
Pepsin as a biomarker for self-diagnosing reflux associated symptoms in UK and USA individuals
Introduction
Reflux is defined as the retrograde movement of gastric contents including the digestive enzyme pepsin, refluxing into the esophagus and beyond (1). Reflux is a common clinical complaint within the UK and USA population, in many cases affecting individuals on a daily basis. Procedures for diagnosing reflux include dual-probe 24-hour pH-monitoring, white light endoscopy and more recently multichannel intraluminal impedance/pHmetry (MII-pH) which are invasive time consuming procedures with poor sensitivity and often with limited availability (2-4), leaving individuals to seek a quick and easy reliable diagnostic method to identify their reflux associated symptoms. Reflux symptoms can typically present as heartburn, regurgitation, or atypically as chronic cough or hoarseness (5,6). These episodes often cause troublesome symptoms for an individual affecting their quality of life (QOL), leading to complications and development of other reflux related diseases including gastroesophageal reflux disease (GERD), laryngopharyngeal reflux (LPR) and also heighten the risk of severe esophageal damage (1,7). The prevalence of these diseases is becoming greater, the increase of GERD in western countries is estimated to affect around 20–40% of the population (8). An estimated 60 million Americans experience GERD like symptoms at least once a month (9), with prevalence rates of 8.1% to 27.8% in North America (10). Heartburn, as a symptom experienced on average once a month is estimated to affect around 25% of the westernized general population (11).
It has been shown that pepsin detection within a sputum and/or saliva sample is a sensitive, non-invasive diagnostic method for proximal reflux of gastric contents (12). Using pepsin as a biomarker for reflux led to the development of Peptest, which is a non-invasive, rapid diagnostic procedure used to identify pepsin within a saliva sample (8). Peptest uses lateral flow technology containing two unique human pepsin monoclonal antibodies, one used to detect pepsin and the other to capture pepsin (13,14). A PepCube reader is used to measure and determine pepsin concentration in ng/mL.
This study involved 793 self-referral individuals from the USA across 41 states including Minnesota, Texas and Massachusetts and a further 739 individuals from the UK all experiencing reflux like symptoms.
The aim of this study was to demonstrate how pepsin can be used as a biomarker for reflux by using Peptest as a first-line non-invasive diagnostic test for self-diagnosis of reflux related symptoms.
We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/aoe-20-79).
Methods
Recruitment
A total of 1,532 (793 USA, 739 UK) individual’s saliva samples were analysed in this study, all individuals were experiencing reflux related symptoms with no clinical diagnosis confirmed. These individuals were made up of 754 males and 778 females with a mean age of 52 years, ages ranged from one month to 90 years.
Sample collection
All individuals were instructed to provide three saliva samples of 1 mL volume, the first on waking prior to eating and cleaning their teeth, maintaining an upright position, the other two samples were provided either post-prandial or post-symptom. The post-prandial samples were collected one hour after the main meal and post-symptom samples were collected within 15 minutes of experiencing reflux like symptoms. All individuals were advised to avoid any medication to treat reflux 48 hours before providing their samples.
All saliva samples (1 mL volume) were collected into 30 mL collection tubes containing 0.5 mL, 0.01 M citric acid and stored at 4 °C prior to pepsin analysis. All samples were analysed for pepsin content within a maximum time period of 7 days from saliva collection.
Sample analysis
Collection tubes containing the saliva samples were centrifuged at 4,000 rpm for 5 minutes until a clear supernatant layer was visible. If no supernatant layer was visible the samples were centrifuged again, and 80 µL from the surface layer of the supernatant sample was drawn up into an automated pipette. The 80 µL sample was transferred to a micro-centrifuge tube containing 240 µL of migration buffer solution (pH 8.2). The sample solution was vortex mixed for 10 seconds. A second pipette was used to transfer 80 µL of the sample into the circular well of the lateral flow device (LFD) (Figure 1) containing two unique human monoclonal antibodies; one to detect and the other to capture pepsin in the saliva samples (Peptest, RD Biomed Limited, UK).
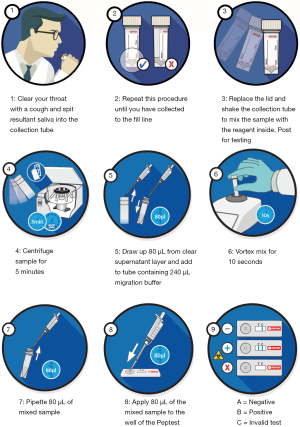
Fifteen minutes after introducing the sample for pepsin analysis into the LFD well, the Peptest LFD was placed into the PepCube reader to determine the intensity of the pepsin test line (ng/mL). Pepsin concentration of ≥25 ng/mL was considered positive, a limit determined by the developer and manufacturer of Peptest (RD Biomed Limited, UK).
Statistical analysis
All individual’s data were anonymised prior to the completion of this study and the analysis performed. Unpaired t-tests were completed between each sample collection time point and age group using the statistical package GraphPad Prism 8.2.0 (GraphPad Software, San Diego, CA 92018, USA). P values <0.05 were considered statistically significant. The mean was displayed as ± standard error of the mean (SEM).
This study is a retrospective study and conducted in individuals seeking a self-referral. These were not patients recruited to take part in a clinical trial. Therefore, the ethical approval of this study was exempt. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all the individual study participants. All participant data were anonymized prior to the final analysis of the data.
Results
A total of 1,532 UK and USA self-referral individuals provided saliva samples for pepsin/Peptest analysis. This included 793 USA individuals and 739 UK individuals with a gender split of 754 males (49%) and 778 females (51%). The mean age of all individuals was 52 years, with an age range of 1 month to 90 years. All self-referral individuals were instructed to produce three saliva samples for pepsin analysis. Seven hundred and seventy-five individuals provided three saliva samples, a further 424 individuals provided two samples and 333 provided one saliva sample. A total of 3,506 saliva samples were analysed for salivary pepsin using Peptest.
Out of the 1,532 individuals tested a total of 981 (64%) individuals produced one or more pepsin positive saliva samples, the remaining 551 (36%) individuals produced pepsin negative saliva samples. The overall mean pepsin concentration for all positive samples from the UK population was 205±4.8 ng/mL and a slightly lower mean of 188.4±4.9 ng/mL was observed from the USA population. All the pepsin positive samples from the UK (n=489) and USA (n=492) population were split into male and female groups and the mean pepsin concentration was compared. Figure 2 shows the mean pepsin concentration in male and females from the UK population compared to the USA population. The highest pepsin concentration was seen in the female UK population at 212.9±9.9 ng/mL with the lowest (184.4±9.8 ng/mL) seen in the USA male population. Overall, the UK population produced higher pepsin concentrations compared to the USA. No statistical difference was observed.
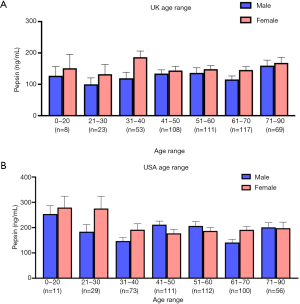
A further breakdown of all self-referral individuals who produced a pepsin positive saliva sample (UK =489, USA =492) was carried out. Individuals were split into groups regarding their age range and sex, represented in Figure 3A,B, Figure 3A shows the pepsin concentration of the UK population and shows a higher pepsin prevalence in females aged between 31–40 years. It also shows individuals in the age range of 41–70 years are seeking self-diagnosis more than other age groups, interestingly the same trend is seen in the USA (Figure 3B). Figure 3B represents the USA population which shows higher pepsin concentrations in the age range of 0–30 years. No statistical difference was seen in pepsin concentration between the age groups in the UK and USA.
An analysis was conducted on self-referral individuals who produced all three saliva samples with pepsin positive values. This consisted of 152 (31%) self-referral individuals in the UK and 144 (29%) individuals in the USA. Individuals with two saliva samples pepsin positive in the UK was 185 (38%) and in the USA 178 (36%). The number of individuals with one pepsin positive sample in the UK was 152 (31%) and in the USA 170 (35%). Figure 4 shows the pepsin analysis of 888 saliva samples collected at each different time point displaying a mean pepsin concentration comparing the UK and USA population.
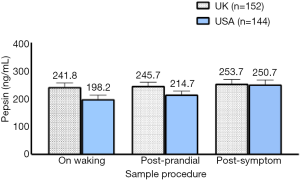
The highest pepsin levels were seen in both the UK (253.7±14.5 ng/mL) and USA (250.7±15.9 ng/mL) population in a post-symptom saliva sample. There was no significant difference in pepsin concentration between the sample collection times in either the UK or USA population.
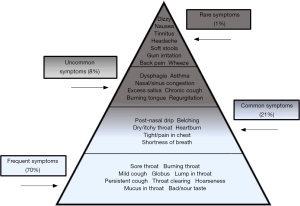
All individuals were asked to record their symptoms before producing a saliva sample. Figure 5 represents the various symptoms reported by self-diagnosing individuals from a combined UK and USA population. Severity of symptoms are illustrated in a severity iceberg, see Figure 6 and showed at least 70% of individuals from both the UK and USA population presented with throat symptoms such as burning throat, globus and throat clearing with only 1% of individuals presenting with symptoms such as dizziness and headache.
Discussion
Reflux symptoms and associated diseases have increased in prevalence worldwide. In the United States GERD is regarded as the most common gastrointestinal diagnosis linked with outpatient clinic visits (15), with a staggering 8.9 million visits alone in 2009 (16). A high prevalence of GERD is also problematic for primary care in the UK (17), LPR has also risen with otolaryngology consultations seeing up to 10% of patients with LPR (18). Koufman et al. reported that 40% of patients in the USA with LPR had episodes of classic reflux symptoms (19). Several studies have demonstrated pepsin to be a good biomarker for GERD and LPR in patients (20-24).
An individual can present with a variety of reflux symptoms giving the potential for reflux symptoms to overlap with other gastrointestinal disorders, making an accurate diagnosis difficult. Procedures used to diagnose reflux involve invasive and time-consuming methods, resulting in a painful and stressful experience for individuals to endure. Currently the waiting time for many of the invasive procedures (25) used to diagnose reflux symptoms becomes a concern for individuals, leading to a preferred self-referral diagnostic and self-management approach to their reflux symptoms.
A non-invasive method of diagnosing reflux was developed (Peptest), which allows for a quick, convenient and easy way of diagnosing reflux with just the collection of three saliva samples collected in the comfort of an individual’s home environment. Peptest has a rapid turnaround for results making the diagnostic method much more efficient compared to other clinical procedures. Peptest was first launched in the UK in 2010 and later introduced to the USA in 2020 as PepsinCheck. The uniqueness of the LFD uses salivary pepsin as a biomarker and has been validated and reviewed in several papers presenting with an overall sensitivity of at least 85% and 60% to 100% specificity (2,8,18,26). Pepsin is a digestive enzyme only produced in the stomach, therefore its presence within saliva is a good indicator of reflux (27).
The self-diagnosing individuals were not seeking assistance of a health professional or wanting medical intervention. They were looking to self-diagnose their symptoms, which are categorised in Figure 5. Many of the symptoms experienced were related to the throat, upper airways, mouth, chest and general gastrointestinal symptoms. However, what is unknown is the length of time the individual had suffered with their symptoms prior to seeking a self-diagnosis. A question could be asked why do they seek to self-diagnose? These individuals may prefer a more relaxed, convenient and low-cost method with no side effects and minimal effort required to fit into their busy day to day life. Some of these individuals may already be self-medicating but only experiencing partial symptom relief, while others might not want to use pharmaceutical products and in turn become reliant on strong medication. Alternatively, they want to confirm whether their symptoms are due to reflux and then seek advice on diet and lifestyle from a professional to help alleviate the symptoms.
The severity of symptoms presented by the individuals seeking self-diagnosis is illustrated in the form of a severity iceberg, showing that the majority of symptoms (70%) experienced by individuals are associated with the throat, for example sore throat, burning throat and lump in throat being popular frequent symptoms amongst all individuals from both the UK and USA. The USA individuals used the term post-nasal drip more than UK individuals, mainly due to post-nasal drip not being routinely used as a descriptive term in the UK. Rarer symptoms reported (1%) included headache, back pain, soft stools and gum irritation. It can be speculated that individuals are more likely to seek help with symptoms such as sore/burning throat, lump in throat, globus sensation and hoarseness. They also may worry their symptoms could be the start of a more serious gastrointestinal disease and require a speedy diagnosis. The symptoms some individuals presented in this study are commonly linked with gastrointestinal diseases (1,28,29) and interestingly, individuals who tested pepsin positive presented with symptoms like globus, hoarseness, throat clearing and cough which are all symptoms of GERD (15,30,31).
The 1,532 individuals in this study included 793 from the USA and 739 individuals from the UK, with an age range of 1 month to 90 years an overall mean age of 52 years, suggesting more middle-aged individuals are suffering and seeking to self-diagnose their reflux symptoms. The UK produced a higher mean pepsin concentration of 205 ng/mL compared to a lower mean of 188.4 ng/mL pepsin concentration from the USA. All individuals from the UK and USA were instructed to provide three saliva samples for pepsin analysis, the data showed higher pepsin concentration in post-symptom and post-prandial collection times compared to a morning saliva collection. The comparison of pepsin concentration in male and females from both the UK and USA showed a higher pepsin concentration in females than males. Both the USA and UK had more individuals in the age range of 41–90 years, highlighting again how reflux symptoms tend to present in an older age range.
This study shows the need for a self-referral diagnostic test for diagnosing reflux like symptoms, and also gives another route for individuals who have not been clinically diagnosed or in fact misdiagnosed by their clinical practitioner. Peptest is shown to be a rapid first-line diagnostic test for reflux disease. The main limitation in this review was the evaluation of only two western populations, the UK and USA. It would be interesting to compare self-diagnosing individuals in other European countries with individuals from Asia, for example China and Japan, and directly compare pepsin profiles.
Peptest is a first-line diagnostic test and uses salivary pepsin as a biomarker for diagnosing reflux. A large number of individuals from the UK and USA seek a self-diagnosis for their reflux like symptoms. Peptest allows individuals to access a non-invasive procedure which is fast and reliable.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Epidemiology, Biomarkers and Modelling of Gastroesophageal Reflux Disease”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/aoe-20-79
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoe-20-79
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-79). The series “Epidemiology, Biomarkers and Modelling of Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. PWD served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from Mar. 2020 to Feb. 2022. KHAB reports other (employed by) from RD Biomed Limited, outside the submitted work. JF reports other (employed by) from RD Biomed Limited, outside the submitted work. ADW reports other (employed by) from RD Biomed, outside the submitted work. PWD reports other (director) from RD Biomed Limited, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study is a retrospective study and conducted in individuals seeking a self-referral. These were not patients recruited to take part in a clinical trial. Therefore, the ethical approval of this study was exempt. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all the individual study participants. All participant data were anonymized prior to the final analysis of the data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Bortoli N, Tolone S, Frazzoni M, et al. Gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome: common overlapping gastrointestinal disorders. Ann Gastroenterol 2018;31:639-48. [Crossref] [PubMed]
- Bor S, Capanoglu D, Vardar R, et al. Validation of Peptest in Patients with Gastro-Esophageal Reflux Disease and Laryngopharyngeal Reflux Undergoing Impedance Testing. J Gastrointestin Liver Dis 2019;28:383-7. [Crossref] [PubMed]
- Sharma N, Agrawal A, Freeman J, et al. An analysis of persistent symptoms in acid-suppressed patients undergoing impedance-pH monitoring. Clin Gastroenterol Hepatol 2008;6:521-4. [Crossref] [PubMed]
- Vaezi MF, Schroeder PL, Richter JE. Reproducibility of proximal probe pH parameters in 24-hour ambulatory esophageal pH monitoring. Am J Gastroenterol 1997;92:825-9. [PubMed]
- Vaezi MF. Atypical manifestations of gastroesophageal reflux disease. MedGenMed 2005;7:25. [PubMed]
- Klimara MJ, Johnston N, Samuels TL, et al. Correlation of salivary and nasal lavage pepsin with MII-pH testing. Laryngoscope 2020;130:961-6. [Crossref] [PubMed]
- . Value of reflux diagnostic questionnaire in the diagnosis of gastroesophageal reflux disease. Chin J Dig Dis 2004;5:51-5. [Crossref] [PubMed]
- Wang YF, Yang CQ, Chen YX, et al. Validation in China of a non-invasive salivary pepsin biomarker containing two unique human pepsin monoclonal antibodies to diagnose gastroesophageal reflux disease. J Dig Dis 2019;20:278-87. [Crossref] [PubMed]
- National Heartburn Alliance. Survey 2000 Results: A Community Perspective. 2000. Accessed April 17, 2020. Available online: http://www.heartburnalliance.org/press-heartburn-survey.php
- Lee YS, Jang BH, Ko SG, et al. Comorbid risks of psychological disorders and gastroesophageal reflux disorder using the national health insurance service-National Sample Cohort: A STROBE-compliant article. Medicine (Baltimore) 2018;97:e0153 [Crossref] [PubMed]
- Lowden M, McGlashan JA, Steel A, et al. Prevalence of symptoms suggestive of extra-oesophageal reflux in a general practice population in the UK. Logoped Phoniatr Vocol 2009;34:32-5. [Crossref] [PubMed]
- Du X, Wang F, Hu Z, et al. The diagnostic value of pepsin detection in saliva for gastro-esophageal reflux disease: a preliminary study from China. BMC Gastroenterology 2017;17:107. [Crossref] [PubMed]
- Strugala V, Woodcock AD, Dettmar PW, et al. Detection of pepsin in sputum: a rapid and objective measure of airways reflux. Eur Respir J 2016;47:339-41. [Crossref] [PubMed]
- Hayat JO, Gabieta-Somnez S, Yazaki E, et al. Pepsin in saliva for the diagnosis of gastro-oesophageal reflux disease. Gut 2015;64:373-80. [Crossref] [PubMed]
- Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:267-76. [Crossref] [PubMed]
- Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179-1187.e3. [Crossref] [PubMed]
- El-Serag H, Hill C, Jones R. Systematic review: the epidemiology of gastro-oesophageal reflux disease in primary care, using the UK General Practice Research Database. Aliment Pharmacol Ther 2009;29:470-80. [Crossref] [PubMed]
- Dettmar P, Watson M, McGlashan J, et al. A Multicentre Study in UK Voice Clinics Evaluating the Non-invasive Reflux Diagnostic Peptest in LPR Patients. SN Compr Clin Med 2019;2:57-65. [Crossref]
- Koufman JA, Aviv JE, Casiano RR, et al. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg 2002;127:32-5. [Crossref] [PubMed]
- Samuels TL, Johnston N. Pepsin as a Marker of Extraesophageal Reflux. Ann otol Rhinol Laryngol 2010;119:203-8. [Crossref] [PubMed]
- Johnston N, Dettmar PW, Strugala V, et al. Laryngopharyngeal reflux and GERD. Ann N Y Acad Sci 2013;1300:71-9. [Crossref] [PubMed]
- Hayat JO, Yazaki E, Moore AT, et al. Objective detection of esophagopharyngeal reflux in patients with hoarseness and endoscopic signs of laryngeal inflammation. J Clin Gastroenterol 2014;48:318-27. [Crossref] [PubMed]
- Calvo-Henríquez C, Ruano-Ravina A, Vaamonde P, et al. Is Pepsin a Reliable Marker of Laryngopharyngeal Reflux? A Systematic Review. Otolaryngol Head Neck Surg 2017;157:385-91. [Crossref] [PubMed]
- Strugala V, Dev Bardhan K, McGlashan J, et al. Differentiation between LPR and GORD with the use of a simple non-invasive diagnostic test for reflux by detection of pepsin in expectorated saliva. 15th British Academic Conference in Otolaryngology, Liverpool 2015.
- de Bortoli N, Martinucci I, Savarino E, et al. Proton pump inhibitor responders who are not confirmed as GERD patients with impedance and pH monitoring: who are they? Neurogastroenterol Motil 2014;26:28-35. [Crossref] [PubMed]
- Bor S, Capangolu DS, Yildirim E, et al. The validation of peptest a new non-invasive technology for the diagnosis of laryngopharyngeal reflux (LPR). Gut 2012;61:A83.
- Ocak E, Kubat G, Yorulmaz I. Immunoserologic Pepsin Detection in The Saliva as a Non-Invasive Rapid Diagnostic Test for Laryngopharyngeal Reflux. Balkan Med J 2015;32:46-50. [Crossref] [PubMed]
- Bardhan KD, Strugala V, Dettmar PW. Reflux revisited: advancing the role of pepsin. Int J Otolaryngol 2012;2012:646901 [Crossref] [PubMed]
- Subramanian CR, Triadafilopoulos G. Refractory gastroesophageal reflux disease. Gastroenterol Rep (Oxf) 2015;3:41-53. [Crossref] [PubMed]
- Wang YJ, Lang XQ, Wu D, et al. Salivary Pepsin as an Intrinsic Marker for Diagnosis of Sub-types of Gastroesophageal Reflux Disease and Gastroesophageal Reflux Disease-related Disorders. J Neurogastroenterol Motil 2020;26:74-84. [Crossref] [PubMed]
- Özdemir P, Erdinç M, Vardar R, et al. The Role of Microaspiration in the Pathogenesis of Gastroesophageal Reflux-related Chronic Cough. J Neurogastroenterol Motil 2017;23:41-8. [Crossref] [PubMed]
Cite this article as: Boulton KHA, Fisher J, Woodcock AD, Dettmar PW. Pepsin as a biomarker for self-diagnosing reflux associated symptoms in UK and USA individuals. Ann Esophagus 2021;4:23.




