
Palliation of malignant dysphagia: stent or radiotherapy?
Introduction
The incidence of esophageal cancer is increasing rapidly (1). Currently, it is the seventh most frequent cancer with annually approximately 572,000 newly diagnosed cases worldwide (2). The majority of patients are diagnosed at an advanced stage and are confronted with palliative treatment options only (3). The initial step in palliation is to relieve dysphagia, which occurs in over 70% of patients and has substantial impact on quality of life (4,5). Unfortunately, optimal management of these patients is still not clear, which has resulted in large practice variation (6). As the median survival of these patients is only four to five months, treatment choice should depend on life-expectancy (as this influences the importance of effect duration and adverse events of a treatment) and severity of dysphagia (as this influences the importance of time until effect on dysphagia) (7). Stent placement and radiotherapy are the two most widely used treatment modalities for reducing and preferably resolving obstructive symptoms. Both have been proven effective and safe (8-11).
Rigid plastic tubes were first introduced for malignant dysphagia in the 1970s (12). Its placement required however esophageal dilatation before placement which was associated with an increased risk of hemorrhage and perforation. Self-expandable metal stents (SEMS) were introduced in the mid-1990s and soon replaced plastic tubes because of fewer stent-related adverse events and better outcomes with regard to dysphagia improvement (13-16). SEMS placement has the advantage that it can almost always be performed under endoscopic and/or fluoroscopic guidance, without the need for prior esophageal dilation. Improvement of dysphagia after SEMS placement is seen directly after stent expansion and technical success rates are high (9,10,17). Nonetheless, adverse events are still seen in 40–50% of patients (10). The most common severe adverse events are hemorrhage, stent migration and retrosternal pain. Importantly, SEMS placement is associated with a relatively high rate of recurrent dysphagia (up to 31%) (10). Recurrent dysphagia is commonly seen after a median of two months and is mostly the result of stent migration or tumor/hyperplastic tissue in- or overgrowth, both of which are influenced by stent design. Whereas fully covered SEMS (fcSEMS) have been shown to have higher migration rates due to lack of anchoring capacity, partially covered SEMS (pcSEMS) are associated with increased tumor/tissue in- or overgrowth (18).
Radiotherapy is usually well-tolerated with only a few serious adverse events reported. It is known for a longer-lasting relief of dysphagia compared to stent placement, although it may take one to two weeks before it becomes clinically noticeable (11). It can be provided externally through external-beam radiotherapy (EBRT) or intraluminal as brachytherapy (BT) using an endoluminal applicator. Whereas EBRT is easier to perform than BT, the latter has gained interest because of a more focused application of radiation energy to the tumor site while sparing normal surrounding tissue. Nonetheless, the endoluminal applicator needs placing inside the esophagus using endoscopic guidance and optimal dosing and fractionation are not completely clear.
To combine advantages of both the SEMS and radiotherapy, an irradiation stent has been developed. This stent is loaded with iodine 125 (125I) beads, resulting in a prolonged local release of radiation in the esophagus while maintaining esophageal lumen patency with the stent. This technique is however not widely available and measuring and planning of the most accurate radiation dosimetry is still not completely elucidated. In line with the rationale of the irradiation stent, combining radiotherapy (EBRT or BT) and esophageal stenting has gained interest, but so far results of larger studies are to be awaited.
The aim of this review is to outline the current literature on stenting, radiotherapy and combination therapies as a palliative treatment of malignant dysphagia.
Methods
We systematically searched the literature on the treatment of malignant dysphagia using PubMed. The following search terms were included: [‘deglutition disorders’, ‘dysphagia’ or ‘esophageal stenosis’] AND [‘stent’, ‘radiotherapy’ or ‘brachytherapy’]. We excluded studies with treatment options other than stent placement or radiotherapy, animal studies and in vitro studies. Furthermore, studies were excluded when full text was not available in English, Dutch or Spanish. Title and abstract of all 2,087 studies published in the last 10 years were screened for eligibility. Full text of 69 studies was evaluated and reference lists of included studies were also screened.
Radiotherapy
Radiotherapy has a direct cytotoxic effect on tumor cells thereby providing palliation of dysphagia by shrinking the esophageal tumor. Retrospective studies on EBRT have shown that patients treated with EBRT had significant improvement of dysphagia in >70% with only limited toxicity reported (19-23). Two systematic reviews that assessed BT as palliative treatment in patients with malignant dysphagia showed a dysphagia-free survival rate of 87% after one month and a median duration of dysphagia relief of 99 days (24,25). However, severe adverse events were seen in 23% of patients, with BT-induced development of esophageal stenosis and fistula most commonly seen (24,25). When comparing dosages and fractions, patients treated with fractionated BT had an increased dysphagia-free period compared to those treated with only a single dose of BT (24).
EBRT vs. BT
Table 1 shows all studies comparing radiotherapy as palliative treatment of malignant dysphagia. Two studies compared EBRT with BT (26,27). A retrospective cohort study comparing EBRT (20–30 Gy in 5–10 fractions) vs. single-dose BT (12 Gy) showed no significant difference in dysphagia scores or adverse events between both groups (26). A recent multicenter non-randomized cohort study comparing short cycle EBRT (5 fractions of 4 Gy) performed in a prospective follow-up study vs. single-dose BT (12 Gy), obtained from a previous study in which BT randomly was compared with stent placement, demonstrated that short cycle EBRT was superior in relieving dysphagia (83% vs. 64%, P<0.05) (27). In addition, dysphagia improved more rapidly after EBRT. Survival rates were not different between both groups. Severe toxicity was more frequently seen in the BT group than the EBRT group (13% vs. 3%, P value not reported), which can possibly be explained by a higher radiation dose at the level of the esophageal mucosa resulting in stenosis and fistula formation.
Table 1
| Comparison | First author (year) | Study design | Interventions | N belonging to study arm | Efficacy | Safety | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Dysphagia relief (short term) | Dysphagia relief (long term) | Median survival (days) | QoL | Adverse events | ||||||
| EBRT vs. BT | Eldeeb (2012) | Retrospective | Study-arm 1: EBRT (20–30 Gy/5–10#), Study-arm 2: BT (1×12 Gy) | 21 | 23 | – | NS | NS | NR | NR | NS | ||
| Jeene (2020) | Prospective | Study-arm 1: EBRT (5×4 Gy), Study-arm 2: BT (1×12 Gy) | 69 | 69 | – | 67% vs. 36% after 2 weeks | 83% vs. 64% after 3 months | NS | NR | 3% vs. 13%* | |||
| BT/EBRT vs. BT + EBRT | Sur (2004) | RCT | Study-arm 1: BT (2×8 Gy), Study-arm 2: BT (2×8 Gy) + EBRT (30 Gy/10#) | 30 | 28 | – | NS | NS | NS | NR | NS | ||
| Rosenblatt (2010) | RCT | Study-arm 1: BT (8 Gy), Study-arm 2: BT (8 Gy) + EBRT (30 Gy/10#) | 109 | 110 | – | 67% vs. 83% after 100 days | NS | NR | NS | ||||
| Welsch (2016) | Retrospective | Study-arm 1: EBRT (30–40.5 Gy total, 2.5–3 Gy per fraction), Study-arm 2: BT (15–25 Gy total, 5–7 Gy per fraction), T3: EBRT (30–40.5 Gy) + BT (10–14 Gy) | 65 | 46 | 28 | Dysphagia free survival: 90% vs. 37% vs. 92% after 6 months | NR | NR | NS | ||||
| Vermeulen (2019) | Retrospective | Study-arm 1: EBRT (5×4 Gy), Study-arm 2: EBRT (10×3 Gy) + BT (1×12 Gy) | 72 | 72 | – | Persistent/recurrent dysphagia: 64% vs. 42% after 6 weeks | 88 vs. 177 | NR | NS | ||||
#, number of fractions; *P value not reported or not applicable. BT, brachytherapy; EBRT, external-beam radiotherapy; N, number of patients; NR, not reported; NS, not significant; QoL, quality of life; RCT, randomized controlled trial; SEMS, self-expandable metal stent.
EBRT combined with BT
Two randomized controlled trials (RCT) compared BT vs. BT combined with EBRT (28,29). One RCT, including a limited number of patients (n=59) reported no significant differences in dysphagia scores, survival or adverse events (29). The other RCT (n=219) showed improved long-term dysphagia scores in the combination therapy group (83% vs. 67%, P<0.05) (28).
A retrospective study that compared EBRT vs. BT vs. combination therapy of EBRT and BT also showed statistically significantly dysphagia free survival scores between groups (90% vs. 37% vs. 92% respectively, P<0.01) (30). Remarkably, dysphagia free survival scores of EBRT alone were comparable to combination therapy of EBRT and BT, suggesting that adding BT to EBRT did not affect outcome in this study. A multicenter retrospective study comparing EBRT (5 fractions of 4 Gy) with combined radiotherapy (10 fractions EBRT of 3 Gy and single-dose 12 Gy BT) however showed lower persistent/recurrent dysphagia rates (64% vs. 42% respectively, P<0.05) in the combination therapy group (31). Although dysphagia scores did not significantly differ between groups, a trend favoring combining EBRT and BT was seen (66% vs. 55% improvement, P=0.066). Furthermore, superior survival rates (177 vs. 88 days, P<0.001) were found in the combination therapy group and adverse event rates were not different between both groups. As all above mentioned studies used a higher radiation dose in the combination therapy group, the difference might well be explained by difference in dosage.
Stent placement
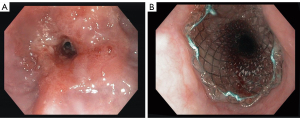
As stent placement does not directly affect tumor viability, its effect on reducing dysphagia is established by restoring luminal patency by mechanical force only (Figure 1). In general, SEMS placement is a relatively easy procedure providing rapid relief of dysphagia (8-10,17). Currently, two types of SEMS are available: pcSEMS and fcSEMS. Based on stent design (a smooth outer surface due to stent cover), higher stent migration rates were expected when using fcSEMS. A systematic review and meta-analysis comparing pcSEMS with fcSEMS showed however no difference in stent migration rates (32). Furthermore, no significant differences were seen between pcSEMS and fcSEMS with regard to reducing dysphagia or adverse events. Therefore, the choice for either pcSEMS or fcSEMS for palliation of malignant dysphagia is primarily based on non-stent related factors, such as pricing of the device, ease of placement and physician preference. Although manufacturers currently focus on developing improved stent designs that prolong palliation of dysphagia and reduce occurrence of adverse events, this seems hard to establish given the progressive course of esophageal cancer with stents having no effect on the natural history of the malignancy.
Stent placement vs. BT
Table 2 shows all studies comparing stent placement and BT or irradiation stent placement as palliative treatment of malignant dysphagia. Two RCTs have compared stent placement with BT (single-dose or fractionated) in a head-to-head design (33,34). One study clearly showed improved long-term (≥ three months) dysphagia relief in the BT group compared to stent placement (34). This was thought to be the result of a high early recurrence rate of dysphagia in the stent group caused by stent migration (17%), tumor/hyperplastic tissue in- and overgrowth (15%) and food-bolus obstruction (15%). As expected, patients treated with a stent had earlier symptom relief (33,34). In line with dysphagia scores, short-term quality-of-life (QoL) of patients was also in favor of the stent group, whereas long-term QoL showed a positive trend towards the BT group (33). For several QoL scales (among others dysphagia, emotional, cognitive and social functioning) these differences were statically significant within groups. The only statistically significant scales in the intergroup analysis were the dysphagia scale after 1 months in favor of the stent group and the trouble with speech score after 6 months in favor of the BT group (33). Survival was not significantly different between both groups (33,34). Whereas one RCT did not report differences in adverse events (33), the other RCT showed a higher adverse event rate in the stent group (33% vs. 21%, P<0.05) (34). The most frequent adverse events in the stent group included late hemorrhage (> seven days), tumor/tissue in- or overgrowth, stent migration and food bolus obstruction. In one of these studies pcSEMS, that are known for higher tumor ingrowth rates compared to fcSEMS, were placed (34). Remarkably, patients in the BT group showed even higher recurrent tumor growth rates in this study (26% in the BT group vs. 15% in the SEMS group). A cost-effectiveness analysis comparing SEMS placement and single-dose BT concluded that total costs of palliative treatment were equal, although the initial costs for SEMS placement were higher than for single-dose BT (35).
Table 2
| Comparison | First author (year) | Study design | Interventions | N belonging to study arm | Efficacy | Safety | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Dysphagia relief (short term) | Dysphagia relief (long term) | Median survival (days) | QoL | Adverse events | |||||||
| SEMS vs. BT | Homs (2004) | RCT | Study-arm 1: pcSEMS, Study-arm 2: BT (1×12 Gy) | 105 | 95 | NS | BT > SEMS after 30 days up till 350 days* | NS | NS | 33% vs. 21% | |||
| Bergquist (2005) | RCT | Study-arm 1: SEMS, Study-arm 2: BT (3×7 Gy) | 28 | 24 | NR | NS | Dysphagia scale SEMS > BT after 1 month, trouble with speech score BT > SEMS after 6 months | NS | |||||
| SEMS vs. IS | Zhongmin (2012) | Prospective (IS) and retrospective (SEMS) | Study-arm 1: SEMS, Study-arm 2: IS | 30 | 28 | NS | 147 vs. 330 | NR | NS | ||||
| Tian (2016) | Prospective | Study-arm 1: SEMS, Study-arm 2: IS | 91 | 40 | NS | NS | NS | NR | NS | ||||
| Zhao (2017) | RCT | Study-arm 1: SEMS, Study-arm 2: IS | 25 | 18 | NS | Mean: 144 vs. 294 | NR | NS | |||||
| Chen (2017) | Meta-analysis | Study-arm 1: SEMS, Study-arm 2: IS | 6 studies including 539 patients | IS > SEMS after 3 and 6 months* | IS > SEMS (pooled weighted mean difference 2.7 months) | NR | NS | ||||||
*P value not reported or not applicable. BT, brachytherapy; IS, irradiation stent; N, number of patients; NR, not reported; NS, not significant; OR, odds ratio; QoL, quality of life; RCT, randomized controlled trial; SEMS, self-expandable metal stent.
Stent placement vs. irradiation stent
A total of nine studies, four of them being RCTs, compared regular SEMS placement with placement of an irradiation stent (see Table 2) (36-39). All studies showed comparable results, including dysphagia relief in both groups. A systematic review and meta-analysis suggested that irradiation stents were superior over SEMS in terms of dysphagia relief at three and six months after placement (39). Performing a meta-analysis for dysphagia relief was not possible however due to limited data available (only mean dysphagia scores provided, no standard errors). All studies except one also showed a prolonged median survival in patients treated with the irradiation stent (ranging from 111–330 vs. 93–147 days; all P<0.05) (37-39). Adverse event rates were not different between both treatment groups in the meta-analysis (36-39). Non-surprisingly, medical costs were significantly lower in the regular SEMS-treated group, mainly because costs in the irradiation stent therapy group were approximately two-thirds higher compared to SEMS placement (36).
Stent placement combined with EBRT
Table 3 shows all studies comparing combination therapies as palliative treatment of malignant dysphagia. In total, five studies were found that combined stent placement with EBRT (40-44), with only one study randomizing patients between SEMS vs. SEMS followed by EBRT (30 Gy in 10 fractions) (41). Short-term dysphagia scores were similar for combination therapy compared to SEMS placement only. As expected, dysphagia relief persisted for a longer time in the group treated with EBRT after SEMS placement (7 vs. 3 months, P<0.05) (41). All studies except two also showed a survival benefit for combined therapy compared to stenting alone (median survival ranging from 161–237 vs. 91–169 days, all P<0.05) (40,41,44). One study showed however higher survival rates in the stent-only group, which could likely be explained by selection bias as this was a retrospective study that compared patients treated with a SEMS because of recurrent dysphagia after prior radiotherapy with curative or palliative intent with patients immediately treated with a stent when presenting with dysphagia (42). The effect of combined EBRT and stenting on adverse event rates is not completely clear. It has been suggested that EBRT after stent placement could increase adverse events up to 85%, specifically with regard to stent migration rates, due to tumor shrinkage as a consequence of radiotherapy (44). In one study more adverse events were seen when EBRT was performed before stent placement (42), specifically a higher incidence of gastrointestinal bleedings (42% vs. 9%, P<0.01) and pneumoniae were seen (56% vs. 9%, P=0.000). Mortality of major gastrointestinal bleeding was associated with a higher radiation dosage and female gender. In contrast, one RCT, one prospective and one retrospective study did not show significant differences in adverse event rates between groups (40,41,43).
Table 3
| Comparison | First author (year) | Study design | Interventions | N belonging to study arm | Efficacy | Safety | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Dysphagia relief (short term) | Dysphagia relief (long term) | Median survival (days) | QoL | Adverse events | ||||||
| SEMS vs. SEMS + EBRT | Song (2002) | Retrospective | Study-arm 1: fcSEMS, Study-arm 2: EBRT + fcSEMS, Study-arm 3: fcSEMS + EBRT (details about EBRT unknown) | 48 | 47 | 13 | NR | Study-arm 1 vs. 3: 91 vs. 161, Study-arm 2 vs. 3: 70 vs. 161 | NR | Study-arm 1 vs. 3:21% vs. 85%Study-arm 2 vs. 3:21% vs. 85% | |||
| Rueth (2012) | Retrospective | Study-arm 1: SEMS, Study-arm 2: EBRT + SEMS, Study-arm 3: SEMS + EBRT (details about EBRT unknown) | 20 | 16 | 8 | NS | NS | NR | NS | ||||
| Eldeeb (2012) | Prospective | Study-arm 1: EBRT (20–30 Gy/5–10#), Study-arm 2: SEMS, Study-arm 3: EBRT (20–30 Gy/5–10#) + SEMS | 30 | 35 | 26 | NS | Study-arm 2 vs. 3: 169 vs. 237, other NSD | NR | NS | ||||
| Javed (2012) | RCT | Study-arm 1: SEMS, Study-arm 2: SEMS + EBRT (30 Gy/10#) | 37 | 42 | – | 3 vs. 7 months of relief | 120 vs. 180 | Declined immediate after EBRT* | NS | ||||
| Qiu (2013) | Retrospective | Study-arm 1: SEMS, Study-arm 2: EBRT (median 60 Gy) + SEMS | 35 | 57 | – | NS | 246 vs. 77 | NR | GI bleeding (9% vs. 42%), pneumoniae (9% vs. 56%) | ||||
| Stent + BT | Bergquist (2012) | Single-arm prospective | Study-arm 1: SEMS + BT (1×12 Gy) | 11 | – | – | 90.9% after 1 month | 198* | Dysphagia-related scores improved* | Only minor* | |||
| Hirdes (2012) | Single-arm prospective | Study-arm 1: biodegradable stent + BT (1×12 Gy) | 19 | – | – | 100% after 1 month | NR | NR | 59% (47% major)* | ||||
| BT vs. SEMS + BT | Amdal (2013) | RCT | Study-arm 1: BT (3×8 Gy), Study-arm 2: SEMS + BT (3×8 Gy) | 20 | 21 | – | 39% vs. 71% after 3 weeks, NS after 7 weeks | NS | NR | NS | |||
#, number of fractions, *P value not reported or not applicable. BT, brachytherapy; EBRT, external-beam radiotherapy; GI, gastrointestinal; N, number of patients; NR, not reported; NS, not significant difference; QoL, quality of life; RCT, randomized controlled trial; SEMS, self-expandable metal stent.
Stent placement combined with BT
Three studies combined stent placement with BT, two of them being single-arm studies (45,46) and one RCT (47). A single-arm prospective study on single-dose BT (12 Gy) followed by biodegradable stent placement was prematurely terminated due to an unacceptably high adverse event rate of 89% (46). Adverse events included pain, vomiting, hematemesis and recurrent dysphagia. Although dysphagia scores improved in all patients, 37% of patients could not tolerate a normal diet due to pain and/or vomiting. Another single-arm prospective study in which SEMS placement was followed by single-dose BT (12 Gy) showed relief of dysphagia without the occurrence of major adverse events (45). The RCT comparing SEMS placement followed by BT to BT alone (3×8 Gy) included only a limited number of patients (n=41) (47). In this trial, a significant improvement of dysphagia scores was seen in the combined therapy group after three weeks of treatment (71% vs. 39%, P<0.05). However, this difference gradually diminished seven weeks after treatment. Survival was not different, and no severe adverse events were reported.
Discussion
Optimal management for palliation of dysphagia in patients with non-curable esophageal cancer remains a challenge. Stent placement and radiotherapy are the two most commonly used treatment modalities. Patients suffering from severe dysphagia or with a life-expectancy of less than three months, clearly benefit from SEMS placement. In case of more than three months life-expectancy, radiotherapy is preferred with short cycle EBRT being superior over single-dose BT. Upcoming therapies include placement of an irradiation stent and combination therapies. Irradiation stent placement appears superior over SEMS in terms of effect duration and could be considered as an alternative in patients with a longer lasting life-expectancy. Combining stent placement with EBRT or BT seem promising; however, adverse events rates are not uncommon and evidence in favor of combination therapy is lacking as only a few RCTs have been published. Table 4 and Figure 2 show characteristics and effect in time of stent placement and radiotherapy, respectively, in palliation of malignant dysphagia.
Table 4
| Variable | Stent placement | EBRT | BT | IS |
|---|---|---|---|---|
| Time until effect on dysphagia | Rapid (within 1 day) | Less rapid (in 1–2 weeks) | Less rapid (in 1–2 weeks) | Rapid (within 1 day) |
| Duration of dysphagia relief | Short (recurrence after 2–3 months) | Relatively long (>3 months) | Relatively long (>3 months) | Relatively long (>3 months) |
| Adverse event rate | Relatively high (40–50%) | Low (around 20%) | Low (around 20%) | Relatively high (equal to stent placement) |
| Survival | No effect on survival | No effect on survival* | No effect on survival* | Prolonged survival |
| Availability | Good | Good | Moderate + complex treatment planning | Moderate + complex treatment planning |
| Costs | Equal to BT | Insufficient evidence | Equal to stent placement | Two-thirds higher than stent placement |
*, combination therapies including EBRT and/or BT have shown prolonged survival rates. BT, brachytherapy; EBRT, external-beam radiotherapy; IS, irradiation stent.
The European Society for Medical Oncology (ESMO) and the European Society of Gastrointestinal Endoscopy (ESGE) both recommend BT as palliative treatment of malignant dysphagia (48,49). BT clearly has shown longer-lasting dysphagia relief when compared to SEMS placement, which makes it a better choice for patients with a longer life-expectancy (> three months) (33,34). In contrast to current guidelines, it was recently shown that short cycle EBRT results in more frequent and faster relief of dysphagia and also showed increased survival rates compared to BT (27). It is thought that the superior results of EBRT are due to a better dose application to the entire tumor compared to BT. Nonetheless, performance of a 3D CT-based treatment planning compared to a 2D X-ray based planning has been suggested to improve BT results (50). In this way, BT isodose can be calculated on the full extent of the tumor and its distance to high-risk surrounding tissues. Although this seems promising, 3D treatment planning is complicated by logistics, complexity of treatment and lack of expertise. Even when radiation planning is optimized, it remains uncertain if BT can compete with EBRT. Logistics for EBRT are much less complicated as EBRT can be offered at almost each radiotherapy facility. Therefore, considering the better outcomes, lower toxicity and less complex logistics, we consider EBRT preferable over BT. Although no differences were found between EBRT schedules (19-23), a higher doses of radiotherapy seems favorable specifically in patients with a life-expectancy of more than six weeks (31). However, the most optimal radiation dosage and total number of fractionations for treatment in patients with incurable esophageal cancer remains to be established.
As stated before, stent placement has been shown to restore luminal patency and improve dysphagia scores within one day compared to only 50% relief of dysphagia within two weeks after radiotherapy, especially BT (17,51). The use of SEMS is largely limited by the high dysphagia recurrence rate as a result of stent migration and tumor ingrowth. Therefore, we consider SEMS placement as the treatment of choice in case of severe dysphagia in patients with a limited life expectancy (< three months).
Results of irradiation stents so far seem very promising, showing rapid and longer-lasting dysphagia relief and prolonged survival compared to SEMS (36-39). Most important advantages include focused radiation to the inner part of an esophageal cancer, protecting surrounding tissues, and endured internal radiation up to 180 days (37). Disadvantages include the availability and the complexity of sophisticated tumor measurement and treatment planning. Although all published studies showed similar outcomes, some studies that did not have access to a dedicated treatment planning system (TPS) for luminal organs have reported uncertainties in these measurements and planning (36,52-54). An accurate measurement technique contributes to optimal calculation of the required number of 125I seeds and their distribution ratio of dosage. As this may affect outcomes, experience and a TPS for luminal organs is warranted. Until now, only studies comparing irradiation stents with regular SEMS with a small sample size and all coming from China have been published. Therefore, there is a need for larger studies comparing irradiation stent treatment with radiotherapy, SEMS placement or a combination of radiotherapy and SEMS in a more heterogeneous population.
Combining stent placement with EBRT has been suggested to provide a longer-lasting relief of dysphagia due to the effect of radiation therapy on the local tumor which may delay tumor ingrowth (40,41,43,44). Although SEMS combined with BT seems safe (45,47), safety outcomes are unclear in case of SEMS combined with EBRT (42,44). In addition, use of a biodegradable stent combined with BT is discouraged as this resulted in an unacceptable high adverse event rate (46). As only two RCTs have been published on combination therapies, results need to be interpreted with caution. Further studies should provide more information on efficacy and safety of combination therapies compared to stenting and/or radiotherapy alone.
The reported prolonged survival rates in favor of the irradiation stent compared to SEMS, combining EBRT and BT compared to EBRT alone and combining EBRT and SEMS compared to SEMS alone seem remarkable at first sight (31,37-41,44). The reason is that survival in incurable esophageal cancer mostly depends on progression of metastases and these treatment modalities probably only affect locoregional disease. Although better relief of dysphagia and subsequent improved nutritional status might contribute to survival rates, some concerns have been put forward that selection bias and/or confounding may have been involved in the favorable results. Moreover, an increase in survival has also been observed in a meta-analysis involving the use of chemotherapy (55). Although the evidence that shows that chemotherapy alone could improve dysphagia is scarce, palliative chemotherapy combined with radiotherapy could be considered in patients with an expected reasonable life-expectancy and good performance status (21,56).
As practice variation in palliative treatment of esophageal cancer has been noted, some guidance in choosing optimal palliative treatment seems warranted (6). A prognostic tool that may help deciding which patients will benefit from stent placement or BT has already been developed (57). This tool is able to differentiate between patients with a predicted poor vs. better prognosis, based on age, gender, tumor length, metastases and World Health Organization (WHO) performance score. More tools like this could contribute to more standardization and thereby improving palliative care in patients with malignant dysphagia.
Conclusions
Although individual patient-related factors should be taken into account when selecting optimal palliative treatment of malignant dysphagia, short cycle EBRT is nowadays the treatment of choice in patients with an expected survival of at least three months. SEMS placement might be reserved for patients with severe dysphagia and short life-expectancy (less than three months). More studies are needed to give irradiation stents and/or combination therapies an established position in the treatment algorithm.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sjoerd Lagarde, Bas Wijnhoven, and Florian Lordick) for the series “Novel Developments in the Multimodality Treatment of Esophageal Cancer” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-2020-08). The series “Novel Developments in the Multimodality Treatment of Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. PDS reports grants from Micro-Tech (Nanjing - China), during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDeri
References
- Arnold M, Laversanne M, Brown LM, et al. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol 2017;112:1247-55. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers 2017;3:17048. [Crossref] [PubMed]
- Diamantis G, Scarpa M, Bocus P, et al. Quality of life in patients with esophageal stenting for the palliation of malignant dysphagia. World J Gastroenterol 2011;17:144-50. [Crossref] [PubMed]
- Brierley JD, Oza AM. Radiation and chemotherapy in the management of malignant esophageal strictures. Gastrointest Endosc Clin N Am 1998;8:451-63. [Crossref] [PubMed]
- Opstelten JL, de Wijkerslooth LR, Leenders M, et al. Variation in palliative care of esophageal cancer in clinical practice: factors associated with treatment decisions. Dis Esophagus 2017;30:1-7. [PubMed]
- Nassri A, Zhu H, Muftah M, et al. Epidemiology and Survival of Esophageal Cancer Patients in an American Cohort. Cureus 2018;10:e2507. [Crossref] [PubMed]
- Dai Y, Li C, Xie Y, et al. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev 2014;CD005048. [PubMed]
- Didden P, Reijm AN, Erler NS, et al. Fully vs. partially covered selfexpandable metal stent for palliation of malignant esophageal strictures: a randomized trial (the COPAC study). Endoscopy 2018;50:961-71. [Crossref] [PubMed]
- Reijm AN, Didden P, Schelling SJC, et al. Self-expandable metal stent placement for malignant esophageal strictures - changes in clinical outcomes over time. Endoscopy 2019;51:18-29. [Crossref] [PubMed]
- Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97-110. [Crossref] [PubMed]
- Atkinson M, Ferguson R. Fibreoptic endoscopic palliative intubation of inoperable oesophagogastric neoplasms. Br Med J 1977;1:266-7. [Crossref] [PubMed]
- O'Donnell CA, Fullarton GM, Watt E, et al. Randomized clinical trial comparing self-expanding metallic stents with plastic endoprostheses in the palliation of oesophageal cancer. Br J Surg 2002;89:985-92. [Crossref] [PubMed]
- Roseveare CD, Patel P, Simmonds N, et al. Metal stents improve dysphagia, nutrition and survival in malignant oesophageal stenosis: a randomized controlled trial comparing modified Gianturco Z-stents with plastic Atkinson tubes. Eur J Gastroenterol Hepatol 1998;10:653-7. [PubMed]
- Sanyika C, Corr P, Haffejee A. Palliative treatment of oesophageal carcinoma--efficacy of plastic versus self-expandable stents. S Afr Med J 1999;89:640-3. [PubMed]
- Siersema PD, Hop WC, Dees J, et al. Coated self-expanding metal stents versus latex prostheses for esophagogastric cancer with special reference to prior radiation and chemotherapy: a controlled, prospective study. Gastrointest Endosc 1998;47:113-20. [Crossref] [PubMed]
- Sabharwal T, Hamady MS, Chui S, et al. A randomised prospective comparison of the Flamingo Wallstent and Ultraflex stent for palliation of dysphagia associated with lower third oesophageal carcinoma. Gut 2003;52:922-6. [Crossref] [PubMed]
- van Rossum PSN, Mohammad NH, Vleggaar FP, et al. Treatment for unresectable or metastatic oesophageal cancer: current evidence and trends. Nat Rev Gastroenterol Hepatol 2018;15:235-49. [Crossref] [PubMed]
- Kassam Z, Wong RK, Ringash J, et al. A phase I/II study to evaluate the toxicity and efficacy of accelerated fractionation radiotherapy for the palliation of dysphagia from carcinoma of the oesophagus. Clin Oncol (R Coll Radiol) 2008;20:53-60. [Crossref] [PubMed]
- Murray LJ, Din OS, Kumar VS, et al. Palliative radiotherapy in patients with esophageal carcinoma: A retrospective review. Pract Radiat Oncol 2012;2:257-64. [Crossref] [PubMed]
- Penniment MG, De Ieso PB, Harvey JA, et al. Palliative chemoradiotherapy versus radiotherapy alone for dysphagia in advanced oesophageal cancer: a multicentre randomised controlled trial (TROG 03.01). Lancet Gastroenterol Hepatol 2018;3:114-24. [Crossref] [PubMed]
- Suzuki G, Yamazaki H, Aibe N, et al. Palliative Radiotherapy in the Local Management of Stage IVB Esophageal Cancer: Factors Affecting Swallowing and Survival. Anticancer Res 2017;37:3085-92. [PubMed]
- Walterbos NR, Fiocco M, Neelis KJ, et al. Effectiveness of several external beam radiotherapy schedules for palliation of esophageal cancer. Clin Transl Radiat Oncol 2019;17:24-31. [Crossref] [PubMed]
- Fuccio L, Mandolesi D, Farioli A, et al. Brachytherapy for the palliation of dysphagia owing to esophageal cancer: A systematic review and meta-analysis of prospective studies. Radiother Oncol 2017;122:332-9. [Crossref] [PubMed]
- Lancellotta V, Cellini F, Fionda B, et al. The role of palliative interventional radiotherapy (brachytherapy) in esophageal cancer: An AIRO (Italian Association of Radiotherapy and Clinical Oncology) systematic review focused on dysphagia-free survival. Brachytherapy 2020;19:104-10. [Crossref] [PubMed]
- Eldeeb H, Reza S, Shmueli U, et al. External beam radiotherapy versus brachytherapy in the management of malignant oesophageal dysphagia: a retrospective study. J BUON 2012;17:508-11. [PubMed]
- Jeene PM, Vermeulen BD, Rozema T, et al. Short course external beam radiotherapy versus brachytherapy for palliation of dysphagia in oesophageal cancer: a matched comparison of two prospective trials. J Thorac Oncol 2020;15:1361-8. [Crossref] [PubMed]
- Rosenblatt E, Jones G, Sur RK, et al. Adding external beam to intra-luminal brachytherapy improves palliation in obstructive squamous cell oesophageal cancer: a prospective multi-centre randomized trial of the International Atomic Energy Agency. Radiother Oncol 2010;97:488-94. [Crossref] [PubMed]
- Sur R, Donde B, Falkson C, et al. Randomized prospective study comparing high-dose-rate intraluminal brachytherapy (HDRILBT) alone with HDRILBT and external beam radiotherapy in the palliation of advanced esophageal cancer. Brachytherapy 2004;3:191-5. [Crossref] [PubMed]
- Welsch J, Kup PG, Nieder C, et al. Survival and Symptom Relief after Palliative Radiotherapy for Esophageal Cancer. J Cancer 2016;7:125-30. [Crossref] [PubMed]
- Vermeulen BD, Jeene PM, Sijben J, et al. Low-Dose Versus High-Dose Radiation Therapy for the Palliation of Dysphagia From Esophageal Cancer: A Multicenter Retrospective Cohort Study. Pract Radiat Oncol 2020;10:e255-63. [Crossref] [PubMed]
- Wang C, Wei H, Li Y. Comparison of fully-covered vs partially covered self-expanding metallic stents for palliative treatment of inoperable esophageal malignancy: a systematic review and meta-analysis. BMC Cancer 2020;20:73. [Crossref] [PubMed]
- Bergquist H, Wenger U, Johnsson E, et al. Stent insertion or endoluminal brachytherapy as palliation of patients with advanced cancer of the esophagus and gastroesophageal junction. Results of a randomized, controlled clinical trial. Dis Esophagus 2005;18:131-9. [Crossref] [PubMed]
- Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364:1497-504. [Crossref] [PubMed]
- Polinder S, Homs MY, Siersema PD, et al. Cost study of metal stent placement vs single-dose brachytherapy in the palliative treatment of oesophageal cancer. Br J Cancer 2004;90:2067-72. [Crossref] [PubMed]
- Tian D, Wen H, Fu M. Comparative study of self-expanding metal stent and intraluminal radioactive stent for inoperable esophageal squamous cell carcinoma. World J Surg Oncol 2016;14:18. [Crossref] [PubMed]
- Zhongmin W, Xunbo H, Jun C, et al. Intraluminal radioactive stent compared with covered stent alone for the treatment of malignant esophageal stricture. Cardiovasc Intervent Radiol 2012;35:351-8. [Crossref] [PubMed]
- Zhao P, Zhang MQ, Zhang YL, et al. Application of esophageal irradiation stents coated with 125I particles in advanced esophageal cancer. J BUON 2017;22:265-9. [PubMed]
- Chen HL, Shen WQ, Liu K. Radioactive self-expanding stents for palliative management of unresectable esophageal cancer: a systematic review and meta-analysis. Dis Esophagus 2017;30:1-16. [Crossref] [PubMed]
- Eldeeb H, El-Hadaad HA. Radiotherapy versus stenting in treating malignant dysphagia. J Gastrointest Oncol 2012;3:322-5. [PubMed]
- Javed A, Pal S, Dash NR, et al. Palliative stenting with or without radiotherapy for inoperable esophageal carcinoma: a randomized trial. J Gastrointest Cancer 2012;43:63-9. [Crossref] [PubMed]
- Qiu G, Tao Y, Du X, et al. The impact of prior radiotherapy on fatal complications after self-expandable metallic stents (SEMS) for malignant dysphagia due to esophageal carcinoma. Dis Esophagus 2013;26:175-81. [Crossref] [PubMed]
- Rueth NM, Shaw D, D'Cunha J, et al. Esophageal stenting and radiotherapy: a multimodal approach for the palliation of symptomatic malignant dysphagia. Ann Surg Oncol 2012;19:4223-8. [Crossref] [PubMed]
- Song HY, Lee DH, Seo TS, et al. Retrievable covered nitinol stents: experiences in 108 patients with malignant esophageal strictures. J Vasc Interv Radiol 2002;13:285-93. [Crossref] [PubMed]
- Bergquist H, Johnsson E, Nyman J, et al. Combined stent insertion and single high-dose brachytherapy in patients with advanced esophageal cancer--results of a prospective safety study. Dis Esophagus 2012;25:410-5. [Crossref] [PubMed]
- Hirdes MM, van Hooft JE, Wijrdeman HK, et al. Combination of biodegradable stent placement and single-dose brachytherapy is associated with an unacceptably high complication rate in the treatment of dysphagia from esophageal cancer. Gastrointest Endosc 2012;76:267-74. [Crossref] [PubMed]
- Amdal CD, Jacobsen AB, Sandstad B, et al. Palliative brachytherapy with or without primary stent placement in patients with oesophageal cancer, a randomised phase III trial. Radiother Oncol 2013;107:428-33. [Crossref] [PubMed]
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. [Crossref] [PubMed]
- Ebigbo A, Karstensen JG, Aabakken L, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Cascade Guideline. Endosc Int Open 2019;7:E833-6. [Crossref] [PubMed]
- Lettmaier S, Strnad V. Intraluminal brachytherapy in oesophageal cancer: defining its role and introducing the technique. J Contemp Brachytherapy 2014;6:236-41. [Crossref] [PubMed]
- Hanna WC, Sudarshan M, Roberge D, et al. What is the optimal management of dysphagia in metastatic esophageal cancer? Curr Oncol 2012;19:e60-6. [Crossref] [PubMed]
- Guo JH, Teng GJ, Zhu GY, et al. Self-expandable esophageal stent loaded with 125I seeds: initial experience in patients with advanced esophageal cancer. Radiology 2008;247:574-81. [Crossref] [PubMed]
- Zhu HD, Guo JH, Mao AW, et al. Conventional stents versus stents loaded with (125)iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncol 2014;15:612-9. [Crossref] [PubMed]
- Dai Z, Zhou D, Hu J, et al. Clinical application of iodine-eluting stent in patients with advanced esophageal cancer. Oncol Lett 2013;6:713-8. [Crossref] [PubMed]
- Janmaat VT, Steyerberg EW, van der Gaast A, et al. Palliative chemotherapy and targeted therapies for esophageal and gastroesophageal junction cancer. Cochrane Database Syst Rev 2017;CD004063. [Crossref] [PubMed]
- Dijksterhuis WPM, Verhoeven RHA, Pape M, et al. Hospital volume and beyond first-line palliative systemic treatment in metastatic oesophagogastric adenocarcinoma: A population-based study. Eur J Cancer 2020;139:107-18. [Crossref] [PubMed]
- Steyerberg EW, Homs MY, Stokvis A, et al. Stent placement or brachytherapy for palliation of dysphagia from esophageal cancer: a prognostic model to guide treatment selection. Gastrointest Endosc 2005;62:333-40. [Crossref] [PubMed]
Cite this article as: Koggel LM, Lantinga MA, Siersema PD. Palliation of malignant dysphagia: stent or radiotherapy? Ann Esophagus 2021;4:41.



