
Long term care after successful endoscopic therapy in Barrett’s esophagus patients: a review of literature
Introduction
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). This metaplasia progresses through low-grade (LGD) and high-grade dysplasia (HGD) eventually to EAC, which carries a 5-year prognosis of less than 20% (1). Recent data predicts an alarming increase in the incidence of EAC, especially among young males (2). Earlier recognition of BE, surveillance, and management of dysplasia at a curative stage is considered key to prevent progression to EAC.
BE surveillance programs were thus designed to identify patients with dysplasia at the earliest to offer endoscopic therapy with hope to prevent progression to invasive cancer (3). Endoscopic eradication therapy (EET) has been proven to be very effective giving successful eradication rates for neoplasia as well as metaplasia (4). Unfortunately, multiple studies have demonstrated that up to 90% of the patients with EAC are detected outside of a surveillance program (5). While this raises several questions regarding the utility of surveillance and capturing appropriate patients effectively in a primary care setting, most patients entering the surveillance program are found to have dysplasia at an early stage, amenable for endoscopic therapy alone, and had better overall outcomes. However, there is limited information on follow-up and care after achieving complete eradication of all intestinal metaplasia (CE-IM), i.e., success of the EET. Also, there is a lack of consensus regarding factors determining success and follow up protocol then onwards.
In this review, we will briefly discuss the goals of EET, immediate and delayed complications, recurrences, and long-term management of patients after the CE-IM.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-86/rc).
EET
EET encompasses any endoscopic therapy aimed at eradication of BE related dysplasia. This includes resection and different forms of ablation therapies as demonstrated in Table 1 with rates of efficacy and adverse events.
Table 1
| Author, year | Modality/indication | Studies | Complication | Recurrence |
|---|---|---|---|---|
| Resective modalities | ||||
| Dan 2019 (6) | EMR—EMR Cap vs. MBM for early/pre-cancerous lesions | 5 studies; 405 patients | Lower bleeding rate (OR =0.45, 95% CI: 0.24–0.83, P=0.01) | Similar local recurrence rate (OR =0.50, 95% CI: 0.09–2.67, P=0.42) |
| Similar perforation rate (OR =0.55, 95% CI: 0.15–2.06, P=0.37) | ||||
| Similar stricture rate (OR =0.77, 95% CI: 0.10–5.84, P=0.80) | ||||
| Tomizawa 2018 (7) | EMR for BE | 8 studies; 676 patients | Stricture: 37.4% | Recurrence of IM: 15.7% |
| Bleeding: 7.9% | Recurrence of neoplasia: 5.8% | |||
| Perforation: 2.3% | ||||
| Desai 2017 (8) | Focal EMR + RFA for HGD/EAC/IMC | 9 studies; 774 patients | Stricture: 10.2% | Recurrence of IM: 16.1% |
| Bleeding: 1.1% | Recurrence of dysplasia: 2.6% | |||
| Perforation: 0.2% | Recurrence of EAC: 1.4% | |||
| Stepwise or complete EMR | 11 studies; 751 patients | Stricture: 33.5% | Recurrence of IM: 12.1% | |
| For HGD/EAC/IMC | Bleeding: 7.5% | Recurrence of dysplasia: 3.3% | ||
| Perforation: 1.3% | Recurrence of EAC: 0.7% | |||
| Lv 2017 (9) | STER for UGI submucosal tumors | 28 studies | Subcutaneous emphysema and pneumomediastinum: 14.8% | |
| Pneumothorax: 6.1% | ||||
| Pneumoperitoneum: 6.8% | ||||
| Perforation: 5.6% | ||||
| Park 2015 (10) | ESD for GEJ cancers | 6 studies; 359 GEJ cancers | Stenosis: 6.9% | 269 curative resections: no local/metastatic recurrences |
| 90 non-curative lesions: 3 local and 2 metastatic recurrences | ||||
| Chadwick 2014 (11) | RFA vs. complete EMR in BE | 28 studies; 1,087 patients; (532-EMR and 555-RFA) | Adverse events (EMR/RFA) | EMR (23-month follow-up): 5% |
| Any short-term AE (12%/2.5%) | RFA (21-month follow-up): 6% | |||
| Esophageal strictures (38%/4%) | ||||
| Ablative modalities | ||||
| Pandey 2018 (12) | RFA in LGD | 8 studies; 619 patients | Recurrence of IM: 5.6% | |
| Recurrence of dysplasia: 9.66% | ||||
| Orman 2013 (13) | RFA for BE | 18 studies; 3,802 patients | Stricture: 5% | Recurrence of IM: 13% |
| Hamade 2019 (14) | Cryotherapy as first line for all BE | 6 studies; 282 patients | Stricture formation: 4.9% | Persistent dysplasia: 7.3% |
| Recurrence of neoplasia: 10.4/100 patient years | ||||
| Recurrence of IM: 19.1/100 patient years | ||||
| Mohan 2019 (15) | Liquid nitrogen cryotherapy for all BE (as a primary modality or in combination) | 9 studies; 386 patients | Any AE: 4.7% | Any BE recurrence: 12.7% |
| Visrodia 2018 (16) | Cryotherapy for persistent IM after RFA | 11 studies; 148 patients | Any AE: 6.7% | |
EET, endoscopic eradication therapy; EMR, endoscopic mucosal resection; MBM, multiband mucosal ligation; BE, Barrett’s esophagus; RFA, radiofrequency ablation; HGD, high-grade dysplasia; EAC, esophageal adenocarcinoma; IMC, intra-mucosal carcinoma; STER, submucosal tunneling endoscopic resection; UGI, upper gastrointestinal; ESD, endoscopic submucosal dissection; GEJ, gastroesophageal junction; AE, adverse events; LGD, low-grade dysplasia; OR, odds ratio; CI, confidence intervals.
EET is being increasingly used for the management of HGD and intramucosal EAC compared to esophagectomy. A systematic review and updated analysis from the ASGE guidelines committee suggested that there was a lower rate of adverse events among patients who underwent EET compared to esophagectomy (RR 0.38; 95% CI, 0.20–0.73). However, there was no difference in survival (1, 3, or 5 years) noted between the 2 groups (RR 0.88; 95% CI, 0.74–1.04) (4). Multimodal EET is used in most cases with a combination of resection (EMR or ESD) and/or ablation modality (i.e., radiofrequency ablation, cryoablation) depending on the presence of visible lesion and therapy of remaining Barrett’s segment to achieve the goal of complete eradication of neoplasia (CE-N)/dysplasia (CE-D) (8).
Complications
Most of the literature on post-EET bleeding suggests that it is easily managed conservatively or endoscopically without significant morbidity or mortality. Complications during and after EET are less frequent in general with improvement in expertise, education, and widespread adoption of minimally invasive methods, however, there is still a risk of adverse events during or after the endoscopic therapy. The major complications that have been reported with the multi-modal EET include bleeding, strictures, and perforation with varying degrees (Table 1).
The most common immediate complications include bleeding and perforation—these appear to be lower for ablative therapies (~0–5%) compared to resective therapies (~0–10%) (8,14-16). Immediate complications can be divided into intra-procedural and post-procedural events. Intra-procedural bleeding is frequent during resection/dissection therapies and can be managed with the use of through-the-scope (TTS) clips, hemostatic forceps (Coagrasper), over the scope (OTS) clip use, use of snare tip soft coagulation, or even hemostatic powder depending on endoscopic practice and expertise. Prompt control of contamination and alternate routes of enteral nutrition (i.e., naso-jejunal feeding) or temporary parenteral nutrition would be mainstay when perforation is noticed post EMR or ESD. In certain cases, the perforation can be closed with the use of an esophageal stent or if smaller, a TTS clip or rarely OTS clip can be used as well with success (17). The involvement of a surgeon early on is important as complications including mediastinal involvement could lead to sepsis and worse outcomes. For patients presenting with bleeding after a few days, endoscopic evaluation and management are performed routinely. Patients presenting with perforation afterward will tend to have complications including mediastinitis and sepsis and likely the thoracic surgeon need to be involved at the earliest.
Delayed complications like stricture and stenosis have been reported for all techniques but the highest have been for EMR/ESD (~30%) (7,8,10). When dysphagia is reported after EET especially after multiple sessions of ablations or when a large area of BE has undergone EMR or ESD, endoscopy should be performed for evaluation of any stricture. Endoscopic dilation with a TTS balloon or bougie can be performed with efficacy and these can be repeated to a desired esophageal luminal diameter or relief of symptoms. There has been a greater focus on the prevention and management of strictures since its relatively more common and causes dysphagia leading to a higher degree of morbidity. Prophylactic endoscopic dilation and stent placement have not demonstrated any benefit in the prevention of strictures (18,19). A systematic review of steroid use (local and systemic) did suggest a 60% lesser risk of strictures as well as a decreased need for endoscopic dilation (20). There have been smaller single-center studies that have examined anti-fibrotic agents like mitomycin C, N-acetylcysteine, and botulinum toxin type A and shown some benefit (21-23). However larger randomized trials will be needed for vetting these agents for widespread use.
Compliance and ongoing use of antisecretory medications for adequate control of acid reflux are also very important to prevent the impact of acid reflux onto resected or ablated mucosa that would drive aggravation of inflammation, stenosis, and recurrence of BE (24,25).
Follow-up and surveillance
Once CE-IM is achieved, the goal is to maintain the complete remission of all neoplasia and IM by detecting and managing recurrences, if any, at the earliest. Close follow up and surveillance endoscopy with a meticulous exam is essential. During surveillance endoscopy, a meticulous, high quality, high definition white light exam, preferably with adjunctive use of electronic chromoendoscopy modality (i.e., NBI or similar) examining each cm. length of previously known Barrett’s with targeted biopsies of any suspicious lesion and random biopsies of the entire segment of previously known BE should be performed.
It must be pointed out that the current practice is based on expert-opinion-based guidelines (3) and scientific literature around this concept is still evolving. There are no unanimous practice parameters established for follow up and care after completion of EET. Historically, removal of all BE was confirmed with the endoscopic absence of salmon-colored mucosa. However, it is well documented that it is imperative to confirm the histological absence of all IM also before stopping EET. It is not entirely clear if completion of EET and achievement of CE-IM should be defined after at least 1 or 2 surveillance endoscopies showing complete absence of IM on biopsies. A recent meta-analysis suggested a higher rate of recurrence of IM after 1 vs. 2 session-defined CE-IM (26). Declaring CE-IM after 2 sets of negative endoscopies and biopsies make reasonable sense but there are practical issues and there is lack of prospective data to support this practice.
Current guidelines recommend continued annual or biennial surveillance with 4-quadrant biopsies every 1–2 cm of the original BE (neo-squamous) segment and targeted sampling of any visible areas (27,28). However, the optimal endoscopic surveillance protocol is yet to be defined and detailed data on the performance of the current methods to capture early recurrence are limited.
A recent study reporting 50 recurrences (29) from a multicenter registry of EET suggested that the majority of recurrence were detected by random biopsy sampling in the distal esophagus. The authors also proposed a modified biopsy protocol with a targeted sampling of visible lesions followed by random biopsy sampling within 2 cm of the neo-squamocolumnar junction (NSCJ) and cardia. However, such a protocol would miss about at least 20% of the esophageal recurrences that we have noted in the literature.
In our opinion, good surveillance should follow a general pattern as shown in Figure 1. Once EET is performed and CE-IM has been confirmed after 2 endoscopies with systematic biopsies, surveillance should be performed at 3, 6 and 12 months and annually after for those lesions that were HGD/EAC at baseline and 1 and 3 years for baseline LGD lesions. The biopsy protocol for surveillance and confirmation of CE-IM should include the tubular esophagus, cardia (within 5–10 mm below the anatomic GEJ)—targeted biopsies from any visible abnormality and random biopsies from visually normal areas of the original BE segment. Documentation is another key component of quality surveillance and should include—the original extent of BE, location of the biopsies as well as the description of the lesion (visible/non-visible). This will give us a better picture over the long term about outcomes and help refine our strategies.
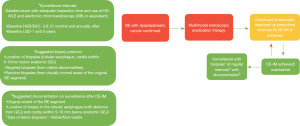
While the longest follow-up of patients after EET in systematic reviews is around 8–9 years (30) and the data shows that the majority of the recurrences would occur in the first 1–2 years (31), there has still not been any recommendation about when to stop surveillance. This is understandable since it would take a randomized controlled study with a long follow-up duration making such an undertaking less feasible and concerning. So, while the finish line after diagnosis of BE and EET appears to be achieving CE-IM, the true finish line for a patient is still at best murky since he/she will need continued surveillance for life as it stands today. We suggest decision on long term surveillance strategy to be based on discussion between the GI provider and the patient explaining utility of ongoing surveillance endoscopies to derive at an informed decision based on the understanding of risks and benefits involved and overall health status of the patient in absence of a consensual guideline recommendation and need for high quality data.
GERD and bile acid reflux
A key strategy in achieving and maintaining CE-IM after EET has been the optimal control of gastroesophageal reflux (GERD) (24). Studies have shown that GERD, a risk factor for BE when not optimally treated has resulted in the need for additional EET treatments and increased persistence of IM (25,32). Thus, proton pump inhibitor (PPI) used to effectively control GERD is essential in the management of dysplastic BE before and after EET. Additionally, role of bile acid reflux in the progression of BE to EAC has also been proposed with lack of targeted therapy for these group of patients currently (33). Animal models have demonstrated an inflammation based pathology cascade that leads to the occurrence and progression of BE (34). Further controlled studies to evaluate this will be necessary.
Recurrence of disease after CE-IM
Despite the meticulous surveillance and medical management with PPI, recurrence after EET and CE-IM is documented well in the literature. Studies have shown recurrence rates of 15–16% for IM and 3–5% for dysplasia (LGD/HGD/EAC) (31). A recent prospective study demonstrated that the rate of recurrence peaked at 1–2 years after CE-IM (31). A recently performed systematic review of 21 studies with 2,921 patients over a follow-up duration of 9,451 patient-years by our group looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus including NSCJ/Cardia (30). Of those that occur in the esophagus, about 80% of them are in the distal 2 cm. Another interesting finding was that only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic (not just random) biopsies.
Once recurrence is detected and confirmed as only IM or dysplasia, management should focus on complete removal of all Barrett’s at the earliest if feasible. Those with intramucosal cancer and HGD can be treated with EMR or ESD with ablation for remaining flat dysplasia and IM in most cases. Invasive cancer should be referred to a multidisciplinary team for review and management. Those with flat dysplasia should have a dedicated effort to find the area and treat with ablation or resection when appropriate. Recurrent IM should be treated with ablation and followed up closely. There is no defined protocol or criteria for treatment of recurrence post-CE-IM, however, most recurrences can be treated endoscopically effectively.
Novel techniques and modalities
Optically enhanced endoscopic techniques like narrow band imaging (35,36) and confocal laser microscopy (37) have been evaluated for detection of BE with promising but mixed results. Optical coherence tomography is another modality that has shown early promising benefit but studies confirming its role are awaited and widescale adoption of such a method is questionable due to the skillset required to use it in daily practice (38). Wide area transepithelial sampling showed incremental yield for dysplasia detection (39) but its role has not been examined in the detection of the recurrence. Novel biomarker like p53 immunostaining has been proposed as an alternate to histologic assessment of dysplasia (40). There have been a studies that have looked at tools like Cytosponge—capsule containing sponge tethered to a string. This in combination with tissue trefoil factor 3, protein biomarkers (p53, c-Myc, Aurora kinase A) or methylation biomarkers (MYOD1, RUNX3) were able to stratify patients with BE into low, moderate or high risk of progression (41-43). While these modalities look promising, none of them have been evaluated in the setting of post EET surveillance and further studies designed to look at their performance in this setting will be needed.
Future directions
With the rising incidence of BE, there has been increasing use of EET with successful eradication of all BE. This cohort of patients is growing with EET in practice for over 20 years. Standardized definitions for complete eradication, surveillance intervals, detection of recurrence, and long term follow up need to be established based on a large-scale high-quality data. In absence of long-term durable efficacy rates of multimodal EET, meticulous endoscopic surveillance should be continued with a formal discussion of benefits and risks involved with patients, and adherence to anti-reflux medications should be emphasized. There is a definite need to examine durable remission rates and protocols to intensify and loosen annual rigorous surveillance endoscopies per risk groups and benefits involved. It would be also interesting to see if the addition of artificial intelligence to detect any recurrence and management has any substantial advantage to remove the subjective bias. Finally, with refinements in minimally invasive anti-reflux procedures, it would be worth exploring in the future if such could be offered and has a role in sustained remission of all BE.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Endoscopic Therapy for Barrett’s Esophagus”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-86/rc
Peer Review File: Available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-86/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aoe.amegroups.com/article/view/10.21037/aoe-20-86/coif). The series “Endoscopic Therapy for Barrett’s Esophagus” was commissioned by the editorial office without any funding or sponsorship. MD served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Then EO, Lopez M, Saleem S, et al. Esophageal Cancer: An Updated Surveillance Epidemiology and End Results Database Analysis. World J Oncol 2020;11:55-64. [Crossref] [PubMed]
- Sharma P, Shaheen NJ, Katzka D, et al. AGA Clinical Practice Update on Endoscopic Treatment of Barrett’s Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020;158:760-9. [Crossref] [PubMed]
- Wani S, Qumseya B, Sultan S, et al. Endoscopic eradication therapy for patients with Barrett’s esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907-931.e9. [Crossref] [PubMed]
- Sabel MS, Pastore K, Toon H, et al. Adenocarcinoma of the esophagus with and without Barrett mucosa. Arch Surg Chic Ill 1960 2000;135:831-5; discussion 836.
- Dan X, Lv XH, San ZJ, et al. Efficacy and Safety of Multiband Mucosectomy Versus Cap-assisted Endoscopic Resection For Early Esophageal Cancer and Precancerous Lesions: A Systematic Review and Meta-Analysis. Surg Laparosc Endosc Percutan Tech 2019;29:313-20. [Crossref] [PubMed]
- Tomizawa Y, Konda VJA, Coronel E, et al. Efficacy, Durability, and Safety of Complete Endoscopic Mucosal Resection of Barrett Esophagus: A Systematic Review and Meta-Analysis. J Clin Gastroenterol 2018;52:210-6. [Crossref] [PubMed]
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017;85:482-495.e4. [Crossref] [PubMed]
- Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc 2017;31:49-63. [Crossref] [PubMed]
- Park CH, Kim EH, Kim HY, et al. Clinical outcomes of endoscopic submucosal dissection for early stage esophagogastric junction cancer: a systematic review and meta-analysis. Dig Liver Dis 2015;47:37-44. [Crossref] [PubMed]
- Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc 2014;79:718-731.e3. [Crossref] [PubMed]
- Pandey G, Mulla M, Lewis WG, et al. Systematic review and meta-analysis of the effectiveness of radiofrequency ablation in low grade dysplastic Barrett’s esophagus. Endoscopy 2018;50:953-60. [Crossref] [PubMed]
- Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245-55. [Crossref] [PubMed]
- Hamade N, Desai M, Thoguluva Chandrasekar V, et al. Efficacy of cryotherapy as first line therapy in patients with Barrett’s neoplasia: a systematic review and pooled analysis. Dis Esophagus 2019;32:doz040. [Crossref] [PubMed]
- Mohan BP, Krishnamoorthi R, Ponnada S, et al. Liquid Nitrogen Spray Cryotherapy in Treatment of Barrett’s Esophagus, where do we stand? A Systematic Review and Meta-Analysis. Dis Esophagus 2019;32:doy130. [Crossref] [PubMed]
- Visrodia K, Zakko L, Singh S, et al. Cryotherapy for persistent Barrett’s esophagus after radiofrequency ablation: a systematic review and meta-analysis. Gastrointest Endosc 2018;87:1396-1404.e1. [Crossref] [PubMed]
- Vermeulen BD, Siersema PD. Esophageal Stenting in Clinical Practice: an Overview. Curr Treat Options Gastroenterol 2018;16:260-73. [Crossref] [PubMed]
- Ezoe Y, Muto M, Horimatsu T, et al. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol 2011;45:222-7. [Crossref] [PubMed]
- Wen J, Lu Z, Yang Y, et al. Preventing stricture formation by covered esophageal stent placement after endoscopic submucosal dissection for early esophageal cancer. Dig Dis Sci 2014;59:658-63. [Crossref] [PubMed]
- Wang W, Ma Z. Steroid Administration is Effective to Prevent Strictures After Endoscopic Esophageal Submucosal Dissection: A Network Meta-Analysis. Medicine (Baltimore) 2015;94:e1664. [Crossref] [PubMed]
- Gusmon-Oliveira CC, Kuboki YM, de Paulo GA, et al. Endoscopic Injection of Mitomycin C for the Treatment of Pharyngoesophageal Stenosis Refractory to Endoscopic Treatment with Dilatation in Patients Treated for Head and Neck Cancer. Gastroenterol Res Pract 2018;2018:5428157. [Crossref] [PubMed]
- Uno K, Iijima K, Koike T, et al. A pilot study of scheduled endoscopic balloon dilation with oral agent tranilast to improve the efficacy of stricture dilation after endoscopic submucosal dissection of the esophagus. J Clin Gastroenterol 2012;46:e76-82. [Crossref] [PubMed]
- Wen J, Lu Z, Linghu E, et al. Prevention of esophageal strictures after endoscopic submucosal dissection with the injection of botulinum toxin type A. Gastrointest Endosc 2016;84:606-13. [Crossref] [PubMed]
- Komanduri S. The importance of optimizing reflux control to improve outcomes after endoscopic eradication therapy for Barrett’s neoplasia [Internet]. AGA Perspectives 2019 [cited 2020 Oct 15]. Available online: http://agaperspectives.gastro.org/the-importance-of-optimizing-reflux-control-to-improve-outcomes-after-endoscopic-eradication-therapy-for-barretts-neoplasia/
- Komanduri S, Kahrilas PJ, Krishnan K, et al. Recurrence of Barrett’s Esophagus is Rare Following Endoscopic Eradication Therapy Coupled With Effective Reflux Control. Am J Gastroenterol 2017;112:556-66. [Crossref] [PubMed]
- Sawas T, Iyer PG, Alsawas M, et al. Higher Rate of Barrett’s Detection in the First Year After Successful Endoscopic Therapy: Meta-analysis. Am J Gastroenterol 2018;113:959-71. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111:30-50; quiz 51. [Crossref] [PubMed]
- Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019;90:335-359.e2. [Crossref] [PubMed]
- Omar M, Thaker AM, Wani S, et al. Anatomic location of Barrett’s esophagus recurrence after endoscopic eradication therapy: development of a simplified surveillance biopsy strategy. Gastrointest Endosc 2019;90:395-403. [Crossref] [PubMed]
- Duvvuri A, Chandrasekar VT, Narimiti A, et al. Mo1273 location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: a systematic review and critical analysis of the published literature. Gastrointest Endosc 2020;91:AB410-1. [Crossref]
- Wani S, Han S, Kushnir V, et al. Recurrence is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol 2020;18:2609-2617.e2. [Crossref] [PubMed]
- Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576-81. [Crossref] [PubMed]
- Masaoka T, Suzuki H. Does Bile Reflux Influence the Progression of Barrett’s Esophagus to Adenocarcinoma? (Gastroenterology 2013;145:1300-1311). J Neurogastroenterol Motil 2014;20:124-6. [Crossref] [PubMed]
- Sun D, Wang X, Gai Z, et al. Bile acids but not acidic acids induce Barrett’s esophagus. Int J Clin Exp Pathol 2015;8:1384-92. [PubMed]
- Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest Endosc 2006;64:167-75. [Crossref] [PubMed]
- Hamamoto Y, Endo T, Nosho K, et al. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett’s esophagus. J Gastroenterol 2004;39:14-20. [Crossref] [PubMed]
- Wallace MB, Crook JE, Saunders M, et al. Multicenter, randomized, controlled trial of confocal laser endomicroscopy assessment of residual metaplasia after mucosal ablation or resection of GI neoplasia in Barrett’s esophagus. Gastrointest Endosc 2012;76:539-547.e1. [Crossref] [PubMed]
- Cobb MJ, Hwang JH, Upton MP, et al. Imaging of subsquamous Barrett’s epithelium with ultrahigh-resolution optical coherence tomography: a histologic correlation study. Gastrointest Endosc 2010;71:223-30. [Crossref] [PubMed]
- Vennalaganti PR, Kaul V, Wang KK, et al. Increased detection of Barrett’s esophagus-associated neoplasia using wide-area trans-epithelial sampling: a multicenter, prospective, randomized trial. Gastrointest Endosc 2018;87:348-55. [Crossref] [PubMed]
- Kastelein F, Biermann K, Steyerberg EW, et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett’s oesophagus. Gut 2013;62:1676-83. [Crossref] [PubMed]
- Kadri SR, Lao-Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341:c4372. [Crossref] [PubMed]
- Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med 2015;12:e1001780. [Crossref] [PubMed]
- Ross-Innes CS, Chettouh H, Achilleos A, et al. Risk stratification of Barrett’s oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017;2:23-31. [Crossref] [PubMed]
Cite this article as: Srinivasan S, Desai M. Long term care after successful endoscopic therapy in Barrett’s esophagus patients: a review of literature. Ann Esophagus 2022;5:8.


