
Role of surgery in the management of synchronous metastatic esophageal cancer
Introduction
Esophageal cancer was newly diagnosed in 572,000 patients in 2018, and 508,000 died from this disease the same year (1). Esophageal cancer remains at the eighth place in the worldwide cancer incidence ranking. It is the sixth cause of cancer-related death. There are two histologic subtypes: squamous cancer cell (SCC) and adenocarcinoma (AC). SCC is predominant in Non-Western countries and more frequently affects the upper and middle third of the esophagus.
AC is more prevalent in Europe and arises more commonly from the distal third of the esophagus and the esogastric junction. In contrast to SCC, AC has still a rising incidence (2).
Surgery is often integrated into a multimodal treatment aimed at cure of the patient (3). However, 50% of patients are unsuitable for surgery at the time of presentation (4). It can be the result of an unresectable tumor (invasion of adjacent structures), distant metastasis or the patient is unfit for surgery (comorbidities or low-performance status) (5). Delay in referral to an expert center can also affect the probability to receive curative treatment. A study from the Netherland showed a strong relation between hospital of diagnosis and chance of referring patients with esophageal cancer for curative treatment (6).
The combination of late diagnosis and advanced age lead to the application of definitive chemoradiotherapy. Long term (5-years) survival increased from 0–14% to 20–25% (7-9) in patients unfit for surgery.
By contrast, despite the development of new chemotherapy agents, metastatic disease is a significant burden associated with poor long-term survival. Esophageal cancer with distant metastases has a lower 5-year survival rate than many other malignancies (10) including breast cancer (26.3%) (11) and colorectal cancer (13.5%) (12). When metastatic disease is present at initial diagnosis, the median survival is one year (13). A distinction should be made between synchronous metastasis (metastasis occurring at the same time of the initial diagnosis) and metachronous (which develop consequently, generally after the initiation of treatment of the primary lesion).
The place of surgery in metastatic esophageal cancer has been limited until recently. Effective chemotherapy, such as the FLOT-regimen (fluorouracil, leucovorin, oxaliplatin, and docetaxel), may achieve good local control and reduce the size of the metastasis. Hence, induction chemotherapy may be applied to select patients that may benefit from a surgical resection.
Metastases in esophageal cancer
The poor outcome of patients with esophageal cancer is caused by the tendency for metastases to occur already at an early stage cancer and the lack of effective treatment. The localization of distant metastasis in esophageal cancer was studied from 9,000 patients using the US Surveillance Epidemiology and End Results (SEER) database. A difference in pattern of metastases was found between the two main histologic types: a higher rate of lung metastases in SCC tumors and a higher rate of liver, bone, and brain metastases in AC. Table 1 summarizes the rate and frequency of metastases according to site.
These results were confirmed in an Asian study (14) with liver metastases were more common in patients with AC, whereas lung metastases were more frequent in patients with SCC. Distant lymph node and bone metastases had a similar rate for SCC and AC. One explanation for the different patterns of metastases may be tumor localization: AC is more frequent in the lower part of the esophagus. This distal localization could have preferential hepatic venous drainage.
The brain is a rare site of metastases (1–5%). Advances in imaging modalities and more extensive imaging may explain the increased incidence seen in recent years: incidence of 6.2% in patients with AC and 2.1% for SCC. This parallels the pattern of metastases in with non-small cell lung cancer: brain metastases are twice as high in patients with AC compared to SCC (15). The number of metastatic sites appeared to have an effect on the overall survival with a hazard ratio of 1.49 (95% CI: 1.23 to 1.8) when two metastatic sites were involved (16). However, surgery could be an option when the metastases affect only a limited number of organs or only a small portion.
Hellman and Weichselbaum first described the concept of oligometastases in 1995 (17).
The most accepted definition of oligometastatic disease is the presence of fewer than five metastases. Individual authors challenge this definition. Some suggest using it to describe 3–5 metastatic foci in a single organ, while other metastases affecting 1 or 2 organs with 1or 2 foci per organ (18). The cut-off size for one is typically less than 3 cm (19).
This category of patients could benefit from an aggressive multimodal treatment with systemic chemo(radio)therapy followed by surgery. Surgical resection for liver metastases in colorectal cancer, lung metastases in soft tissue sarcoma, and cerebral metastases in lung cancer is accepted practice currently (20).
The difficulty of metastatic disease is to offer the appropriate treatment without futile management. Surgery in an aggressive tumor will accelerate the propagation by delaying systemic treatment. However, if surgery can control all metastatic foci with less morbidity and allow the systemic treatment to be started quickly, it could offer a survival advantage.
Gastric cancer and surgery for M1 disease
Because there is a limited data on the role of surgery in metastatic esophageal cancer, there is some evidence for its benefit derived from gastric cancer. No actual guidelines recommend surgery for metastases. Liver resections for gastric metastases however are feasible in less than 1% of the cases. When patients are well selected, liver metastasis in gastric cancer can be treated with moderate outcomes. Five-year survival after surgical R0 resection is reported to be as high as 27–37% (21,22). Some 5,185 cases from the SEER database (23) with distant metastases were retrospectively analyzed. Survival improvement was noted when surgical resection was performed for the primary tumor and the metastases. An aggressive treatment, even in disseminated cases, can be beneficial for the patient.
The place of surgery for metastatic gastric cancer was analyzed by the multicentric, randomized, Asian REGATTA trial (24). The authors included patients with advanced gastric cancer with a single metastasis site (liver, peritoneal, lymph nodes). Patients were randomly assigned to chemotherapy alone or gastrectomy and adjuvant chemotherapy. There was no difference in overall survival at two years between the two groups (31.7% in chemotherapy alone, 25.1% in surgery and chemotherapy group) and in median overall survival (16.6 months in chemotherapy alone and 14.3 months in surgery and chemotherapy). The authors concluded that gastrectomy followed by chemotherapy did not offer a survival advantage and should not be recommended.
However, two main remarks can be opposed for this trial: first, the lymphadenectomy was restricted to D1, which is insufficient in terms of oncological resection criteria. Second, there was no resection of the metastatic lesions.
An observational study, FLOT-3 (25), confirmed the feasibility of combined chemotherapy and surgery for gastric and esophagogastric junction patients with oligometastic disease.
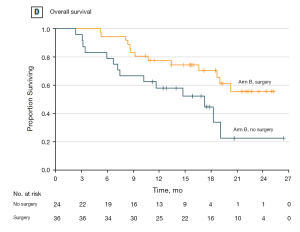
This study included three patient categories (locally advanced tumors, tumors with limited metastases, non-resectable metastasis tumors) and the results showed a favorable survival in the group of patients with limited metastatic disease who received neoadjuvant chemotherapy and proceeded to surgery. In this limited metastatic group, the median overall survival was 31.3 months (95% CI, 18.9–upper level not achieved) for patients who proceeded to surgery and 15.9 months (95% CI, 7.1–22.9 months) for the other patients (Figure 1).
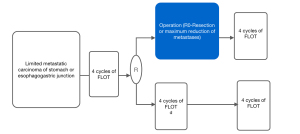
The FLOT-3 trial set the evidence basis for the ongoing trial FLOT 5-RENAISSANCE (26). This multicenter randomized trial is including patients with limited metastatic gastric and junction AC. Patients will receive four cycles of chemotherapy alone or with trastuzumab if HER-2 positive. After the four cycles, a re-staging is performed, and if no sign of disease progression is proved, the patients are randomized to receive either additional chemotherapy cycles or surgical resection of primary and metastases (Figure 2). The results of this trial could lead to a new standard in the management of metastatic gastric and junction cancer.
Esophageal cancer
Local and distant disease control for patients with the unresectable disease is complicated and non-satisfying. Despite significant progress in chemo(radio)therapy regiments, tumor downstaging in order to become eligible for surgery is rare. Some authors report in selected patients curative surgical resection for T4-disease stages after downstaging with chemoradiotherapy (27,28). A systematic review of studies published in 2011 (29) compared chemoradiotherapy and surgery versus chemoradiotherapy alone. The conclusion of available studies is that chemoradiotherapy with additional surgery was superior to chemotherapy alone in esophageal cancer with T4bNxM0 stage in terms of local control and short-term prognosis (median one-year survival: 57% versus 39.5%). Chemoradiotherapy seems to have sufficient local and systemic control to achieve a surgical resection.
Chemotherapy is still the first choice of treatment for patients with metastatic disease (30). However, in patients with a limited number of metastases, localized therapy directed at the metastases is an alternative management (31). Treatment includes surgery (for example, for liver metastases or lung metastases) (32,33) radiofrequency ablation, cryoablation or radiotherapy. This management is accepted in colorectal, lung and breast cancer metastases. For esophageal cancer, the evidence is growing, and these situations are increasingly treated locally with curative intent (34,35).
In a systematic review of the literature, Chiapponi et al. (36) included studies on the management of oligometastatic disease in esophageal cancer. They found two studies with radiotherapy of metastases from any primary tumor. In this series, patients with esophageal cancer had the worst overall survival (31,37). A retrospective study included in their review reported (38) about the metastatic disease in patients with esophageal cancer. They examined the survival of patients who underwent esophagectomy for locally advanced esophageal cancer with foci that were suspicious for metastatic disease on initial imaging but whose disease did not progress after induction chemoradiation treatment. They found that 32% of the patients who underwent esophagectomy had a suspicion of metastasis on initial imaging. None of the ten patients who had a pathologic confirmation of metastatic disease during the esophageal resection survived longer than 2.5 years. However, the authors did not mention if the histological confirmation was a biopsy or the complete resection of the metastases. As in the REGATTA Trial, a complete resection of the metastatic disease, if possible, could have improved the patient survival.
Resection of lung metastases may prolong survival in selected patients. The data from several Spanish centers were retrospectively analyzed (18). They included patients with AC of the esophagus, eso-gastric junction, and gastric with distant metastases. The primary aim of the study was to describe the frequency of metastasectomies nationwide. In their results, metastasectomy was performed in 92 patients at a median time of 5 months after chemotherapy. Negative resection margin was described in the majority of cases (64%). R1 and R2 resection was described in respectively 14% and 22%. The three-year survival rate for patients who underwent metastasectomy was 30.6% compared to 8.4% in the total group of patients with metastases. This retrospective study confirmed that in selected patients, (oligometastatic disease, no progression, good general/nutritional status), surgical resection of metastases could improve the long-term survival.
Schmidt et al. (39) also conducted a retrospective analysis of 123 patients with metastatic esophagogastric cancer undergoing surgery. In their cohort, 63 patients underwent complete resection (R0) of the tumor and the metastases. The patients with complete resection of the primary tumour and the metastases had a statistically significant longer median survival of 29.5 months (±6.7, 95% CI, 16.4–42.7 months) (P=0.003) compared to the patient with no resection of the metastases. When all the patients with metastatic cancer were analyzed, surgical resection, complete resection and preoperative chemoradiotherapy were associated with prolonged survival (all P<0.001).
In a systematic review, Schizas et al. (40) included six retrospective studies on outcomes of patients who underwent metastasectomy for limited metastatic disease in esophageal cancer, with a total of 420 patients.
Metastasectomy was performed during the same operative time of primary tumor resection in 73.5% of the cases. AC was the most frequent histological type (77.3%) compared to squamous cell carcinoma (22.7%). A negative resection margin (R0) for the metastases was obtained in 69.8% of the patients. Authors used a bootstrap methodology to approach the estimation of prognosis. They calculated a mean survival of 24.5 months and 5-year survival of 36.3%. These survivals were 2 to 6 times longer compared to the results of patients undergoing maximal medical therapy reported in the literature (4–11 months) (41).
Jamel et al. (42) conducted another systematic review about the management of patients with oligometastatic esophageal cancer. They identified and included 14 publications (12 on metachronous and two on synchronous oligometastasis). In the synchronous studies, one study (43) reported the management of primary tumor and limited brain metastases in seven patients. Definitive chemoradiotherapy for the esophageal cancer was performed with local treatment of the cerebral metastases (radiotherapy or surgery). Median survival in this series was 18.9 months.
These two reviews seem to favor surgical resection in patients with oligometastases from esophageal cancer. However, an essential element when considering surgical treatment for the distant lesion is patient selection. Prognostic factors associated with survival in 56 patients were studied by Ghaly et al. (44). The patients had undergone multimodal management for limited metastatic esophageal cancer recurrence in the liver, bone, brain or adrenal glands. In the 56 patients with metachronous metastases, 31 were treated surgically with or without chemoradiotherapy, and 25 received definitive chemoradiotherapy alone. The median disease-free survival was 19 months. The 1- and 3- year post-recurrence survival rate was 78% and 38%. Time to tumor recurrence was found to be the only independent factor that predicted survival in the multivariable analysis model. A cut-off of 12 months provided the best-combined sensitivity and specificity. Time to recurrence more than a year after the primary treatment of esophageal cancer is an indicator of less aggressive tumor behavior (45).
A multicentric retrospective study (46) from Germany proposed and validated a prognostic score for patients with metastatic esophageal and gastric cancer disease. The prognostic score includes the grade, clinical response to chemotherapy, and extent of resection. Patients were categorized in low and high risk depending on their score. The median survival time for the low versus high-risk group was 35.3 and 12.0 months.
The authors concluded that the prognosis of patients undergoing resection for metastatic esophagogastric cancer could be predicted with three simple factors (grading, clinical response and R0-resection). These factors can be determined preoperatively with relatively high accuracy by an experienced interdisciplinary team. Patients with at least two favorable factors could have a survival advantage with surgical resection.
Other studies about prognostic factors will help clarify and identify the patient population, which will benefit the most from aggressive treatment of their metastatic disease. Table 2 summarizes the principal studies with survival data on surgical resection for synchronous metastases of esophageal cancer.
Table 2
| Study | Year | Type of metastasis | Metastases localization | Number of patients | Survival |
|---|---|---|---|---|---|
| Adam (47) | 2006 | Mixed (synchronous and metachronous) | Liver: 20 | 20 | Median survival: 16 months; three year survival rate: 32% |
| Schmidt (39) | 2015 | Synchronous only | Lymph node: 27; liver: 14; lung: 8; peritoneum: 4; >1 site: 4; others: 6 | 63 | Median overall survival: 29.5 months |
| Van Daele (48) | 2018 | Synchronous only | Liver, lymph node | 12 | Median: 22 months |
| Carmona-Bayonas (18) | 2018 | Synchronous only | Peritoneal: 27; liver: 22; lymph node: 11 | 92 | Median: 16.7 months; three year survival rate: 30.6% |
| Depypere (49) | 2018 | Synchronous only | Lung: 5; peritoneal: 1; adrenal: 2; pleural: 1; pancreatic: 1 | 10 | Median overall survival: 21.4; three-year survival rate: 26.7% |
| Seesing (50) | 2019 | Mixed (synchronous and metachronous) | Liver: 19; lung :15 | 34 | Median overall survival: 28 months; 1 year survival rate: 84%; 3 year survival rate: 41%; 5 year survival rate: 31% |
There is no clear guideline in regards to the management of esophageal cancer metastases. According to the guidelines of the European Society for Medical Oncology (ESMO) (30), different options of palliative treatment for patients with metastatic esophageal cancer can be considered. German guidelines (51) confirm this statement and do not recommend surgical resection if the metastases are diagnosed preoperatively. The guidelines admit one exception, and it is when a limited metastatic disease is discovered intraoperatively. If negative resection margin of the tumor and the metastasis can be achieved, the guidelines advise that the tumor and the metastasis should be resected. The decision to resect distant metastases should be mostly a personalized treatment recommended by tumor boards for patients in good general condition with prolonged tumor-free survival.
Conclusions
Esophageal cancer is an aggressive tumor with rapid local and distant invasion. The improvement of local and systemic treatment could help control tumor progression. The dogma that metastatic disease is incurable is now being questioned. Selected patients with limited metastatic disease can benefit from aggressive local therapy. High-quality studies to determine the efficacy of such approaches in patients with esophageal cancer are lacking. The results of the ongoing RENAISSANCE trial will help elucidate this debate for gastric and eso-gastric junction cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sjoerd Lagarde, Bas Wijnhoven, and Florian Lordick) for the series “Novel Developments in the Multimodality Treatment of Esophageal Cancer” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-2020-07). The series “Novel Developments in the Multimodality Treatment of Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer 2018. Available online: https://gco.iarc.fr/today
- Bollschweiler E, Plum P, Mönig SP, et al. Current and future treatment options for esophageal cancer in the elderly. Expert Opin Pharmacother 2017;18:1001-10. [Crossref] [PubMed]
- Mönig SP, Chevallay M, Niclauss N, et al. Esophageal and esophago-gastric junction can-cer: management and multimodal treatment. Rev Med Suisse 2020;16:1292-9. [PubMed]
- Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in pa-tients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 2013;14:627-37. [Crossref] [PubMed]
- van Rossum PSN, Mohammad NH, Vleggaar FP, et al. Treatment for unresectable or metastatic oesophageal cancer: current evidence and trends. Nat Rev Gastroenterol Hepatol 2018;15:235-49. [Crossref] [PubMed]
- Koëter M, van Steenbergen LN, Lemmens VEPP, et al. Hospital of diagnosis and proba-bility to receive a curative treatment for oesophageal cancer. Eur J Surg Oncol 2014;40:1338-45. [Crossref] [PubMed]
- Versteijne E, van Laarhoven HW, van Hooft JE, et al. Definitive chemoradiation for pa-tients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern. Dis Esophagus 2015;28:453-9. [Crossref] [PubMed]
- Kleinberg L, Gibson MK, Forastiere AA. Chemoradiotherapy for localized esophageal cancer: regimen selection and molecular mechanisms of radiosensitization. Nat Clin Pract Oncol 2007;4:282-94. [Crossref] [PubMed]
- Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8. [Crossref] [PubMed]
- Institute NC. SEER Stat Fact Sheets: Esophageal Cancer 2016. Available online: http://seer.cancer.gov/statfacts/html/esoph.html. Accessed 09.09 2020.
- Institute NC. SEER Stat Fact Sheets: Female Breast Cancer 2016. Accessed 09.09.2020 2016.http://seer.cancer.gov/statfacts/html/breast.html
- Institute NC. SEER Stat Fact Sheets: Colon and Rectum Cancer 2016. Accessed 09.09 2020.http://seer.cancer.gov/statfacts/html/breast.html
- Parry K, Visser E, van Rossum PSN, et al. Prognosis and treatment after diagnosis of re-current esophageal carcinoma following esophagectomy with curative intent. Ann Surg Oncol 2015;22:S1292-300. [Crossref] [PubMed]
- Ai D, Zhu H, Ren W, et al. Patterns of distant organ metastases in esophageal cancer: a population-based study. J Thorac Dis 2017;9:3023-0. [Crossref] [PubMed]
- Wu SG, Zhang WW, Sun JY, et al. Patterns of distant metastasis between histological types in esophageal cancer. Front Oncol 2018;8:302. [Crossref] [PubMed]
- Shi AA, Digumarthy SR, Temel JS, et al. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung cancer? J Thorac Oncol 2006;1:205-10. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Carmona-Bayonas A, Jiménez-Fonseca P, Echavarria I, et al. surgery for metastases for esophagealgastric cancer in the real world: data from the AGAMENON national registry. Eur J Surg Oncol 2018;44:1191-8. [Crossref] [PubMed]
- Procopio F, Marano S, Gentile D, et al. Management of liver oligometastatic esophageal cancer: overview and critical analysis of the different loco-regional treatments. Cancers 2019;12:20. [Crossref] [PubMed]
- Broomfield JA, Greenspoon JN, Swaminath A. Utilization of stereotactic ablative radio-therapy in the management of oligometastatic disease. Curr Oncol 2014;21:115-7. [Crossref] [PubMed]
- Song A, Zhang X. Surgical resection for hepatic metastasis from gastric cancer: a multi- institution study. Oncotarget 2017;8:71147-53. [Crossref] [PubMed]
- Markar SR, Mikhail S, Malietzis G, et al. Influence of Surgical Resection of Hepatic Metas-tases From Gastric Adenocarcinoma on Long-term Survival: Systematic Review and Pooled Analysis. Ann Surg 2016;263:1092-101. [Crossref] [PubMed]
- Chen J, Kong YY, Weng SS, et al. Outcomes of surgery for gastric cancer with distant metastases: a retrospective study from the SEER database. Oncotarget 2017;8:4342-51. [Crossref] [PubMed]
- Fujitani K, Yang HK, Mizusawa J, et al. gastrectomy plus chemotherapy versus chemo-therapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomized controlled trial. Lancet Oncol 2016;17:309-18. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Effect of Neoadjuvant Chemotherapy Fol-lowed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gas-troesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol 2017;3:1237-44. [Crossref] [PubMed]
- Al-Batran SE, Goetze TO, Mueller DW, et al. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction - a phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer 2017;17:893. [Crossref] [PubMed]
- Shimoji H, Karimata H, Nagahama M, et al. Induction chemotherapy or chemoradio-therapy followed by radical esophagectomy for T4 esophageal cancer: results of a prospec-tive cohort study. World J Surg 2013;37:2180-8. [Crossref] [PubMed]
- Jung MK, Schmidt T, Chon SH, et al. Current surgical treatment standards for esophage-al and esophagogastric junction cancer. Ann N Y Acad Sci 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Makino T, Doki Y. Treatment of T4 esophageal cancer: Definitive chemoradiotherapy versus chemoradiotherapy followed by surgery. Ann Thorac Cardiovasc Surg 2011;17:221-8. [Crossref] [PubMed]
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. [Crossref] [PubMed]
- Milano MT, Katz AW, Zhang H, et al. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys 2012;83:878-86. [Crossref] [PubMed]
- Al-Asfoor A, Fedorowicz Z, Lodge M. Resection versus no intervention or other surgical interventions for colorectal cancer liver metastases. Cochrane Database Syst Rev 2008;CD006039. [PubMed]
- Sternberg DI, Sonett JR. Surgical therapy of lung metastases. Semin Oncol 2007;34:186-96. [Crossref] [PubMed]
- Martel G, Hawel J, Rekman J, et al. Liver Resection for Non-Colorectal, Non-Carcinoid, Non-Sarcoma Metastases: A Multicenter Study. PLoS One 2015;10:e0120569. [Crossref] [PubMed]
- Huddy JR, Ni MZ, Markar SR, et al. Point-of-care testing in the diagnosis of gastrointes-tinal cancers: current technology and future directions. World J Gastroenterol 2015;21:4111-20. [Crossref] [PubMed]
- Chiapponi C, Berlth F, Plum P, et al. Oligometastatic Disease in Upper Gastrointestinal Cancer - How to Proceed? Visc Med 2017;33:31-4. [Crossref] [PubMed]
- Omae K, Hiraki T, Gobara H, et al. Longterm survival after radiofrequency ablation of lung oligometastases from five types of primary lesions: a retrospective evaluation. J Vasc Interv Radiol 2016;27:1362-70. [Crossref] [PubMed]
- Erhunmwunsee L, Englum BR, Onaitis MW, et al. Impact of pretreatment imaging on survival of esophagectomy after induction therapy for esophageal cancer: who should be given the benefit of the doubt?: esophagectomy outcomes of patients with suspicious metastatic lesions. Ann Surg Oncol 2015;22:1020-5. [Crossref] [PubMed]
- Schmidt T, Alldinger I, Blank S, et al. Surgery in oesophago-gastric cancer with metastatic disease: treatment, prognosis and preoperative patient selection. Eur J Surg Oncol 2015;41:1340-7. [Crossref] [PubMed]
- Schizas D, Mylonas KS, Kapsampelis P, et al. Patients undergoing surgery for oligometa-static oesophageal cancer survive for more than 2 years: bootstrapping systematic review data. Interact CardioVasc Thorac Surg 2020;31:299-304. [Crossref] [PubMed]
- Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers 2017;3:17048. [Crossref] [PubMed]
- Jamel S, Tukanova K, Markar S. Detection and management of oligometastatic disease in oesophageal cancer and identification of prognostic factors: A systematic review. World J Gastrointest Oncol 2019;11:741-9. [Crossref] [PubMed]
- Onal C, Akkus Yildirim B, Guler OC. Outcomes of aggressive treatment in esophageal cancer patients with synchronous solitary brain metastasis. Mol Clin Oncol 2017;7:107-12. [Crossref] [PubMed]
- Ghaly G, Harrison S, Kamel MK, et al. Predictors of survival after treatment of oligome-tastases after esophagectomy. Ann Thorac Surg 2018;105:357-62. [Crossref] [PubMed]
- Mönig S, van Hootegem S, Chevallay M, et al. The role of surgery in advanced disease for esophageal and junctional cancer. Best Pract Res Clin Gastroenterol 2018;36-37:91-6. [Crossref] [PubMed]
- Blank S, Lordick F, Dobritz M, et al. A reliable risk score for stage IV esophagogastric cancer. Eur J Surg Oncol 2013;39:823-30. [Crossref] [PubMed]
- Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006;244:524-35. [PubMed]
- Van Daele E, Scuderi V, Pape E, et al. Long-term survival after multimodality therapy including surgery for metastatic esophageal cancer. Acta Chir Belg 2018;118:227-32. [Crossref] [PubMed]
- Depypere LP, Moons J, Lerut TE, et al. Palliative esophagectomy in unexpected meta-static disease: sense or nonsense? Asian Cardiovasc Thorac Ann 2018;26:552-7. [Crossref] [PubMed]
- Seesing MFJ, van der Veen A, Brenkman HJF, et al. Resection of hepatic and pulmonary metastasis from metastatic esophageal and gastric cancer: a nationwide study. Dis Esoph-agus 2018;31:9. [Crossref]
- Hölscher AH, Gockel I, Porschen R. Updated German S3 guidelines on esophageal can-cer and supplements from a surgical perspective. Chirurg 2019;90:398-402. [PubMed]
Cite this article as: Chevallay M, Jung M, Wassmer CH, Mönig S. Role of surgery in the management of synchronous metastatic esophageal cancer. Ann Esophagus 2021;4:39.


