patients with BE (Figure 1). However, prospective trials are warranted to establish the efficacy and safety of this approach.
Endoscopic treatment of non-dysplastic Barrett’s
The average risk of progression from non-dysplastic BE to EAC is estimated to be very low, at about 0.3% per year (26). Therefore, with a strategy based on endoscopic ablation of all patients with BE, the prevention of a single case of cancer would require a high number-needed-to-treat. In addition, according to a recent multicentre study, there is evidence that after successful eradication of intestinal metaplasia with radiofrequency ablation (RFA), BE can relapse in up to a quarter of patients (27). Hence, on the one hand, treating all non-dysplastic BE cases could prevent repeating gastroscopies performed as part of endoscopic surveillance, but on the other there is uncertainty whether it is possible to safely discharge patients after successful eradication of BE, even in the absence of dysplasia. Therefore a blanket ablation strategy of non-dysplastic BE is not cost-effective (28). An optimal solution would be to limit the treatment to those patients at highest risk to progress to cancer. The current ‘gold standard’ of pathological dysplasia has proved to be fraught with technical and inter-observer discrepancies and thus there is a clear need for better methods to stratify patients based on their risk of progression. In the era of genomics research advances in our understanding of molecular mechanisms underlying disease pathogenesis and progression have given significant insight in the potential usefulness of molecular biomarkers to quantify cancer risk. However, finding a single molecular biomarker to be used as diagnostic test to guide clinical management has proven to be a tremendous challenge (29) and it is likely a combination of biomarkers will be needed. Recent studies have shown that the combination of clinical parameters (e.g., gender, age and BE segment length) (30) and both genetic (loss of heterozygosity at chromosome 9p and 17p, tetraploidy and aneuploidy) (31-34) and/or epigenetic (methylation pattern) (35,36) alterations increase the predictive value through the development of risk models.
The p53 gene located on chromosome 17p codes for a tumour suppressor that provides a major barrier against neoplastic transformation. P53 is the single most commonly mutated gene in EAC (14). Genomic events at the p53 locus are known to occur in pre-cancerous stages, including 1–5% of non-dysplastic BE, 65% of LGD and 75% of HGD (37-39). In addition, mutations in p53 have been associated with worse prognosis and reduced overall survival following surgical resection (40,41). A case-control study on a prospective cohort demonstrated that aberrant p53 protein expression on endoscopic biopsies correlated to an odds ratio (OR) for progression to HGD/EAC of 5.6 (95% CI: 3.1–10.3) (42). Similarly, a more recent prospective study showed that aberrant p53 increased the risk of short-term progression within 12 months, with an OR of 6.0 (95% CI: 3.1–11.2) (43). A large multi-centre study showed that aberrant p53 can also be detected on cytological samples obtained with a non-endoscopic cell-collection device (CytospongeTM). A model including glandular atypia, P53 abnormality, Aurora kinase A positivity, and the interaction of age, waist-to-hip ratio, and length of the BE segment identified with a 96% (99% CI: 73.8–99.99) probability patients at low-risk of progression, which could be monitored less intensively or even with non-endoscopic technologies (44).
Recent sequencing data demonstrated that EAC is characterised by high degree of genomic instability (13). In keeping with this, a measure of aneuploidy by flow cytometry has been demonstrated in prospective studies to be an accurate biomarker of progression. Patients with BE and baseline aneuploidy have a risk of cancer progression which is 3 to 6 times higher (43,45). However, flow cytometry is laborious and requires fresh biopsies, which limits its diagnostic applicability. A recent study investigated genome-wide copy number chromosomal instability in patients with BE using shallow whole-genome sequencing. The authors assessed 777 biopsies, from 88 patients with BE, including patients with stable non-dysplastic BE as well as those that progressed during surveillance, and were able to show that genomic events can distinguish progressive from stable disease even 10 years before histopathological transformation (46). Of note, 55% of samples from patients who did not progress during follow-up were classified as low risk, whereas 77% of samples from patients who progressed were classified as high risk. One of the interesting aspects of this molecular test is the compatibility with formalin-fixed paraffin embedded material resulting from conventional histopathological diagnosis, making this technology applicable to standard clinical practice. Therefore, we can clearly envisage a future scenario whereby a combination of clinical and molecular data will be integrated to generate a risk score to quantify the individual risk of BE-related cancer (Figure 1). In this context ablation strategies in patients at high risk could meet the threshold for cost-effectiveness. Prospective studies with longitudinal outcome as well as biomarker-based randomised controlled studies are required to validate the utility in clinical practice of prognostic biomarkers.
Do all patients with dysplasia need treatment?
The presence of dysplasia, classified into LGD and HGD, remains the best clinical biomarker to guide treatment decisions (10,12,47). However, dysplasia has significant limitations as it can be easily missed by the endoscopist during random sampling and is diagnosed by pathologists with the use of subjective criteria. The field of endoscopic diagnosis is moving very fast with the development of technologies that assist the physician in detection of subtle lesions. However, dysplasia is often a microscopic diagnosis and is truly invisible even on magnified view. A detailed discussion of novel imaging modalities is outside the scope of this manuscript (48). The other challenge in clinical practice is the high rate of inter-observer variability among pathologists, particularly in the case of LGD where the kappa values for agreement can be as low as 0.14 (standard error 0.1) (49). A pathological diagnosis of HGD associates with a significant risk of progression to cancer. Two randomised controlled trials evaluating photodynamic therapy and RFA showed that patients with HGD randomised to the control arm had a short-term rate of progression to cancer ranging from 19% to 28% (6,50). These data represent the basis of current ablation strategies in patients with HGD. However, the natural history of LGD is less clear and is likely to be heavily influenced by the histopathological stringency of the diagnosis as annual neoplastic progression rates vary hugely between 0.5% (51) and 13.4% (52). The SURF trial, which compared the outcomes of RFA versus surveillance in BE patients with confirmed LGD over 3 years of follow-up, showed progression rates to HGD/EAC of 1.5% and 26.5%, respectively (7). Long term follow up (median 73 months) of LGD patients from the same trial showed that, despite a 32.4% cancer risk reduction in the ablation arm (95% CI: 22.4–44.2), approximately three quarters of LGD patients had stable disease or were downgraded to non-dysplastic BE at follow up (53). Therefore, it is unclear whether an intervention strategy founded on ablation of all patients with LGD is fully justified. Two Dutch studies clearly show that the robustness of the pathological diagnosis of LGD is the key to avoid over diagnosis. When a community LGD is confirmed by more than one expert GI pathologists the risk of progression can be as high as 9–13% per year, as opposed to 0.5–0.6% in patients whose LGD is down-graded (52,54). However, given that the LGD diagnosis in patients enrolled in the SURF trial was confirmed by the same panel of expert pathologists the question remains whether all LGD diagnoses, particularly in elderly patients should be ablated. In the future it is likely that a biomarker-based risk stratification will be applied to guide management decision and avoid exposing elderly patients to un-necessary risks of endoscopic interventions (Figure 1).
How to achieve high rate of BE remission with endotherapy
The endoscopic techniques that are used for the treatment of BE can broadly be categorized into: (I) resection and (II) ablation techniques. These may also be combined to increase the effectiveness of endoscopic eradication therapy.
In recent years, there has been an impressive investment from industry and research bodies in the development and validation of novel endoscopic devices for the treatment of BE. Methods studied include photodynamic therapy, argon-plasma coagulation (APC) (55), hybrid APC (56), spray cryotherapy (57), balloon-based cryotherapy (58), RFA (6,7) and non-thermal mechanical resection (Endorotor®) (59). Detailed description of evidence in support of safety and effectiveness of individual techniques is beyond the scope of this manuscript. Different techniques can be used alone or in combination. One multimodal approach for the treatment of BE with HGD and intramucosal EAC that showed excellent eradication rates is the combination of focal endoscopic resection and ablation. The main rationale behind ablation of residual BE after successful removal of neoplastic lesions is the high rate of neoplastic recurrence (14.5–36.7%) within the flat residual BE (8,60). RFA is by far the most investigated ablation technique and the level of evidence together with the good safety profile has made it the modality of choice in the majority of Countries based on specialist guideline recommendations (10,12,61). However, RFA is far from perfect, given that the rate of complete BE eradication ranges from 62% in registry data (62) to 87% in well-designed prospective studies (63). This suggests that in real life clinical practice approximately a quarter of patients receiving RFA will not achieve the ultimate endpoint, i.e., the complete remission of intestinal metaplasia (CR-IM). In addition to this, after apparent endoscopic remission following RFA there remains a small risk of buried glands, which have been described in less than 1% of cases (63), with anecdotal evidence of EAC developing from sub-squamous glandular metaplasia (64). Clearly the efficacy of emerging techniques will need to be compared with RFA to prove non-inferiority or ideally superiority in order to rescue patients that might not achieve CR-IM with RFA or shift the treatment paradigm towards more effective techniques. A recent pilot RCT compared RFA and APC in 76 BE patients with early BE-related neoplasia. In this study, the patients treated with endoscopic resection were randomized to receive endoscopic ablation by APC or RFA. Each technique showed similar efficacy and safety, although APC was shown to be more cost-effective (65). However, this conclusion can be challenged for two main reasons. Firstly, the primary endpoint of the study was complete remission of dysplasia (CR-D) and not CR-IM, which should be the ultimate goal of the endoscopic ablation pathway; secondly, the sample size was underpowered to compare efficacy and safety of both techniques. One suggested advantage of APC is its wider availability, however, this may be of limited benefit as there is broad consensus that the treatment of BE-related neoplasia should be restricted to expert centres (10-12).
Cryotherapy may become an attractive alternative for cases refractory to RFA. A small retrospective study, which assessed a response to liquid nitrogen spray cryotherapy in 16 patients with persistent dysplasia after a median number of 3 RFA sessions, showed that CR-D could be achieved in 75% of refractory cases (66). A meta-analysis of 11 studies reporting on efficacy of different cryotherapy techniques in patients with persistent dysplasia or IM after RFA found that CR-D and CR-IM can be achieved in 76% and 46% of cases, respectively (67). A novel balloon-based nitrous oxide cryotherapy device is available on the market and has showed promising results in a recent single arm multicentre prospective study, where CR-D and CR-IM were achieved in 76% and 72% of patients with dysplastic BE (68). The availability of different treatment modalities to the managing physician will enhance in the future the opportunity to achieve disease remission in patients with neoplastic BE.
However, it remains unclear the reason why a proportion of patients with BE fails to respond to the ablation treatments. One possible explanation is that the persistence of acid and non-acid reflux after the endoscopic treatment creates a hostile environment for tissue healing. In a small retrospective study on 45 patients, normal esophageal acid exposure after RFA correlated with rate of response to treatment (69). However, there is no data on the usefulness of pharmacological or surgical interventions to improve reflux control and enhance response to endoscopic treatment. In a large US-based registry collecting data from 5,537 patients undergoing RFA, previous fundoplication did not correlate to higher rates of CR-IM (70). One possible way to improve CR-IM rates would be pharmacologically boost epithelial regeneration after treatment towards the squamous phenotype. It can be postulated that after thermal injury (heat or cold) of the superficial mucosa, progenitor cells located in the deeper mucosal or submucosal layers are responsible of esophageal re-epithelisation. There is evidence that embryological pathways such as Hedgehog and Bone Morphogenetic Proteins (BMPs) are implicated both in the development of the embryonic human esophagus (71,72), as well as in the onset of BE (73). In particular, inhibition of the Hedgehog pathway has been shown to prevent BE-like lesions and cancer in a rat surgical model of reflux disease (74). Even though there remain uncertainty about the cell compartment that acts as progenitor to BE epithelium, it is reasonable to hypothesize that, after endoscopic ablation, the activation of embryological pathways that support the intestinal metaplastic phenotype could be responsible of the regeneration with BE rather than neosquamous epithelium. This could lead to reduced efficacy of the therapeutic intervention. Therefore, systemic or topical inhibition of BMP or Hedgehog could represent in the future a viable opportunity to interfere with this cell fate decision and drive regeneration with normal squamous phenotype (Figure 1). This represents an exciting new avenue of translational research that might lead to improvement of outcomes after endoscopic ablation.
The future of endoscopic resection: endoscopic mucosal resection (EMR) vs. endoscopic submucosal dissection (ESD)
BE-related neoplasia associated to endoscopically visible lesions is an indication to endoscopic resection. The European Society of Gastrointestinal Endoscopy guidelines recommend piecemeal EMR over ESD as preferred technique in most cases of BE-related early neoplasia (10). This recommendation is founded on two pieces of evidence. First, a treatment strategy based on the combination of EMR and RFA leads to remission of neoplasia in 98% of cases according to a European multicentre study including 132 patients (63). Second, a small RCT comparing effectiveness and safety of EMR and ESD did not find superiority of a routine ESD-based strategy (75). In particular, this trial, despite a higher radical resection rate in the ESD group, failed to show a difference in complete remission of neoplasia during a mean follow-up period of 23 months. In addition, ESD was associated with a significantly longer procedure time (mean 54 versus 22 minutes; P=0.0002). Nevertheless, this was a small study, which was not powered to show differences in the curative rate, particularly in the case of larger lesions which might bear a significant risk of deeper invasion. It is estimated that ESD is performed for less than 10 percent of all early Barrett’s neoplasia cases (76). It is not easy to predict whether in the future we will observe an increase in the proportion of ESD during BE endotherapy given the fact that this technique has a longer learning curve and more limited training availability in the Western centres compared to EMR. On the other hand, it is becoming more popular in Europe and North America, which brings about a larger training resource for the future.
An analogy could be drawn however with the management of the squamous cell cancer (SCC) where ESD is the technique of choice to completely resect the early cancer and the entire dysplastic field (77,78). In the context of SCC, this strategy has a 95% of long term remission rate and has been proven to be superior to an EMR-based approach (79,80). The question arises whether in the future for an optimal management of BE-related neoplasia a radical endoscopic resection should include the adjacent non-dysplastic BE, which would overcome the issue of BE refractory to ablation. This therapeutic approach was compared to a multimodal strategy (EMR followed by RFA) in a small randomised controlled trial. In this study the stepwise radical resection resulted in a significantly higher rate of moderate and severe complication compared to EMR followed by RFA, including a stricture rate of 88% (81). This mirrors retrospective and prospective data from cohorts of patients with SCC treated with ESD, where esophageal stricture occurs at significant rate after resection of mucosal areas larger than three-quarters of the circumference of the esophagus (82-84). A meta-analysis of data from 3 studies revealed that the risk ratio for the development of stenosis following endoscopic resection was 30.9 (95% CI: 18.8–50.8; P<0.001) for lesions involving > 3/4 as compared to lesions involving ≤ 3/4 of the esophageal circumference (40). The incidence of post-procedural stricture is almost 100% if patients require circumferential mucosal resection (40). This suggests that preventive interventions against severe stricture are required in order to make radical resection safe and feasible in clinical practice.
Temporary placement of a self-expandable metallic stent has been investigated (85). However, stenting carries disadvantages of migration, need for removal and high costs. Recently, the use of biodegradable stents made of synthetic polymers that decompose in situ and do not require removal was described. Studies have reported efficacy in preventing post-ESD esophageal stricture, however the sample size of these studies is small (86,87). Several previous studies showed that endoscopic steroid injection into the ESD bed reduces the risk of stricture by approximately 70% (88). Yamaguchi et al. reported that oral prednisolone is an effective treatment for preventing esophageal strictures after esophageal ESD (89), but this can associate to the risk of severe complications, especially in elderly patients with moderate to severe comorbidities. Topical steroids, such as orodispersible budesonide could be a safer alternative to systemic steroids and an RCT is ongoing (90). Some reports show potential efficacy of anti-fibrosis and anti-scarring drugs such as botulinum toxin A (91), mitomycin C (92), 5-fluorouracil (93), tranilast (94), N-acetylcysteine (95) and small interfering RNA (96). However, none of these treatments have been investigated in multicentre trials with a sufficient sample size and some are still highly experimental.
Regenerative medicine is evolving as an emerging field in the prevention of post-procedural endoscopic strictures. The application of an autologous cell suspension that involves the use of cells extracted from autologous fat, skin, and oral mucosa has shown promising results in animal studies (97). A recent study that included 10 patients with superficial esophageal squamous cell carcinoma who had undergone ESD with mucosal defects greater than 3/4 of the esophageal circumference, showed feasibility of the cell sheets technology, however the incidence of esophageal stricture remained high at 40% (98). Trials using autologous tissue transplantation showed very promising initial results. To date, transplantation of gastric (99) and esophageal mucosa (100) and skin graft placement on patients after extensive endoscopic resection of esophageal neoplasia showed feasibility and efficacy. However, the number of patients included into all these experiments is small, and there is a lack of controls.
In summary, while EMR appears the preferred resection modality within a multimodal strategy in combination with endoscopic ablation, in the future radical ESD could become an option as training becomes more available and preventive intervention against stricture might allow a drastic reduction in the complication rate. A particular subgroup of patients who might benefit from ESD is those with T1b EAC, which is an increasing indication for endoscopic treatment as discussed in the next paragraph.
Shifting paradigm of minimally invasive treatment: the case of T1b
Endoscopic resection is considered curative for completely removed dysplastic lesions and early-stage EAC at low risk of lymph node metastases. Since lymph nodes are not removed during endoscopic resection, the risk of lymph node metastases must be predicted based on histopathological criteria. Uniformly accepted criteria for curative resection of BE related neoplasia include invasion within the muscularis mucosa, good or moderate differentiation (G1/G2), no lymph vascular invasion (lv-) and resection margin clear from cancer (R0 resection) (10,101). When these criteria are met, the risk of lymph node metastases has been proven to be very low (0–2%) (102) and long-term disease specific survival after endoscopic treatment is comparable to surgery, but with significantly lower morbidity and mortality rates (103). Although invasion into the submucosal layer historically represent an indication to surgery, recent series of patients with T1b disease treated with endoscopic resection indicate that the incidence of lymph node metastasis in submucosal tumours is lower than in the previously reported in surgical series (104-107). There is evidence that superficial submucosal invasion (≤500 µm) harbour an overall risk of lymph node metastases of 8–9% (106,108) and when limited to cancers with no other risk factors (G1/G2, lv-, and R0) this risk may be as low as 2%, which is similar to that of mucosal cancers (106). A multicentre retrospective study showed that lv+ and poor differentiation are the strongest risk factors for metastasis with OR of 6.2 (95% CI: 3.1–12.3) and 3.7 (95% CI: 1.9–7.1), respectively (109). As a consequence, both European and Japanese guidelines consider sm1 EAC without other histological risk factors (G1/G2, lv-) an extended criteria for curative resection, particularly in patients with borderline fitness to surgery (10,110).
A recent propensity score matched study which, analysed data from the US National Cancer Database, compared endoscopic and surgical resections in 2,545 patients with T1a and 1,281 patients with T1b EAC (111). While it was not surprising that the 5-year survival between endoscopically and surgically treated patients did not differ (70% and 74%, respectively, P=0.1), it was somehow unexpected that overall surgery did not confer survival benefit in the T1b group where 5-year overall survival were 53% and 61% after endoscopic resection and esophagectomy, respectively (P=0.3). In this cohort, 23% of T1b patient treated by endoscopic resection received adjuvant treatment.
The rationale behind the standard approach of radical surgical treatment in patients with high risk T1b disease lies in the 20% risk for lymph node metastasis (108), which can increase to 50% when multiple histopathological risk factors are present (112). This means that as many as 80% of these patients may receive unnecessary surgery. How can the situation change in a foreseeable future (Figure 1)? First, new histopathological predictors may help stratify patients based on their risk of nodal involvement. The most promising one is tumour budding, defined as the presence of single cells or small groups of less than 5 undifferentiated cells at the invasive front of the invasive cancer. Tumour budding has been shown to be independent risk factor for lymph node metastasis in T1 EAC (113) and has been associated with poor overall survival in esophageal cancer (113,114). Second, already identified and emerging histological risk factors will likely be converted into quantitative models to predict the risk of lymph node metastases for patients with endoscopically treated T1 EAC. The benefit of the models is that they take into account a weighted risk of each individual risk factor. Such model, including tumour size, depth of invasion, grade and lymph vascular invasion has already been developed and shown to effectively stratify patients into four risk categories for lymph node involvement (<5%, 5–10%; 15–20%; and >20%) (115). Furthermore, it also predicted survival and risk of recurrence among patients without lymph node involvement at the time of esophagectomy, which may call into question the dogma that surgical resection is required to effectively manage lymph node metastases (115). The next steps are to incorporate more detailed and new histopathological features along with clinical factors, including comorbidities to estimate the potential benefit of additional treatment and to validate the model in other cohorts. One may also expect that artificial intelligence systems will outperform such model and replace it in predicting the risk of lymph node metastasis through automated analysis of histopathological specimens. Such model has already been developed and internally validated for pT1 colorectal cancers (116) and it may become routine for EAC in the future.
It remains unclear whether oncological treatments with chemotherapy or chemoradiation therapy (CRT) could be a better, organ saving alternative to the surgery for patients with non-curative resection due to high risk pT1b EAC. In the field of SCC, several studies investigated the role CRT in patients with high risk T1 disease and showed similar overall survival and progression free survival to surgery (117-120). The first phase III RCT comparing esophagectomy and CRT in patients with N0-pT1b esophageal SCC after ESD is ongoing (121). In the context of EAC, a recent systematic review which included 285 patients with early submucosal EAC suggested that surgery might be superior to CRT, with 5-year overall survival between 90–100% after esophagectomy and 75–85% after adjuvant CRT (122). However, available studies might be limited by selection bias, in that patients undergoing surgery might be fitter that those treated by CRT, which could impact on overall survival. Therefore, RCTs are highly warranted to compare the efficacy of the two approaches. Meanwhile a prospective study is ongoing, where patients with pT1b EAC who have undergone radical endoscopic resection will be offered a strict endoscopic and radiological follow-up as alternative to an esophagectomy (123). This study will provide invaluable insight into the natural history of T1b disease.
Endotherapy as adjuvant treatment for more advanced neoplasia
Although EAC is less chemo- and radio-sensitive than SCC, the rate of complete pathological response has increased with recent advances in neo-adjuvant treatment. A prospective study on 700 patients with esophageal cancer, of which 112 received neoadjuvant CRT, showed complete pathological response rates of 21% and 42% for EAC and SCC respectively (124). Interestingly the rate of pathologically negative lymph nodes was 52% and 65% in EAC and SCC respectively. The question arises then whether endoscopic resection post CRT has a role to control residual local disease. Studies from Japan indicate that salvage endoscopic resection could be a minimally invasive strategy for persistent luminal disease after definitive CRT (125,126). A recent European multicentre case series including 25 patients showed feasibility of this approach with a 92% en bloc resection rate for ESD (127).
Differently from SCC, there is very scarce evidence on the role of endotherapy as salvage resection after definitive oncological treatment (126,128). Given an increasing incidence of EAC and the longer life expectancy, this cancer is increasingly diagnosed in elderly patients who might be unfit for surgery or refuse it for personal reasons, therefore further research on the combination of CRT and salvage endoscopic resection is warranted. Although it is difficult to design an RCT with the current level of evidence, there is an increasing patient population that might benefit from this approach within well designed prospective studies, which take into account patients’ comorbidities and preference.
Esophageal cancer is associated with a complex pattern of lymph node metastasis, from the cervical to the abdominal stations. As a consequence, in order to reduce the risk of recurrence an extensive lymphadenectomy is required, which carries a high risk of complications. Another interesting area of future research on multimodal therapy is based on advances in imaging to better identify early nodal metastasis in patients with EAC. This can be achieved with sentinel node (SN) mapping, which has been widely applied to the surgical staging of breast cancer or melanoma. However, SN mapping for esophageal cancer is technically difficult because of the multidirectional lymphatic networks of the esophagus and mediastinum. SN mapping and biopsy in the context of esophageal cancer has been investigated and might have a role to inform decision-making process in minimally invasive treatment of early stage esophageal cancer.
A meta-analysis including 18 studies showed sensitivity to predict nodal metastasis of 91% in EAC and 81% in SCC. Interestingly the sensitivity tended to be lower according to tumor depth therefore patients with superficial primary tumors (T1 or T2) would benefit the most from SN mapping (129). A study with 134 patients showed an excellent sensitivity and accuracy for T1 tumours (sensitivity 91.7%, accuracy 98.2%) using post-surgical histopathology as gold standard (130). A recent pilot study assessed the feasibility of SN navigation surgery in combination with thoracolaparoscopic lymphadenectomy without concomitant esophagectomy in early EAC without neoadjuvant therapy. The results of the study suggested that this hybrid technique is feasible in patients with high-risk submucosal early EAC (131). If the results of this current line of research will prove successful, they will open new and exciting avenues for endotherapy in early stage EAC.
Conclusions
Tremendous progress has been made over the last two decades in the understanding of the natural history, molecular make up and endoscopic management of BE. This has informed recent guidelines which corroborated a step change toward minimally invasive treatment of BE-related early neoplasia. Currently, endoscopy is the main stay for treatment of patients with BE disease, however, further efforts are required to translate research advances into a more personalised and patient-centred clinical management. New targeted pharmacological therapies are awaited to reduce cancer risk and improve response to endoscopic treatments. At the same time, better molecular stratification can be seen at the horizon. Several biomarkers are at late stage of validation and will allow soon to focus therapeutic interventions on patients at high risk and to reduce the burden of endoscopic surveillance in patients at low risk of progression. Finally, increasing understanding of natural history and patterns of progression of T1b EAC will allow better tailoring of the curative pathway, with increased use of minimally invasive endoscopic therapy in combination with oncological treatments.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Madhav Desai) for the series “Endoscopic Therapy for Barrett’s Esophagus” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-93/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-93/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-93/coif). The series “Endoscopic Therapy for Barrett’s Esophagus” was commissioned by the editorial office without any funding or sponsorship. MDP reports personal fees (consultancy) from Medtronic, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. Jama 2013;310:627-36. [Crossref] [PubMed]
- Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett's esophagus. N Engl J Med 1976;295:476-80. [Crossref] [PubMed]
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375-83. [Crossref] [PubMed]
- Burke ZD, Tosh D. Barrett's metaplasia as a paradigm for understanding the development of cancer. Curr Opin Genet Dev 2012;22:494-9. [Crossref] [PubMed]
- Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394-8. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. Jama 2014;311:1209-17. [Crossref] [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200-6. [Crossref] [PubMed]
- Wani S, Drahos J, Cook MB, et al. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population-based study. Gastrointest Endosc 2014;79:224-232.e1. [Crossref] [PubMed]
- Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017;49:191-8. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol 2016;111:30-50; quiz 1. [Crossref] [PubMed]
- Wani S, Qumseya B, Sultan S, et al. Endoscopic eradication therapy for patients with Barrett's esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907-931.e9. [Crossref] [PubMed]
- Secrier M, Li X, de Silva N, et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet 2016;48:1131-41. [Crossref] [PubMed]
- Weaver JMJ, Ross-Innes CS, Shannon N, et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat Genet 2014;46:837-43. [Crossref] [PubMed]
- Kawanishi S, Ohnishi S, Ma N, et al. Crosstalk between DNA Damage and Inflammation in the Multiple Steps of Carcinogenesis. Int J Mol Sci 2017;18:1808. [Crossref] [PubMed]
- Kunze B, Wein F, Fang HY, et al. Notch Signaling Mediates Differentiation in Barrett's Esophagus and Promotes Progression to Adenocarcinoma. Gastroenterology 2020;159:575-90. [Crossref] [PubMed]
- Quante M, Bhagat G, Abrams JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 2012;21:36-51. [Crossref] [PubMed]
- Tan MC, El-Serag HB, Yu X, et al. Acid suppression medications reduce risk of oesophageal adenocarcinoma in Barrett's oesophagus: a nested case-control study in US male veterans. Aliment Pharmacol Ther 2018;48:469-77. [Crossref] [PubMed]
- Krishnamoorthi R, Borah B, Heien H, et al. Rates and predictors of progression to esophageal carcinoma in a large population-based Barrett's esophagus cohort. Gastrointest Endosc 2016;84:40-46.e7. [Crossref] [PubMed]
- Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut 2014;63:1229-37. [Crossref] [PubMed]
- Galipeau PC, Oman KM, Paulson TG, et al. NSAID use and somatic exomic mutations in Barrett's esophagus. Genome Med 2018;10:17. [Crossref] [PubMed]
- Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): a randomised factorial trial. Lancet 2018;392:400-8. [Crossref] [PubMed]
- Solaymani-Dodaran M, Card TR, West J. Cause-specific mortality of people with Barrett's esophagus compared with the general population: a population-based cohort study. Gastroenterology 2013;144:1375-83, 83.e1.
- Ogunwobi OO, Beales IL. Statins inhibit proliferation and induce apoptosis in Barrett's esophageal adenocarcinoma cells. Am J Gastroenterol 2008;103:825-37. [Crossref] [PubMed]
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors Associated With Progression of Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:1046-55.e8. [Crossref] [PubMed]
- Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut 2012;61:970-6. [Crossref] [PubMed]
- Sami SS, Ravindran A, Kahn A, et al. Timeline and location of recurrence following successful ablation in Barrett's oesophagus: an international multicentre study. Gut 2019;68:1379-85. [Crossref] [PubMed]
- Gordon LG, Mayne GC, Hirst NG, et al. Cost-effectiveness of endoscopic surveillance of non-dysplastic Barrett's esophagus. Gastrointest Endosc 2014;79:242-256.e6. [Crossref] [PubMed]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89-95. [Crossref] [PubMed]
- Gatenby PA, Caygill CP, Ramus JR, et al. Short segment columnar-lined oesophagus: an underestimated cancer risk? A large cohort study of the relationship between Barrett's columnar-lined oesophagus segment length and adenocarcinoma risk. Eur J Gastroenterol Hepatol 2007;19:969-75. [Crossref] [PubMed]
- Sanz-Ortega J, Hernández S, Saez MC, et al. 3p21, 5q21, 9p21 and 17p13.1 allelic deletions are potential markers of individuals with a high risk of developing adenocarcinoma in Barrett's epithelium without dysplasia. Hepatogastroenterology 2003;50:404-7.
- Helm J, Enkemann SA, Coppola D, et al. Dedifferentiation precedes invasion in the progression from Barrett's metaplasia to esophageal adenocarcinoma. Clin Cancer Res 2005;11:2478-85. [Crossref] [PubMed]
- Chaves P, Crespo M, Ribeiro C, et al. Chromosomal analysis of Barrett's cells: demonstration of instability and detection of the metaplastic lineage involved. Mod Pathol 2007;20:788-96. [Crossref] [PubMed]
- Casson AG, Evans SC, Gillis A, et al. Clinical implications of p53 tumor suppressor gene mutation and protein expression in esophageal adenocarcinomas: results of a ten-year prospective study. J Thorac Cardiovasc Surg 2003;125:1121-31. [Crossref] [PubMed]
- Brock MV, Gou M, Akiyama Y, et al. Prognostic importance of promoter hypermethylation of multiple genes in esophageal adenocarcinoma. Clin Cancer Res 2003;9:2912-9.
- Sato F, Jin Z, Schulmann K, et al. Three-tiered risk stratification model to predict progression in Barrett's esophagus using epigenetic and clinical features. PLoS One 2008;3:e1890. [Crossref] [PubMed]
- Snyder P, Dunbar K, Cipher DJ, et al. Aberrant p53 Immunostaining in Barrett's Esophagus Predicts Neoplastic Progression: Systematic Review and Meta-Analyses. Dig Dis Sci 2019;64:1089-97. [Crossref] [PubMed]
- Sepulveda JL, Komissarova EV, Kongkarnka S, et al. High-resolution genomic alterations in Barrett's metaplasia of patients who progress to esophageal dysplasia and adenocarcinoma. Int J Cancer 2019;145:2754-66. [Crossref] [PubMed]
- Ishikawa K, Okimoto K, Matsumura T, et al. Comprehensive Analysis of Barrett's Esophagus: Focused on Carcinogenic Potential for Barrett's Cancer in Japanese Patients. Dig Dis Sci 2021;66:2674-81. [Crossref] [PubMed]
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 2019;16:1-24.
- Iyer PG, Taylor WR, Johnson ML, et al. Accurate Nonendoscopic Detection of Barrett's Esophagus by Methylated DNA Markers: A Multisite Case Control Study. Am J Gastroenterol 2020;115:1201-9. [Crossref] [PubMed]
- Kastelein F, Biermann K, Steyerberg EW, et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett's oesophagus. Gut 2013;62:1676-83. [Crossref] [PubMed]
- Hadjinicolaou AV, van Munster SN, Achilleos A, et al. Aneuploidy in targeted endoscopic biopsies outperforms other tissue biomarkers in the prediction of histologic progression of Barrett's oesophagus: A multi-centre prospective cohort study. EBioMedicine 2020;56:102765.
- Ross-Innes CS, Chettouh H, Achilleos A, et al. Risk stratification of Barrett's oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017;2:23-31. [Crossref] [PubMed]
- Galipeau PC, Li X, Blount PL, et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med 2007;4:e67. [Crossref] [PubMed]
- Killcoyne S, Gregson E, Wedge DC, et al. Genomic copy number predicts esophageal cancer years before transformation. Nat Med 2020;26:1726-32. [Crossref] [PubMed]
- Spechler SJ. Dysplasia in Barrett's esophagus: limitations of current management strategies. Am J Gastroenterol 2005;100:927-35. [Crossref] [PubMed]
- Sutton RA, Sharma P. Imaging for Barrett's esophagus: state of the art. Curr Opin Gastroenterol 2019;35:395-400. [Crossref] [PubMed]
- Wani S, Falk GW, Post J, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett's esophagus. Gastroenterology 2011;141:1179-86, 86.e1.
- Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62:488-98. [Crossref] [PubMed]
- Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:897-909.e4; quiz 83.e1, 83.e3.
- Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol 2010;105:1523-30. [Crossref] [PubMed]
- Pouw RE, Klaver E, Phoa KN, et al. Radiofrequency ablation for low-grade dysplasia in Barrett's esophagus: long-term outcome of a randomized trial. Gastrointest Endosc 2020;92:569-74. [Crossref] [PubMed]
- Duits LC, Lao-Sirieix P, Wolf WA, et al. A biomarker panel predicts progression of Barrett's esophagus to esophageal adenocarcinoma. Dis Esophagus 2019;32:doy102. [Crossref] [PubMed]
- Sie C, Bright T, Schoeman M, et al. Argon plasma coagulation ablation versus endoscopic surveillance of Barrett's esophagus: late outcomes from two randomized trials. Endoscopy 2013;45:859-65. [Crossref] [PubMed]
- Manner H, May A, Kouti I, et al. Efficacy and safety of Hybrid-APC for the ablation of Barrett's esophagus. Surg Endosc 2016;30:1364-70. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Canto MI, Shaheen NJ, Almario JA, et al. Multifocal nitrous oxide cryoballoon ablation with or without EMR for treatment of neoplastic Barrett's esophagus (with video). Gastrointest Endosc 2018;88:438-446.e2. [Crossref] [PubMed]
- Knabe M, Blößer S, Wetzka J, et al. Non-thermal ablation of non-neoplastic Barrett's esophagus with the novel EndoRotor® resection device. United European Gastroenterol J 2018;6:678-83. [Crossref] [PubMed]
- Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett's epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6-12. [Crossref] [PubMed]
- di Pietro M, Fitzgerald RC. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett's oesophagus with low-grade dysplasia. Gut 2018;67:392-3. [Crossref] [PubMed]
- Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett's esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology 2013;145:87-95. [Crossref] [PubMed]
- Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut 2016;65:555-62. [Crossref] [PubMed]
- Kohoutova D, Haidry R, Banks M, et al. Esophageal neoplasia arising from subsquamous buried glands after an apparently successful photodynamic therapy or radiofrequency ablation for Barrett's associated neoplasia. Scand J Gastroenterol 2015;50:1315-21. [Crossref] [PubMed]
- Peerally MF, Bhandari P, Ragunath K, et al. Radiofrequency ablation compared with argon plasma coagulation after endoscopic resection of high-grade dysplasia or stage T1 adenocarcinoma in Barrett's esophagus: a randomized pilot study (BRIDE). Gastrointest Endosc 2019;89:680-9. [Crossref] [PubMed]
- Sengupta N, Ketwaroo GA, Bak DM, et al. Salvage cryotherapy after failed radiofrequency ablation for Barrett's esophagus-related dysplasia is safe and effective. Gastrointest Endosc 2015;82:443-8. [Crossref] [PubMed]
- Visrodia K, Zakko L, Singh S, et al. Cryotherapy for persistent Barrett's esophagus after radiofrequency ablation: a systematic review and meta-analysis. Gastrointest Endosc 2018;87:1396-1404.e1. [Crossref] [PubMed]
- Canto MI, Trindade AJ, Abrams J, et al. Multifocal Cryoballoon Ablation for Eradication of Barrett's Esophagus-Related Neoplasia: A Prospective Multicenter Clinical Trial. Am J Gastroenterol 2020;115:1879-90. [Crossref] [PubMed]
- Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra-esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett's esophagus. Dig Dis Sci 2012;57:2625-32. [Crossref] [PubMed]
- Shaheen NJ, Kim HP, Bulsiewicz WJ, et al. Prior fundoplication does not improve safety or efficacy outcomes of radiofrequency ablation: results from the U.S. RFA Registry. J Gastrointest Surg 2013;17:21-8; discussion p.28-9. [Crossref] [PubMed]
- Litingtung Y, Lei L, Westphal H, et al. Sonic hedgehog is essential to foregut development. Nat Genet 1998;20:58-61. [Crossref] [PubMed]
- Crowley AR, Mehta SS, Hembree MJ, et al. Bone morphogenetic protein expression patterns in human esophageal atresia with tracheoesophageal fistula. Pediatr Surg Int 2006;22:154-7. [Crossref] [PubMed]
- Wang DH, Clemons NJ, Miyashita T, et al. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett's metaplasia. Gastroenterology 2010;138:1810-22. [Crossref] [PubMed]
- Gibson MK, Zaidi AH, Davison JM, et al. Prevention of Barrett esophagus and esophageal adenocarcinoma by smoothened inhibitor in a rat model of gastroesophageal reflux disease. Ann Surg 2013;258:82-8. [Crossref] [PubMed]
- Terheggen G, Horn EM, Vieth M, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett's neoplasia. Gut 2017;66:783-93. [Crossref] [PubMed]
- Belghazi K, Pouw RE, Bergman JJ. In the expanding arena of endoscopic management for Barrett's neoplasia, how should we fit in endoscopic submucosal dissection? Gastrointest Endosc 2018;87:1394-5. [Crossref] [PubMed]
- di Pietro M, Canto MI, Fitzgerald RC. Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy. Gastroenterology 2018;154:421-36. [Crossref] [PubMed]
- Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 2015;12:1-30.
- Berger A, Rahmi G, Perrod G, et al. Long-term follow-up after endoscopic resection for superficial esophageal squamous cell carcinoma: a multicenter Western study. Endoscopy 2019;51:298-306. [Crossref] [PubMed]
- Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 2010;72:255-64, 64.e1-2.
- van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut 2011;60:765-73. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860-6. [Crossref] [PubMed]
- Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol 2011;11:46. [Crossref] [PubMed]
- Katada C, Muto M, Manabe T, et al. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003;57:165-9. [Crossref] [PubMed]
- Ye LP, Zheng HH, Mao XL, et al. Complete circular endoscopic resection using submucosal tunnel technique combined with esophageal stent placement for circumferential superficial esophageal lesions. Surg Endosc 2016;30:1078-85. [Crossref] [PubMed]
- Saito Y, Tanaka T, Andoh A, et al. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World J Gastroenterol 2007;13:3977-80. [Crossref] [PubMed]
- Imaz-Iglesia I, García-Pérez S, Nachtnebel A, et al. Biodegradable stents for the treatment of refractory or recurrent benign esophageal stenosis. Expert Rev Med Devices 2016;13:583-99. [Crossref] [PubMed]
- Yu JP, Liu YJ, Tao YL, et al. Prevention of Esophageal Stricture After Endoscopic Submucosal Dissection: A Systematic Review. World J Surg 2015;39:2955-64. [Crossref] [PubMed]
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011;73:1115-21. [Crossref] [PubMed]
- ESD Botvptposa. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-002617-35/DE. EudraCT number 2018-002617-35.
- Bhutani MS. EUS-guided botulinum toxin injection into the pyloric sphincter for the treatment of gastroparesis. Endosc Ultrasound 2019;8:350-1. [Crossref] [PubMed]
- Zhang Y, Wang X, Liu L, et al. Intramuscular injection of mitomycin C combined with endoscopic dilation for benign esophageal strictures. J Dig Dis 2015;16:370-6. [Crossref] [PubMed]
- Mizutani T, Tadauchi A, Arinobe M, et al. Novel strategy for prevention of esophageal stricture after endoscopic surgery. Hepatogastroenterology 2010;57:1150-6.
- Wang H, Shuai Q, Tang J, et al. Local Thymosin β4 Gel Injection Prevents Esophageal Stricture after Circumferential Endoscopic Submucosal Dissection in a Porcine Model. Dig Dis 2019;37:87-92. [Crossref] [PubMed]
- Barret M, Batteux F, Beuvon F, et al. N-acetylcysteine for the prevention of stricture after circumferential endoscopic submucosal dissection of the esophagus: a randomized trial in a porcine model. Fibrogenesis Tissue Repair 2012;5:8. [Crossref] [PubMed]
- Sato H, Sagara S, Nakajima N, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using RNA-based silencing of carbohydrate sulfotransferase 15 in a porcine model. Endoscopy 2017;49:491-7. [Crossref] [PubMed]
- Zuercher BF, George M, Escher A, et al. Stricture prevention after extended circumferential endoscopic mucosal resection by injecting autologous keratinocytes in the sheep esophagus. Surg Endosc 2013;27:1022-8. [Crossref] [PubMed]
- Yamaguchi N, Isomoto H, Kobayashi S, et al. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep 2017;7:17460. [Crossref] [PubMed]
- Hochberger J, Koehler P, Wedi E, et al. Transplantation of mucosa from stomach to esophagus to prevent stricture after circumferential endoscopic submucosal dissection of early squamous cell. Gastroenterology 2014;146:906-9. [Crossref] [PubMed]
- Liao Z, Liao G, Yang X, et al. Transplantation of autologous esophageal mucosa to prevent stricture after circumferential endoscopic submucosal dissection of early esophageal cancer (with video). Gastrointest Endosc 2018;88:543-6. [Crossref] [PubMed]
- Lam AK. Updates on World Health Organization classification and staging of esophageal tumors: implications for future clinical practice. Hum Pathol 2021;108:100-12. [Crossref] [PubMed]
- Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. Am J Gastroenterol 2012;107:850-62; quiz 63. [Crossref] [PubMed]
- Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann Surg 2011;254:67-72. [Crossref] [PubMed]
- Schölvinck D, Künzli H, Meijer S, et al. Management of patients with T1b esophageal adenocarcinoma: a retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc 2016;30:4102-13. [Crossref] [PubMed]
- Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch 2005;446:497-504. [Crossref] [PubMed]
- Manner H, Pech O, Heldmann Y, et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc 2015;29:1888-96. [Crossref] [PubMed]
- Künzli HT, Belghazi K, Pouw RE, et al. Endoscopic management and follow-up of patients with a submucosal esophageal adenocarcinoma. United European Gastroenterol J 2018;6:669-77. [Crossref] [PubMed]
- Liu L, Hofstetter WL, Rashid A, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol 2005;29:1079-85.
- Ishihara R, Oyama T, Abe S, et al. Risk of metastasis in adenocarcinoma of the esophagus: a multicenter retrospective study in a Japanese population. J Gastroenterol 2017;52:800-8. [Crossref] [PubMed]
- Ishihara R, Goda K, Oyama T. Endoscopic diagnosis and treatment of esophageal adenocarcinoma: introduction of Japan Esophageal Society classification of Barrett's esophagus. J Gastroenterol 2019;54:1-9. [Crossref] [PubMed]
- Kamarajah SK, Phillips AW, Hanna GB, et al. Is Local Endoscopic Resection a Viable Therapeutic Option for Early Clinical Stage T1a and T1b Esophageal Adenocarcinoma?: A Propensity-Matched Analysis. Ann Surg 2022;275:700-5. [Crossref] [PubMed]
- Boys JA, Worrell SG, Chandrasoma P, et al. Can the Risk of Lymph Node Metastases Be Gauged in Endoscopically Resected Submucosal Esophageal Adenocarcinomas? A Multi-Center Study. J Gastrointest Surg 2016;20:6-12; discussion 12. [Crossref] [PubMed]
- Landau MS, Hastings SM, Foxwell TJ, et al. Tumor budding is associated with an increased risk of lymph node metastasis and poor prognosis in superficial esophageal adenocarcinoma. Mod Pathol 2014;27:1578-89. [Crossref] [PubMed]
- Almangush A, Karhunen M, Hautaniemi S, et al. Prognostic value of tumour budding in oesophageal cancer: a meta-analysis. Histopathology 2016;68:173-82. [Crossref] [PubMed]
- Davison JM, Landau MS, Luketich JD, et al. A Model Based on Pathologic Features of Superficial Esophageal Adenocarcinoma Complements Clinical Node Staging in Determining Risk of Metastasis to Lymph Nodes. Clin Gastroenterol Hepatol 2016;14:369-377.e3. [Crossref] [PubMed]
- Kudo SE, Ichimasa K, Villard B, et al. Artificial Intelligence System to Determine Risk of T1 Colorectal Cancer Metastasis to Lymph Node. Gastroenterology 2021;160:1075-84.e2. [Crossref] [PubMed]
- Kawaguchi G, Sasamoto R, Abe E, et al. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat Oncol 2015;10:31. [Crossref] [PubMed]
- Hamada K, Ishihara R, Yamasaki Y, et al. Efficacy and Safety of Endoscopic Resection Followed by Chemoradiotherapy for Superficial Esophageal Squamous Cell Carcinoma: A Retrospective Study. Clin Transl Gastroenterol 2017;8:e110. [Crossref] [PubMed]
- Suzuki G, Yamazaki H, Aibe N, et al. Endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer: choice of new approach. Radiat Oncol 2018;13:246. [Crossref] [PubMed]
- Minashi K, Nihei K, Mizusawa J, et al. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2019;157:382-90.e3. [Crossref] [PubMed]
- Yang Y, Su Y, Zhang X, et al. Esophagectomy versus definitive chemoradiotherapy for patients with clinical stage N0 and pathological stage T1b esophageal squamous cell carcinoma after endoscopic submucosal dissection: study protocol for a multicenter randomized controlled trial (Ad-ESD Trial). Trials 2020;21:603. [Crossref] [PubMed]
- Tsou YK, Lee CH, Le PH, et al. Adjuvant therapy for pT1a-m3/pT1b esophageal squamous cell carcinoma after endoscopic resection: Esophagectomy or chemoradiotherapy? A critical review. Crit Rev Oncol Hematol 2020;147:102883. [Crossref] [PubMed]
- ClinicalTrials.gov. Identifier NCT03222635. Prospective Endoscopic Follow-up of Patients With Submucosal Esophageal Adenocarcinoma (The PREFER Trial); Last updated January 9,2020.
- Stiles BM, Kamel MK, Harrison SW, et al. Neoadjuvant Therapy for Locally Advanced Esophageal Cancer Should Be Targeted to Tumor Histology. Ann Thorac Surg 2019;107:187-93. [Crossref] [PubMed]
- Takeuchi M, Kobayashi M, Hashimoto S, et al. Salvage endoscopic submucosal dissection in patients with local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Scand J Gastroenterol 2013;48:1095-101. [Crossref] [PubMed]
- Yano T, Muto M, Hattori S, et al. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy 2008;40:717-21. [Crossref] [PubMed]
- Al-Kaabi A, Schoon EJ, Deprez PH, et al. Salvage endoscopic resection after definitive chemoradiotherapy for esophageal cancer: a Western experience. Gastrointest Endosc 2021;93:888-98.e1. [Crossref] [PubMed]
- Noordzij IC, Curvers WL, Huysentruyt CJ, et al. Salvage endoscopic resection in patients with esophageal adenocarcinoma after chemoradiotherapy. Endosc Int Open 2018;6:E1126-E1129. [Crossref] [PubMed]
- Dabbagh Kakhki VR, Bagheri R, Tehranian S, et al. Accuracy of sentinel node biopsy in esophageal carcinoma: a systematic review and meta-analysis of the pertinent literature. Surg Today 2014;44:607-19. [Crossref] [PubMed]
- Uenosono Y, Arigami T, Yanagita S, et al. Sentinel node navigation surgery is acceptable for clinical T1 and N0 esophageal cancer. Ann Surg Oncol 2011;18:2003-9. [Crossref] [PubMed]
- Künzli HT, van Berge Henegouwen MI, Gisbertz SS, et al. Pilot-study on the feasibility of sentinel node navigation surgery in combination with thoracolaparoscopic lymphadenectomy without esophagectomy in early esophageal adenocarcinoma patients. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
Cite this article as: Pilonis ND, di Pietro M. The future of therapy of Barrett’s esophagus and related cancer: a narrative review. Ann Esophagus 2023;6:8.


