
Minimally invasive esophagectomy: anastomotic techniques
Introduction
Most patients with esophageal cancer present with locally advanced disease, for which esophagectomy remains the gold standard treatment. The goals of esophagectomy include resection of the diseased esophagus with negative margins, lymphadenectomy, and restoration of gastrointestinal continuity.
Historically, open esophagectomy was performed with different techniques, most often including a right thoracotomy and laparotomy. This approach was associated with very high rates of postoperative morbidity and mortality (1). The most common complications following open esophagectomy are respiratory, which occur in up to 40% of patients and may significantly increase the risk of mortality (2,3). Pneumonia has repeatedly been reported as an independent risk factor for morbidity and mortality following esophagectomy and is associated with a 20% mortality (2-7).
Minimally invasive techniques were first applied to the surgical treatment of esophageal cancer in the early 1990s as an attempt to mitigate the morbidity associated with open resection (8,9). The first approach to minimally invasive esophagectomy (MIE) was developed as a hybrid operation utilizing thoracoscopic techniques for esophageal resection as a method to avoid thoracotomy, followed by open laparotomy and preparation of the gastric conduit (8,10-12). A few years later in 2005, laparoscopic transhiatal esophagectomy emerged as the first totally minimally invasive approach to esophagectomy that avoided a thoracotomy and laparotomy (11-14). Further development of minimally invasive techniques expanded rapidly, giving rise to transthoracic esophagectomy techniques, of which the thoracoscopic and laparoscopic 3-stage McKeown esophagectomy and the thoracoscopic-laparoscopic Ivor Lewis esophagectomy are the most common.
MIE is preferred to the traditional open techniques because it provides an equivalent oncological resection with no difference in disease-free or overall survival but with significantly lower rates of postoperative transfusion, wound infection, ileus, pneumonia, and vocal cord palsy as well as a shorter length of hospital stay (1,15-20). Despite these advantages, several large scale systematic reviews of more than 40 studies failed to show any significant difference in the incidence of anastomotic leak between patients undergoing open resection when compared to those treated with MIE (21-23). Robot-assisted minimally invasive esophagectomy (RAMIE) is an additional approach to MIE that provides a similar oncological resection to traditional MIE approaches with no difference in perioperative morbidity and mortality (20,24).
Restoration of gastrointestinal continuity is most commonly accomplished using the stomach with an esophagogastric anastomosis for esophageal reconstruction. The stomach has an abundant intramural vascular network that allows mobilization of the whole organ and permits it to be brought up as a conduit to the chest or neck so that only a single anastomosis is required. While constructing the gastric conduit, the left gastric, left gastroepiploic, and short gastric vessels are divided, thus it is crucial to preserve the right gastroepiploic artery throughout gastric mobilization to avoid ischemia of the gastric conduit (25). Reconstruction can also be achieved with colon and jejunum, which may be useful in patients with previous foregut surgery.
Creation of the anastomosis is arguably the most critical step regardless of the conduit or surgical approach chosen. The esophagus does not hold sutures or staples well due to the lack of serosa and friability of the muscularis propria that occurs as a result of the longitudinal orientation of the muscle fibers. Anastomotic complications are the most feared complications following esophagectomy, as they contribute to a significant morbidity and mortality in these patients (26-29). These complications may be immediately life-threatening and can also result in substantial reductions in patient quality of life due to stricture formation and severe gastric reflux (28,30,31). Additionally, anastomotic leak decreases the oncologic value of the operation in patients with esophageal cancer and is an independent risk factor for mortality in this population (32-34). Anastomotic leak and gastric conduit necrosis increase the risk for post-esophagectomy trachea-bronchial-esophageal fistula, which is a rare but devastating complication associated with high rates of morbidity and mortality. Therefore, meticulous technique is essential to prevent postoperative anastomotic complications and the optimal anastomotic technique is frequently debated.
Anastomosis location
The location of the esophagogastric anastomosis divides surgical approach into two broad categories. The transhiatal and transthoracic McKeown approaches create a cervical anastomosis, whereas the Ivor Lewis and left thoracoabdominal approaches create an intrathoracic anastomosis (35).
Cervical anastomosis is most commonly performed in the left neck due to the slight curve to the of the cervical esophagus and the longer course of the left recurrent laryngeal nerve as it travels near or in the tracheoesophageal groove, where it is at risk for injury during the thoracic phase of the operation, to avoid inadvertent bilateral injury to the recurrent laryngeal nerves (36,37).
Intrathoracic anastomosis is created after laparoscopic creation of the gastric conduit, thoracoscopic esophageal mobilization, and mediastinal lymph node resection. The proximal esophagus is divided at the level of the azygous vein and the tubularized gastric conduit is carefully positioned in the chest above the divided azygos and under the divided esophagus, taking care to ensure correct orientation without tension (38-41). The intrathoracic gastroesophageal anastomosis is created between the proximal esophagus and a chosen portion of the gastric conduit with a rich vascular supply, enabling adequate healing while reducing the risk of anastomotic leak (42,43). The anastomosis is performed high in the chest at the thoracic inlet to prevent conduit redundancy and reflux (42,44).
With the introduction of minimally invasive techniques, a cervical anastomosis was typically utilized as it could still be performed without significant technical changes. Conversely, creation of an intrathoracic anastomosis using minimally invasive techniques required new techniques or variations of existing techniques to be devised and as a result, it is technically more challenging and time consuming compared to creation of a cervical anastomosis. While more challenging, intrathoracic anastomosis creation offers significant advantages including reduced incidence of anastomotic leak, benign anastomotic stricture, and of recurrent laryngeal nerve palsy, decreased blood loss, improved R0 resection, higher lymph node yield (21,35,45-48). Increased stretch of the gastric conduit and inability to discard areas of ischemia at the conduit tip are thought to explain the higher rate of anastomotic leak reported with cervical anastomosis.
Although the overall incidence of anastomotic leak is lower, cases of intrathoracic anastomotic leak are difficult to manage and are associated with high rates of morbidity and mortality, whereas cervical anastomotic leaks are often easy to clinically manage and rarely are life threatening (45). While cervical anastomotic leaks may be managed with a conservative approach consisting of nil by mouth, antibiotics, gastric drainage, and enteral or parenteral feeding, intrathoracic anastomoses may lead to devastating consequences requiring more aggressive interventions, such as operative exploration, thoracotomy, thoracoscopic drainage, and even complete gastrointestinal diversion (46,49).
There have been significant advances in interventional treatments of anastomotic leak, including endoscopic treatment techniques with stents or endoscopic vacuum-assisted closure devices, which have led to a more confident attitude towards intrathoracic anastomosis creation by surgeons. As a result, Ivor Lewis MIE now ranks first as the most common approach to MIE used clinically (50). Listed on Table 1 are the most relevant studies which have compared outcomes in relationship to location of the anastomosis. While some studies are limited by small study numbers, overall the following reports suggest that neck anastomoses are associated with higher rates of anastomotic leak with similar rates of cardiopulmonary complication, perioperative mortality, and benign stricture formation.
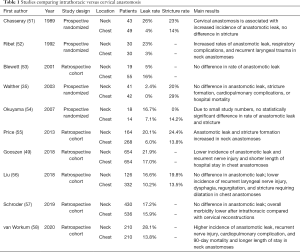
Full table
Techniques for anastomosis creation
There are three broad methods used to construct the esophagogastric anastomosis (see Figure 1), including manual (hand-sewn), mechanical (stapled), and hybrid (semi-mechanical) techniques (59-62). Regardless of technique chosen, it is imperative to ensure adequate apposition of the submucosal layer as collagen within the esophageal submucosa is crucial to maintain the integrity and strength of anastomosis (21,63). Three different configurations can be implemented when fashioning the esophagogastric anastomosis, including end-to-end, end-to-side and side-to-side anastomosis. An end-to-end anastomosis describes when the end of the esophageal stump is connected to end of the gastric conduit at the site of the anastomosis whereas an end-to-side anastomosis is constructed by connecting the end of the esophageal stump to the side of the gastric conduit at the site of the anastomosis. A side-to-side anastomosis is constructed by connecting a transverse gastrotomy made on the anterior wall of the gastric conduit with the adjacent posterior wall of the proximal esophageal stump (64). All three techniques can be used to create either a cervical or intrathoracic esophagogastrostomy, which will be further discussed in detail.
Handsewn anastomosis techniques
Generally, handsewn techniques are often preferred when creating a cervical anastomosis as the length of the conduit may prohibit use of a mechanical stapler. Conversely, creation of an intrathoracic handsewn anastomosis requires considerable technical skill and is often time-consuming, thus a stapled technique is most often utilized (35,65-67).
Hand-sewn anastomoses may be constructed using absorbable or nonabsorbable sutures in a continuous or interrupted fashion. Most commonly the continuous technique is typically considered to be superior as it is generally easier technically, cheaper, and can be performed faster when compared to the interrupted technique (63,68), however, ultimately choice is based on surgeon preference.
A single- or double-layer is used to create an esophagogastric anastomosis. The single layer technique uses absorbable or nonabsorbable sutures with full-thickness bites of the mucosa and muscularis propria in a circumferential fashion to ensure adequate mucosal apposition (63). The double-layer technique also uses an outer row of absorbable or nonabsorbable sutures on the seromuscular layer, either in a running or interrupted fashion, but has an additional inner layer of absorbable suture to invert the mucosa (69). One retrospective study reported reduced incidence of anastomotic leak and stricture with the double layer technique (70), however, this was not supported in subsequent randomized controlled trials (71,72). Often, the single-layer technique is preferred as it is commonly associated with a shorter operative time and lower cost than the double-layer technique. Listed on Table 2 are some relevant studies comparing single layer and double layer techniques for cervical anastomosis.
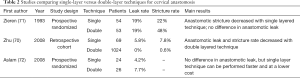
Full table
Cervical handsewn anastomosis techniques
Handsewn cervical anastomoses are most often performed using either an end-to-end or end-to-side configuration. Choice is guided by length of the gastric conduit and surgeon preference. An end-to-side approach requires more length of gastric conduit as compared to an end-to-end technique, which is thought to increase the risk of conduit tip ischemia and subsequently lead to a higher chance of dehiscence of the suture and staple line (73). This mechanism is also thought to explain why end-to-side anastomoses are associated with increased incidence of anastomotic leak and longer in-hospital stay (73). Conversely, the incidence of anastomotic stricture has been reported to be higher with an end-to-end technique due to the decreased diameter of anastomosis itself (73,74). Listed on Table 3 are some relevant studies comparing different configurations techniques for cervical anastomosis.
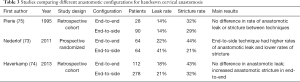
Full table
Thoracic handsewn anastomosis techniques
The technique used to create a handsewn intrathoracic anastomosis is similar to the methods used to create a cervical handsewn anastomosis. Typically, a single- or double-layered, end-to-end or end-to-side anastomosis is created between the distal esophagus and the gastric conduit along the greater curvature (76). Beginning with the outer posterior layer, interrupted seromuscular sutures are placed starting in the center of the posterior wall of the proximal esophagus and working toward both ends in the middle anteriorly, and subsequently a gastrotomy is made on the greater curvature prior to completion of the anterior wall (13,44,77,78). However, unlike the neck, creation of a thoracoscopic handsewn anastomosis can be very challenging so that as a result, it is rarely performed with conventional thoracoscopic techniques (50,79,80).
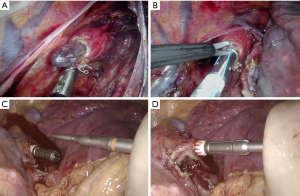
A handsewn thoracoscopic anastomosis is much more commonly accomplished using a robotic approach due to the improved view of the surgical field and range of motion of the instruments (41) (Figure 2). As utilization of robotic-assisted techniques continues to increase, postoperative outcomes continue to improve, and recent reports suggest that when compared to traditional MIE, RAMIE is associated with higher total lymph node yield, reduced intraoperative blood loss, reduced incidence of vocal cord palsy, with comparable oncological outcomes and rate of R0 resection (41,48,81-84). However, it is associated with a steep learning curve and more randomized controlled studies with larger sample sizes are needed to confirm observed benefits.
Mechanical anastomosis techniques
Mechanical techniques emerged following introduction of circular staplers in the 1970s (85). There are numerous variations of mechanical staplers used, which are divided into two subgroups based on the specific anastomotic configuration (end-to-end, side-to-side, or end-to-side) and suturing mechanism of the device (59-62). Clinically, the circular end-to-end anastomosis stapler (EEA stapler) and the linear cutting gastrointestinal stapler (GIA stapler) are the most commonly used. Many surgeons prefer mechanical staplers to hand-sewn anastomosis as they reduce intraoperative time significantly, are less operator-dependent, and require less surgical skill to use (63).
Linear side-to-side stapled anastomosis
A linear stapled anastomosis is most commonly performed using a 30- or 45-mm gastrointestinal anastomosis (GIA) stapler, which places a triple staggered row of titanium staples to create a side-to-side anastomosis (86,87). Depending on the approach, the linear stapler can be used to create a totally mechanical or a hybrid anastomosis.
Hybrid side-to-side cervical anastomosis techniques
Inspired by the high risk of technical complications with manual techniques of cervical esophagogastrostomy creation, a terminalized semi-mechanical side-to-side suture technique was developed for cervical anastomosis (88). If the length of the conduit is sufficient (>5 cm gastric conduit and esophageal stump overlap), an Orringer or modified Collard side-to-side anastomosis using a linear cutting stapler is typically preferred (63,82,88,89).
After delivery of the gastric conduit into the neck, a gastrotomy is made on the anterior wall of the gastric conduit, which is then opposed to the posterior wall of the proximal esophageal stump (65). The large end of an Endo GIA stapler is inserted into the gastrotomy and the thin blade is inserted into the open esophagotomy. After ensuring adequate alignment, the stapler is fired, creating a V-shaped opening between the stomach and esophagus that forms the posterior wall of the anastomosis (88,90). This triangular or V-shaped anastomosis provides a wide anastomosis with a low stricture rate. After completion of the posterolateral aspect of the anastomosis, a nasogastric tube is inserted and guided toward the hiatus for gastric decompression. To create a total mechanical anastomosis, the anterior defect is closed with a 30-mm or 45-mm stapler, whereas a semi-mechanical anastomosis involves using handsewn technique with interrupted 3-0 silk Lembert sutures (9,82). Table 4 describes various studies comparing handsewn and modified Collard approaches to cervical esophagogastric anastomosis for reconstruction during esophagectomy in patients with esophageal cancer.
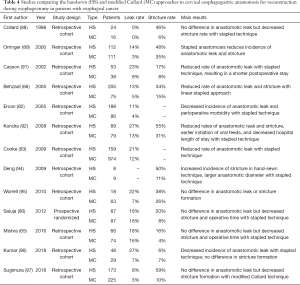
Full table
Hybrid side-to-side thoracic anastomosis techniques
Similarly, to create an intrathoracic anastomosis, following mobilization of the esophagus, the gastric conduit is placed posterior to the divided esophagus to allow the esophagus to overlap 4 to 5 cm onto the stomach. The anterior wall of the gastric conduit is aligned with the posterior wall of the esophageal stump, and an enterotomy is made approximately 4 cm inferior from the tip of the conduit. The jaws of a 30- or 45-mm endoscopic linear cutting stapler are then inserted into the esophagus and gastric conduit to create a side-to-side, functional end-to-end, anastomosis (9,90). The anterior defect is then closed either with another firing of the linear stapler or with a hand-sewn technique. The are some advantages of this technique: the linear stapler is easy to insert through the ribs and easy to use, and the size of the anastomosis is large. However, the main limitation is the length of the esophageal stump needed to align the esophagus with the stomach and the difficulty of creating an anastomosis high in the thoracic cavity.
Circular stapled anastomosis
Circular stapled methods are commonly utilized to create both cervical and intrathoracic anastomoses. An intraluminal EAA stapler with a built-in cylindrical knife creates an end-to-side esophagogastrostomy by placing a detachable anvil into the proximal esophagus, producing a double row of staplers in a circular fashion. There are various anvil sizes available, and choice is guided by the size of the esophagus. Notably, the size of the anvil has been shown to an important risk factor for anastomotic stricture formation in patients without anastomotic leakage, and recent data suggests that the use of a large-sized circular stapler does not lead to an increased rate of anastomotic leakage but may decrease the incidence of stricture (98,99).
The circular stapler can be introduced into the esophagus either using the transthoracic or transoral method. Technically, cervical and intrathoracic anastomoses can be constructed using either of these two methods, however, in clinical practice, the transoral route is rarely used to construct cervical esophagogastrostomies (35).
Transthoracic route
Regardless of location, the anvil of an end-to-end anastomosis (EEA) stapler is inserted into the cut end of the proximal esophagus, and two purse string sutures are placed to secure the esophagus around the stem of the anvil (35). A 2–2.5 cm gastrotomy is created on the anterior gastric wall 5 cm distal to the tip of the fundus along the staple line. The base of the EEA stapler is then inserted into the conduit through the gastrotomy, which is then docked to the anvil (65,69). The anvil and the stapler are subsequently engaged, and the stapler is fired to complete the end-to-side (esophagus to stomach) circular anastomosis (63,100). A nasogastric tube is passed by the anesthetist and advanced downward using manual guidance of the surgeon through the intrathoracic stomach to the antrum for postoperative gastric decompression. The gastrotomy opening is closed by stapling off the excess gastric tissue proximal to the anastomosis (including the anterior gastrotomy site) with an Endo-GIA stapler.
Transoral route
Launched in 2008, the transoral circular stapling device permits transoral passage of a 25-mm anvil, which is mounted on nasogastric tubing (101). This pre-packaged commercially available device contains an anvil head secured in the tilted position that is mounted and secured on a 90cm long PVC delivery tube with a suture (102). This device is given to the anesthesiologist only after the esophagus is transected, as transoral placement of the anvil requires that the proximal esophagus be divided by means of linear stapler (102,103). The PVC delivery tube in then inserted through the patient's mouth by the anesthesiologist until pressure from the orogastric tube is visualized at the staple line of the esophageal stump.
Once the tip of the oral-gastric tube is observed within the esophageal stump, a small esophagotomy is performed perpendicular to the staple-line of the esophageal stump, allowing advancement of the orogastric tube until it can be grasped by the surgeon, who pulls it out through a thoracic trocar until the EEA anvil is comfortably positioned in the proximal esophagus (103,104). While holding the anvil in place, the tubing is disconnected from the anvil and an EEA anastomosis is performed in standard fashion (see Figure 3). This technique is especially useful when creating an intrathoracic anastomosis as it avoids the need to secure the anvil in the esophagus with purse string sutures (9,42). As a result, less technical skill is required, and the anastomosis can be created more quickly as compared to hand-sewn techniques. In a prospective randomized controlled trial, when compared with the hand-sewn method, the circular stapler method for esophagogastric anastomoses was associated with reduced incidence of anastomotic leak, shorter operative times, and increased risk of anastomotic strictures (100). Tables 5 and 6 highlight relevant studies comparing semi-mechanical linear stapled (LS), circular stapled (CS), and handsewn (HS) intrathoracic anastomoses.
Full table
Full table
Other factors to improve anastomotic outcomes
Perfusion assessment
Successful esophageal anastomosis following esophagectomy relies on preservation of the right gastric and right gastroepiploic arteries for adequate perfusion and is critical for wound healing and prevention of postoperative anastomotic complications. Often, anastomotic leaks are attributed to technical errors that stem from a perfusion deficiency of the gastric conduit or tension on the anastomosis as a result of rough handling, poor preparation, and suboptimal technique, and ultimately compromised perfusion of the gastric conduit perioperatively has been reported to be a major risk factor for benign anastomotic stricture following esophagectomy (21,112,113). Prevention strategies are aimed at correction of patient related factors and systemic variables can influence anastomotic integrity, including patient nutritional status, medical comorbidities, and fluid balance and precise gastroesophageal mobilization and dissection to ensure the formation of a tension-free anastomosis (35,65,114).
The proximal portion of the conduit is particularly prone to ischemia because the gastroduodenal artery rarely reaches the tip of the graft (115). Therefore, intraoperative assessment of perfusion is critical for the early detection of compromised perfusion and may be used to guide surgical decision making with regards to the location of the anastomosis or may highlight the need for additional surgical intervention (115,116). Traditionally, assessment of intraoperative conduit perfusion was with visual clinical inspection of the intestinal color, pulsations of the vessels, bleeding from the cut edge, and temperature of the anastomosis site (115,117). However, the accuracy of this method is greatly limited as it relies on subjective assessment of parameters that do not reliably correspond to perfusion (17,18,117).
Newer methods have subsequently been developed to aid assessment of gastric conduit viability and currently, there are several noninvasive optical techniques that allow intraoperative assessment of perfusion in real time. The first of these optical techniques developed was intraoperative laser doppler flowmetry (LDF), which uses a low-power laser to measure the Doppler shift of moving red blood cells within the microcirculation (118). Using this technique, perfusion is assessed prior to creating the anastomosis so that the optimal location can be chosen, taking care to minimize tension in the conduit (119).
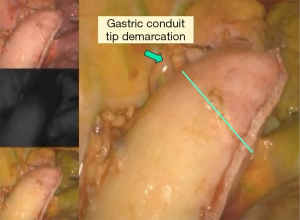
Intraoperative fluorescence angiography (FA) using near-infrared imaging (NIFI) with indocyanine green (ICG) is the most commonly used method at our institution. This method provides visual mapping and accurate quantitative measurement of the arterial blood flow and venous return of the reconstructed gastric tube in patients undergoing esophagectomy (120-122). The system provides four images to aid in the assessment of perfusion (Figures 4,5).

Omental flap or other reinforcement techniques
The greater omentum is a free-hanging mesenteric tissue that hangs down from the greater curve of the stomach to cover the surface of the intra-peritoneal organs. As a result of unique inherent anatomic and physiologic properties, the omentum is often exploited during various surgical procedures to promote local control of infection, wound healing, and tissue regeneration (117,118). Regardless of the approach chosen, whenever feasible, often surgeons will perform an omentoplasty during esophagectomy to reinforce the esophagogastric anastomosis. During this procedure, a pedicled omental flap based of the right gastroepiploic arcade is created by dividing the omentum off the transverse colon in the avascular plane, which is then used to envelop the entire anastomosis as well as the gastric staple line (123,124). The omental pedicle promotes healing and regeneration of the injured tissue as a result of its rich blood supply, innate immune function, high absorptive capacity, and secretion of pro-angiogenic and chemotactic factors that promote angiogenesis and neovascularization (125-129).
A recent metanalysis that included 6 randomized controlled trials with a total of 1,608 patients reported a significant reduction in the incidence of anastomotic leak and length of hospital stay when an omentoplasty was performed (130). Notably, the addition of omentoplasty was not associated with a significant change in hospital mortality, duration of hospital stay, or incidence of anastomotic stricture, pulmonary and cardiac complication, infection, vocal cord palsy, and peri-jejunostomy leakage. While some retrospective reviews have reported similar results, citing reduced cases of anastomotic leak in both cervical and intrathoracic anastomoses when an omentoplasty was performed (126,128,131-133), others have failed to show any significant difference in anastomotic leak rate with the addition of omentoplasty (134), Larger clinical trials are needed to further define the role of omentoplasty after esophagectomy.
Conclusion
Anastomosis creation remains the most critical step during esophagectomy. As perioperative outcomes continue to improve, further emphasis is placed on the construction of a durable anastomosis without complication. Despite a large volume of research investigating this topic, considerable debate remains on the optimal technique. While continued research is needed to ensure adequate conduit perfusion and prevent anastomotic complications, it is probably more important for surgeons to have a standardized method that they can confidently perform.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christopher R. Morse and Uma M. Sachdeva) for the series “Minimally Invasive Esophagectomy” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-40). The series “Minimally Invasive Esophagectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Atkins BZ, Shah AS, Hutcheson KA, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg 2004;78:1170-6; discussion 1170-6. [Crossref] [PubMed]
- Zingg U, Smithers BM, Gotley DC, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol 2011;18:1460-8. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Flanagan JC, Batz R, Saboo SS, et al. Esophagectomy and Gastric Pull-through Procedures: Surgical Techniques, Imaging Features, and Potential Complications. Radiographics 2016;36:107-21. [Crossref] [PubMed]
- Weijs TJ, Ruurda JP, Nieuwenhuijzen GA, et al. Strategies to reduce pulmonary complications after esophagectomy. World J Gastroenterol 2013;19:6509-14. [Crossref] [PubMed]
- Vrba R, Vrána D, Neoral Č, et al. Respiratory complications following mini-invasive laparoscopic and thoracoscopic esophagectomy for esophageal cancer. Experience in 215 patients. Wideochir Inne Tech Maloinwazyjne 2019;14:52-9. [Crossref] [PubMed]
- Giugliano DN, Berger AC, Rosato EL, et al. Total minimally invasive esophagectomy for esophageal cancer: approaches and outcomes. Langenbecks Arch Surg 2016;401:747-56. [Crossref] [PubMed]
- Murthy RA, Clarke NS, Kernstine KH Sr. Minimally Invasive and Robotic Esophagectomy: A Review. Innovations (Phila) 2018;13:391-403. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Collard JM, Lengele B, Otte JB, et al. En bloc and standard esophagectomies by thoracoscopy. Ann Thorac Surg 1993;56:675-9. [Crossref] [PubMed]
- Vaghjiani RG, Molena D. Surgical management of esophageal cancer. Chin Clin Oncol 2017;6:47. [Crossref] [PubMed]
- Watson DI, Davies N, Jamieson GG. Totally endoscopic Ivor Lewis esophagectomy. Surg Endosc 1999;13:293-7. [Crossref] [PubMed]
- DePaula AL, Hashiba K, Ferreira EA, et al. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc 1995;5:1-5. [PubMed]
- Ahmadi N, Crnic A, Seely AJ, et al. Impact of surgical approach on perioperative and long-term outcomes following esophagectomy for esophageal cancer. Surg Endosc 2018;32:1892-900. [Crossref] [PubMed]
- Gottlieb-Vedi E, Kauppila JH, Malietzis G, et al. Long-term Survival in Esophageal Cancer After Minimally Invasive Compared to Open Esophagectomy: A Systematic Review and Meta-analysis. Ann Surg 2019;270:1005-17. [Crossref] [PubMed]
- Booka E, Takeuchi H, Kikuchi H, et al. Recent advances in thoracoscopic esophagectomy for esophageal cancer. Asian J Endosc Surg 2019;12:19-29. [Crossref] [PubMed]
- Lv L, Hu W, Ren Y, et al. Minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: a meta-analysis. Onco Targets Ther 2016;9:6751-62. [Crossref] [PubMed]
- Sihag S, Kosinski AS, Gaissert HA, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Comparison of Early Surgical Outcomes From The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101:1281-8; discussion 1288-9. [Crossref] [PubMed]
- Taurchini M, Cuttitta A. Minimally invasive and robotic esophagectomy: state of the art. J Vis Surg 2017;3:125. [Crossref] [PubMed]
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Zhou C, Ma G, Li X, et al. Is minimally invasive esophagectomy effective for preventing anastomotic leakages after esophagectomy for cancer? A systematic review and meta-analysis. World J Surg Oncol 2015;13:269. [Crossref] [PubMed]
- Markar SR, Arya S, Karthikesalingam A, et al. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol 2013;20:4274-81. [Crossref] [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg 1992;54:1110-5. [Crossref] [PubMed]
- Chadi SA, Fingerhut A, Berho M, et al. Emerging Trends in the Etiology, Prevention, and Treatment of Gastrointestinal Anastomotic Leakage. J Gastrointest Surg 2016;20:2035-51. [Crossref] [PubMed]
- Aoyama T, Atsumi Y, Hara K, et al. Risk Factors for Postoperative Anastomosis Leak After Esophagectomy for Esophageal Cancer. In Vivo 2020;34:857-62. [Crossref] [PubMed]
- Agzarian J, Visscher SL, Knight AW, et al. The cost burden of clinically significant esophageal anastomotic leaks-a steep price to pay. J Thorac Cardiovasc Surg 2019;157:2086-92. [Crossref] [PubMed]
- Jones CE, Watson TJ. Anastomotic Leakage Following Esophagectomy. Thorac Surg Clin 2015;25:449-59. [Crossref] [PubMed]
- Lerut T, Coosemans W, Decker G, et al. Anastomotic Complications after Esophagectomy. Dig Surg 2002;19:92-8. [Crossref] [PubMed]
- Tanaka K, Makino T, Yamasaki M, et al. An analysis of the risk factors of anastomotic stricture after esophagectomy. Surg Today 2018;48:449-54. [Crossref] [PubMed]
- Gujjuri RR, Kamarajah SK, Markar SR. Effect of anastomotic leaks on long-term survival after oesophagectomy for oesophageal cancer: systematic review and meta-analysis. Dis Esophagus 2021;34:doaa085.
- Andreou A, Biebl M, Dadras M, et al. Anastomotic leak predicts diminished long-term survival after resection for gastric and esophageal cancer. Surgery 2016;160:191-203. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. The Impact of Severe Anastomotic Leak on Long-term Survival and Cancer Recurrence After Surgical Resection for Esophageal Malignancy. Ann Surg 2015;262:972-80. [Crossref] [PubMed]
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. [Crossref] [PubMed]
- Myssiorek D. Recurrent laryngeal nerve paralysis: anatomy and etiology. Otolaryngol Clin North Am 2004;37:25-44. v. [Crossref] [PubMed]
- Scholtemeijer MG, Seesing MFJ, Brenkman HJF, et al. Recurrent laryngeal nerve injury after esophagectomy for esophageal cancer: incidence, management, and impact on short- and long-term outcomes. J Thorac Dis 2017;9:S868-78. [Crossref] [PubMed]
- Noble F, Kelly JJ, Bailey IS, et al. A prospective comparison of totally minimally invasive versus open Ivor Lewis esophagectomy. Dis Esophagus 2013;26:263-71. [Crossref] [PubMed]
- Cadiere GB, Dapri G, Himpens J, et al. Ivor Lewis esophagectomy with manual esogastric anastomosis by thoracoscopy in prone position and laparoscopy. Surg Endosc 2010;24:1482-5. [Crossref] [PubMed]
- Hoppo T, Jobe BA, Hunter JG. Minimally invasive esophagectomy: the evolution and technique of minimally invasive surgery for esophageal cancer. World J Surg 2011;35:1454-63. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [Crossref] [PubMed]
- Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc 2012;26:1795-802. [Crossref] [PubMed]
- Nguyen NT, Follette DM, Lemoine PH, et al. Minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2001;72:593-6. [Crossref] [PubMed]
- Agasthian T, Shabbir A. VATS hand sewn intrathoracic esophagogastric anastomosis. J Vis Surg 2017;3:90. [Crossref] [PubMed]
- van Workum F, van der Maas J, van den Wildenberg FJ, et al. Improved Functional Results After Minimally Invasive Esophagectomy: Intrathoracic Versus Cervical Anastomosis. Ann Thorac Surg 2017;103:267-73. [Crossref] [PubMed]
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011;28:29-35. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Meredith K, Blinn P, Maramara T, et al. Comparative outcomes of minimally invasive and robotic-assisted esophagectomy. Surg Endosc 2020;34:814-20. [Crossref] [PubMed]
- Gooszen JAH, Goense L, Gisbertz SS, et al. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. Br J Surg 2018;105:552-60. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Chasseray VM, Kiroff GK, Buard JL, et al. Cervical or thoracic anastomosis for esophagectomy for carcinoma. Surg Gynecol Obstet 1989;169:55-62. [PubMed]
- Ribet M, Debrueres B, Lecomte-Houcke M. Resection for advanced cancer of the thoracic esophagus: cervical or thoracic anastomosis? Late results of a prospective randomized study. J Thorac Cardiovasc Surg 1992;103:784-9. [Crossref] [PubMed]
- Blewett CJ, Miller JD, Young JE, et al. Anastomotic leaks after esophagectomy for esophageal cancer: a comparison of thoracic and cervical anastomoses. Ann Thorac Cardiovasc Surg 2001;7:75-8. [PubMed]
- Okuyama M, Motoyama S, Suzuki H, et al. Hand-sewn cervical anastomosis versus stapled intrathoracic anastomosis after esophagectomy for middle or lower thoracic esophageal cancer: a prospective randomized controlled study. Surg Today 2007;37:947-52. [Crossref] [PubMed]
- Price TN, Nichols FC, Harmsen WS, et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg 2013;95:1154-60; discussion 1160-1. [Crossref] [PubMed]
- Liu YJ, Fan J, He HH, et al. Anastomotic leakage after intrathoracic versus cervical oesophagogastric anastomosis for oesophageal carcinoma in Chinese population: a retrospective cohort study. BMJ Open 2018;8:e021025 [Crossref] [PubMed]
- Schroder W, Raptis DA, Schmidt HM, et al. Anastomotic Techniques and Associated Morbidity in Total Minimally Invasive Transthoracic Esophagectomy: Results From the EsoBenchmark Database. Ann Surg 2019;270:820-6. [Crossref] [PubMed]
- van Workum F, Slaman AE, van Berge Henegouwen MI, et al. Propensity Score-Matched Analysis Comparing Minimally Invasive Ivor Lewis Versus Minimally Invasive Mckeown Esophagectomy. Ann Surg 2020;271:128-33. [Crossref] [PubMed]
- Kim RH, Takabe K. Methods of esophagogastric anastomoses following esophagectomy for cancer: A systematic review. J Surg Oncol 2010;101:527-33. [Crossref] [PubMed]
- Law S, Fok M, Chu KM, et al. Comparison of hand-sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg 1997;226:169-73. [Crossref] [PubMed]
- Beitler AL, Urschel JD. Comparison of stapled and hand-sewn esophagogastric anastomoses. Am J Surg 1998;175:337-40. [Crossref] [PubMed]
- Honda M, Kuriyama A, Noma H, et al. Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Ann Surg 2013;257:238-48. [Crossref] [PubMed]
- Yuan Y, Wang KN, Chen LQ. Esophageal anastomosis. Dis Esophagus 2015;28:127-37. [Crossref] [PubMed]
- Gorenstein LA, Bessler M, Sonett JR. Intrathoracic linear stapled esophagogastric anastomosis: an alternative to the end to end anastomosis. Ann Thorac Surg 2011;91:314-6. [Crossref] [PubMed]
- Mishra PK, Shah H, Gupta N, et al. Stapled versus hand-sewn cervical esophagogastric anastomosis in patients undergoing esophagectomy: A Retrospective Cohort Study. Ann Med Surg (Lond) 2016;5:118-24. [Crossref] [PubMed]
- Behzadi A, Nichols FC, Cassivi SD, et al. Esophagogastrectomy: the influence of stapled versus hand-sewn anastomosis on outcome. J Gastrointest Surg 2005;9:1031-40; discussion 1040-2. [Crossref] [PubMed]
- Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part I surgical techniques. Dis Esophagus 2009;22:195-202. [Crossref] [PubMed]
- Bardini R, Bonavina L, Asolati M, et al. Single-layered cervical esophageal anastomoses: a prospective study of two suturing techniques. Ann Thorac Surg 1994;58:1087-9; discussion 1089-90. [Crossref] [PubMed]
- Linden PA, Sugarbaker DJ. Section V: techniques of esophageal resection. Semin Thorac Cardiovasc Surg 2003;15:197-209. [Crossref] [PubMed]
- Zhu ZJ, Zhao YF, Chen LQ, et al. Clinical application of layered anastomosis during esophagogastrostomy. World J Surg 2008;32:583-8. [Crossref] [PubMed]
- Zieren HU, Muller JM, Pichlmaier H. Prospective randomized study of one- or two-layer anastomosis following oesophageal resection and cervical oesophagogastrostomy. Br J Surg 1993;80:608-11. [Crossref] [PubMed]
- Aslam V, Bilal A, Khan A, et al. Gastroesophageal anastomosis: single-layer versus double-layer technique--an experience on 50 cases. J Ayub Med Coll Abbottabad 2008;20:6-9. [PubMed]
- Nederlof N, Tilanus HW, Tran TC, et al. End-to-end versus end-to-side esophagogastrostomy after esophageal cancer resection: a prospective randomized study. Ann Surg 2011;254:226-33. [Crossref] [PubMed]
- Haverkamp L, van der Sluis PC, Verhage RJ, et al. End-to-end cervical esophagogastric anastomoses are associated with a higher number of strictures compared with end-to-side anastomoses. J Gastrointest Surg 2013;17:872-6. [Crossref] [PubMed]
- Pierie JP, De Graaf PW, Poen H, et al. End-to-side and end-to-end anastomoses give similar results in cervical oesophagogastrostomy. Eur J Surg 1995;161:893-6. [PubMed]
- Blackmon SH, Correa AM, Wynn B, et al. Propensity-Matched Analysis of Three Techniques for Intrathoracic Esophagogastric Anastomosis. Ann Thorac Surg 2007;83:1805-13. [Crossref] [PubMed]
- Elshaer M, Gravante G, Tang CB, et al. Totally minimally invasive two-stage esophagectomy with intrathoracic hand-sewn anastomosis: short-term clinical and oncological outcomes. Dis Esophagus 2018;31. [Crossref] [PubMed]
- Caso R, Wee JO. Esophagogastric Anastomotic Techniques for Minimally Invasive and Robotic Ivor Lewis Operations. Oper Tech Thorac Cardiovasc Surg 2020;25:105-23. [Crossref]
- Kingma BF, de Maat MFG, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy (RAMIE) improves perioperative outcomes: a review. J Thorac Dis 2019;11:S735-S42. [Crossref] [PubMed]
- Charalabopoulos A, Lorenzi B, Kordzadeh A, et al. Role of 3D in minimally invasive esophagectomy. Langenbecks Arch Surg 2017;402:555-61. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol 2015;22:S1350-6. [Crossref] [PubMed]
- Ercan S, Rice TW, Murthy SC, et al. Does esophagogastric anastomotic technique influence the outcome of patients with esophageal cancer? J Thorac Cardiovasc Surg 2005;129:623-31. [Crossref] [PubMed]
- Harbison GJ, Vossler JD, Yim NH, et al. Outcomes of robotic versus non-robotic minimally-invasive esophagectomy for esophageal cancer: An American College of Surgeons NSQIP database analysis. Am J Surg 2019;218:1223-8. [Crossref] [PubMed]
- Jin D, Yao L, Yu J, et al. Robotic-assisted minimally invasive esophagectomy versus the conventional minimally invasive one: A meta-analysis and systematic review. Int J Med Robot 2019;15:e1988 [Crossref] [PubMed]
- Liboni A, Mari C, Zamboni P, et al. A new technic for esophago-enteral anastomosis with a mechanical stapler without purse-string sutures. Ann Ital Chir 1989;60:125-7; discussion 128. [PubMed]
- Muguruma K, Tanaka H, Sakurai K, et al. Laparoscopy-assisted total gastrectomy: a simplified approach. Int Surg 2014;99:79-85. [Crossref] [PubMed]
- Kimura M, Mitsui A, Kuwabara Y. Creation of the ideal gastric tube: Comparison of three methods: A prospective cohort study. Ann Med Surg (Lond) 2016;6:42-5. [Crossref] [PubMed]
- Collard JM, Romagnoli R, Goncette L, et al. Terminalized semimechanical side-to-side suture technique for cervical esophagogastrostomy. Ann Thorac Surg 1998;65:814-7. [Crossref] [PubMed]
- Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 2000;119:277-88. [Crossref] [PubMed]
- Saluja SS, Ray S, Pal S, et al. Randomized trial comparing side-to-side stapled and hand-sewn esophagogastric anastomosis in neck. J Gastrointest Surg 2012;16:1287-95. [Crossref] [PubMed]
- Casson AG, Porter GA, Veugelers PJ. Evolution and critical appraisal of anastomotic technique following resection of esophageal adenocarcinoma. Dis Esophagus 2002;15:296-302. [Crossref] [PubMed]
- Kondra J, Ong SR, Clifton J, et al. A change in clinical practice: a partially stapled cervical esophagogastric anastomosis reduces morbidity and improves functional outcome after esophagectomy for cancer. Dis Esophagus 2008;21:422-9. [Crossref] [PubMed]
- Cooke DT, Lin GC, Lau CL, et al. Analysis of cervical esophagogastric anastomotic leaks after transhiatal esophagectomy: risk factors, presentation, and detection. Ann Thorac Surg 2009;88:177-84; discussion 184-5. [Crossref] [PubMed]
- Deng B, Wang RW, Jiang YG, et al. Functional and menometric study of side-to-side stapled anastomosis and traditional hand-sewn anastomosis in cervical esophagogastrostomy. Eur J Cardiothorac Surg 2009;35:8-12. [Crossref] [PubMed]
- Worrell S, Mumtaz S, Tsuboi K, et al. Anastomotic complications associated with stapled versus hand-sewn anastomosis. J Surg Res 2010;161:9-12. [Crossref] [PubMed]
- Kumar T, Krishanappa R, Pai E, et al. Completely Linear Stapled Versus Handsewn Cervical Esophagogastric Anastomosis After Esophagectomy. Indian J Surg 2018;80:134-9. [Crossref] [PubMed]
- Sugimura K, Miyata H, Matsunaga T, et al. Comparison of the modified Collard and hand-sewn anastomosis for cervical esophagogastric anastomosis after esophagectomy in esophageal cancer patients: A propensity score-matched analysis. Ann Gastroenterol Surg 2019;3:104-13. [Crossref] [PubMed]
- Hosoi T, Abe T, Uemura N, et al. The Impact of Circular Stapler Size on the Incidence of Cervical Anastomotic Stricture After Esophagectomy. World J Surg 2019;43:1746-55. [Crossref] [PubMed]
- Allen W, Wells CI, Greenslade M, et al. Association Between Circular Stapler Diameter and Stricture Rates Following Gastrointestinal Anastomosis: Systematic Review and Meta-analysis. World J Surg 2018;42:3097-105. [Crossref] [PubMed]
- Liu QX, Qiu Y, Deng XF, et al. Comparison of outcomes following end-to-end hand-sewn and mechanical oesophagogastric anastomosis after oesophagectomy for carcinoma: a prospective randomized controlled trial. Eur J Cardiothorac Surg 2015;47:e118-23. [Crossref] [PubMed]
- Campos GM, Jablons D, Brown LM, et al. A safe and reproducible anastomotic technique for minimally invasive Ivor Lewis oesophagectomy: the circular-stapled anastomosis with the trans-oral anvil. Eur J Cardiothorac Surg 2010;37:1421-6. [Crossref] [PubMed]
- Nguyen NT, Nguyen X-MT, Masoomi H. Minimally Invasive Intrathoracic Esophagogastric Anastomosis: Circular Stapler Technique with Transoral Placement of the Anvil. Semin Thorac Cardiovasc Surg 2010;22:253-5. [Crossref] [PubMed]
- Jaroszewski DE, Williams DG, Fleischer DE, et al. An Early Experience Using the Technique of Transoral OrVil EEA Stapler for Minimally Invasive Transthoracic Esophagectomy. Ann Thorac Surg 2011;92:1862-9. [Crossref] [PubMed]
- Valmasoni M, Capovilla G, Pierobon ES, et al. A Technical Modification to the Circular Stapling Anastomosis Technique During Minimally Invasive Ivor Lewis Procedure. J Laparoendosc Adv Surg Tech A 2019;29:1585-91. [Crossref] [PubMed]
- Craig SR, Walker WS, Cameron EW, et al. A prospective randomized study comparing stapled with handsewn oesophagogastric anastomoses. J R Coll Surg Edinb 1996;41:17-9. [PubMed]
- Luechakiettisak P, Kasetsunthorn S. Comparison of hand-sewn and stapled in esophagogastric anastomosis after esophageal cancer resection: a prospective randomized study. J Med Assoc Thai 2008;91:681-5. [PubMed]
- Wang WP, Gao Q, Wang KN, et al. A prospective randomized controlled trial of semi-mechanical versus hand-sewn or circular stapled esophagogastrostomy for prevention of anastomotic stricture. World J Surg 2013;37:1043-50. [Crossref] [PubMed]
- Harustiak T, Pazdro A, Snajdauf M, et al. Anastomotic leak and stricture after hand-sewn versus linear-stapled intrathoracic oesophagogastric anastomosis: single-centre analysis of 415 oesophagectomies. Eur J Cardiothorac Surg 2016;49:1650-9. [Crossref] [PubMed]
- Xu QR, Wang KN, Wang WP, et al. Linear stapled esophagogastrostomy is more effective than hand-sewn or circular stapler in prevention of anastomotic stricture: a comparative clinical study. J Gastrointest Surg 2011;15:915-21. [Crossref] [PubMed]
- Yanni F, Singh P, Tewari N, et al. Comparison of Outcomes with Semi-mechanical and Circular Stapled Intrathoracic Esophagogastric Anastomosis following Esophagectomy. World J Surg 2019;43:2483-9. [Crossref] [PubMed]
- Zhang H, Wang Z, Zheng Y, et al. Robotic Side-to-Side and End-to-Side Stapled Esophagogastric Anastomosis of Ivor Lewis Esophagectomy for Cancer. World J Surg 2019;43:3074-82. [Crossref] [PubMed]
- Prasetya H, Jansen SM, Marquering HA, et al. Estimation of microvascular perfusion after esophagectomy: a quantitative model of dynamic fluorescence imaging. Med Biol Eng Comput 2019;57:1889-900. [Crossref] [PubMed]
- Wang X, Pei X, Li X, et al. Predictive Value of Anastomotic Blood Supply for Anastomotic Stricture After Esophagectomy in Esophageal Cancer. Dig Dis Sci 2019;64:3307-13. [Crossref] [PubMed]
- Messager M, Warlaumont M, Renaud F, et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 2017;43:258-69. [Crossref] [PubMed]
- Koyanagi K, Ozawa S, Oguma J, et al. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: New predictive evaluation of anastomotic leakage after esophagectomy. Medicine (Baltimore) 2016;95:e4386 [Crossref] [PubMed]
- Schlottmann F, Patti MG. Evaluation of Gastric Conduit Perfusion During Esophagectomy with Indocyanine Green Fluorescence Imaging. J Laparoendosc Adv Surg Tech A 2017;27:1305-8. [Crossref] [PubMed]
- Athanasiou A, Hennessy M, Spartalis E, et al. Conduit necrosis following esophagectomy: An up-to-date literature review. World J Gastrointest Surg 2019;11:155-68. [Crossref] [PubMed]
- Jansen SM, de Bruin DM, van Berge Henegouwen MI, et al. Optical techniques for perfusion monitoring of the gastric tube after esophagectomy: a review of technologies and thresholds. Dis Esophagus 2018;31: [Crossref] [PubMed]
- Boyle NH, Pearce A, Owen WJ, et al. Validation of scanning laser Doppler flowmetry against single point laser Doppler flowmetry in the measurement of human gastric serosal/muscularis perfusion. Int J Surg Investig 2000;2:203-11. [PubMed]
- Ladak F, Dang JT, Switzer N, et al. Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 2019;33:384-94. [Crossref] [PubMed]
- Ishige F, Nabeya Y, Hoshino I, et al. Quantitative Assessment of the Blood Perfusion of the Gastric Conduit by Indocyanine Green Imaging. J Surg Res 2019;234:303-10. [Crossref] [PubMed]
- Slooter MD, Eshuis WJ, Cuesta MA, et al. Fluorescent imaging using indocyanine green during esophagectomy to prevent surgical morbidity: a systematic review and meta-analysis. J Thorac Dis 2019;11:S755-65. [Crossref] [PubMed]
- Liu QX, Deng XF, Hou B, et al. Preventing and localizing esophagogastric anastomosis leakage by sleeve-wrapping of the pedicled omentum. World J Gastroenterol 2014;20:16282-6. [Crossref] [PubMed]
- Dai JG, Zhang ZY, Min JX, et al. Wrapping of the omental pedicle flap around esophagogastric anastomosis after esophagectomy for esophageal cancer. Surgery 2011;149:404-10. [Crossref] [PubMed]
- Shrager JB, Wain JC, Wright CD, et al. Omentum is highly effective in the management of complex cardiothoracic surgical problems. J Thorac Cardiovasc Surg 2003;125:526-32. [Crossref] [PubMed]
- Di Nicola V. Omentum a powerful biological source in regenerative surgery. Regen Ther 2019;11:182-91. [Crossref] [PubMed]
- Wang AW, Prieto JM, Cauvi DM, et al. The Greater Omentum-A Vibrant and Enigmatic Immunologic Organ Involved in Injury and Infection Resolution. Shock 2020;53:384-90. [Crossref] [PubMed]
- Shelton EL, Poole SD, Reese J, et al. Omental grafting: a cell-based therapy for blood vessel repair. J Tissue Eng Regen Med 2013;7:421-33. [Crossref] [PubMed]
- Bhat MA, Dar MA, Lone GN, et al. Use of pedicled omentum in esophagogastric anastomosis for prevention of anastomotic leak. Ann Thorac Surg 2006;82:1857-62. [Crossref] [PubMed]
- Yuan Y, Zeng X, Hu Y, et al. Omentoplasty for esophagogastrostomy after esophagectomy. Cochrane Database Syst Rev 2012;11:CD008446 [PubMed]
- Zhou D, Liu QX, Deng XF, et al. Anastomotic reinforcement with omentoplasty reduces anastomotic leakage for minimally invasive esophagectomy with cervical anastomosis. Cancer Manag Res 2018;10:257-63. [Crossref] [PubMed]
- Tuo G, Jin G, Pang Y, et al. Omentoplasty Decreases Leak Rate After Esophagectomy: a Meta-analysis. J Gastrointest Surg 2020;24:1237-43. [Crossref] [PubMed]
- Lu M, Luketich JD, Levy RM, et al. Anastomotic complications after esophagectomy: Influence of omentoplasty in propensity-weighted cohorts. J Thorac Cardiovasc Surg 2020;159:2096-105. [Crossref] [PubMed]
- Kamarajah SK, Boyle C, Bundred JR, Tan BH. Critical appraisal of gastric conduit ischaemic conditioning (GIC) prior to oesophagectomy: A systematic review and meta-analysis. Int J Surg 2020;77:77-82. [Crossref] [PubMed]
Cite this article as: Carr RA, Molena D. Minimally invasive esophagectomy: anastomotic techniques. Ann Esophagus 2021;4:19.




