
Surgery and adjuvant therapy after esophagectomy
Introduction
Ranking as the sixth leading cause of cancer deaths (1), the prognosis for esophageal cancer remains dismal. Two major histological subtypes exist: adenocarcinoma (AC), which is most prevalent in North America and Europe, and esophageal squamous cell carcinoma (ESCC), which is predominant in Asia, Africa, and South America. ESCC and AC differ in terms of location of the tumor and tumor biology. Successful treatment of esophageal cancer requires a multidisciplinary approach consisting of surgery, chemotherapy, and radiotherapy (2-4). Moreover, as treatment strategies increase, individualization of treatment will likely achieve the best outcomes. Here, we review future perspective on adjuvant and perioperative multimodality treatments for resectable esophageal cancer.
Surgical procedures for esophageal cancer
Esophagectomy has been the mainstay in the treatment of curable esophageal cancer. A safe and effective surgical approach is important to eradicate the primary tumor and possible metastases. However, there are many different approaches. For AC, the standard procedure is an Ivor Lewis esophagectomy including a lower mediastinal and perigastric lymph node (LN) dissection. An intrathoracic anastomosis is conducted. The second operation technique is the transhiatal esophagectomy (5). Kurokawa et al. conducted a multicenter prospective interventional study to evaluate the distribution of LN metastasis of esophagogastric junction (EGJ) cancer (6). The study reported that esophageal invasion longer than 4 cm is a risk factor for upper mediastinal LN metastasis, and they suggested upper mediastinal lymphadenectomy for these population. Via a transhiatal approach, these nodes cannot be removed. The authors then conclude that there is an indication for upper mediastinal LN dissection for AC of the EGJ. This is in contrast to the Dutch randomized controlled trial which compared transhiatal with a transthoracic approach as this study did not show a benefit of extended mediastinal lymphadenectomy (7).
Esophageal squamous cell carcinoma (ESCC) often resides at mid-thoracic esophagus and can metastasize widely from the neck to the abdominal nodes, even in the early stage of the disease (8). Because the LNs around the recurrent laryngeal nerve are one of the most frequent locations for metastasis (8-10), upper mediastinal LN dissection is required. So-called three-field LN dissection, which is an extended LN dissection including the supraclavicular LNs, has been shown to be beneficial and is standard in Japan (11-13). In this procedure, an anastomosis is performed mostly in the neck.
Regardless what surgical approach is chosen, postoperative pneumonia and anastomotic leakage are relatively common complications that worsen postoperative patients’ conditions (14,15). In order to minimize surgical invasiveness, minimally invasive esophagectomy (MIE) and robot-assisted MIE (RAMIE) were introduced. MIE reduces the incidence of postoperative complications, including pneumonia, when compared to open esophagectomy (16,17). More recently, van der Sluis et al. conducted a randomized controlled trial to investigate the safety of RAMIE compared to open esophagectomy, and they reported that RAMIE exhibited a significantly lower percentage of surgery-related and cardiopulmonary complications with less postoperative pain (18). For ESCC, a nationwide retrospective study using a Japanese national clinical database demonstrated that MIE was associated with a lower incidence of pneumonia (19). Conversely, long-term outcomes of MIE have not been reported yet. We are currently running a phase III trial to compare overall survival between open and MIE (20).
Adjuvant chemotherapy for esophageal cancer
Rationale and historical perspective
The rationale of adjuvant therapy is to eradicate residual tumor cells outside the regional field and suppress postoperative recurrence. Since esophagectomy is highly invasive and could delay postoperative recovery, treatment needs to be tolerable. Several decades ago, since the improvement of the safety of surgical procedure and perioperative care, surgery had been established as the only curative treatment for surgically resectable esophageal and gastric cancer. In order to eradicate the residual disease after surgery and further improve survival, the efficacy of adjuvant treatment provided after surgery was examined. With regard to ESCC, the Japanese JCOG9204 trial evaluated the superiority of adjuvant therapy using fluorouracil (F) and cisplatin (C) rather than surgery alone (21). Consequently, disease-free survival was shown to be significantly longer in the adjuvant therapy group. Concerning overall survival, the risk reduction by adjuvant CF therapy was notable in patients diagnosed with LN metastasis in the resected specimens. For AC, adjuvant chemoradiotherapy was recognized as a standard since the intergroup 0116 trial in 2001 proved its efficacy in comparison to surgery alone (22). On the contrary, despite criticisms on the relatively lower rate of D2 lymph node dissection in that trial, the efficacy of fluorouracil and radiation as adjuvant therapy was maintained by CALGB 80101 trial. However, it failed to prove the superiority of epirubicin, cisplatin, and infusion 5-fluorouracil (ECF) with radiation for gastric and AC of EGJ (23). In East Asia, the development of adjuvant chemotherapy without radiation was mainly focused on gastric cancer. Surgery alone versus adjuvant capecitabin plus oxaliplatin (CLASSIC) and surgery alone versus S-1 monotherapy (ACTS-GC) which compared adjuvant chemotherapy with surgery alone remarkably demonstrated the survival advantages of adjuvant chemotherapy (24,25). On the contrary, due to the relatively lower incidence of AC of EGJ in Asia, adjuvant chemotherapy without radiation for GEJ lacked full investigation.
Adjuvant, neoadjuvant, and perioperative therapies
Despite the reported survival advantage of adjuvant therapy compared to surgery alone, the treatment is not ideal to all candidates for adjuvant therapy. Furthermore, postoperative complications negatively impact cancer relapse and overall survival (26). Our group previously reported that the postoperative systemic inflammatory response is associated with disease recurrence independent of infectious complications (27). These previous findings further suggested reducing the amount of tumor cells prior to esophagectomy, which led the introduction of neoadjuvant treatment.
For ESCC, the Japan Clinical Oncology Group conducted a multicenter phase III trial comparing the efficacy of neoadjuvant CF therapy with adjuvant CF therapy (28). Results demonstrated the survival advantage of neoadjuvant CF based on the planned interim analysis after patient accrual. Based on these results, neoadjuvant CF became a standard in Japan (29,30). For AC, instead of adjuvant chemotherapy, perioperative treatment (neoadjuvant and adjuvant treatment combined) has been tested. In the 2000s, the British MAGIC trial established the survival benefits of perioperative epirubicin, cisplatin, and fluorouracil (ECF) (31). In parallel, the French FFCD9703 trial evaluated the impact of perioperative fluorouracil and cisplatin on AC, where 25% of tumors were located in the distal stomach (32).
In the last decade, several landmark trials have demonstrated that the intensification of neoadjuvant and perioperative therapies could further improve outcomes. Moreover, in 2012, the Dutch CROSS trial proved the efficacy of neoadjuvant chemoradiotherapy using carboplatin and paclitaxel (33). In 2019, the German FLOT4 trial reported the efficacy of triplet chemotherapy using fluorouracil, oxaliplatin, and docetaxel compared to ECF or epirubicin, cisplatin, and capecitabine therapy (34). In the FLOT4 trial, which focused on AC, the overall survival was extended with a hazard ratio of 0.7. Based on a number of well-designed epoch-making clinical trials from various esophageal societies, multidisciplinary treatments improved. However, the 5-year overall survival remains unsatisfactory: 45% in the FLOT4 trial, 47% in the CROSS trial, and 55% in the JCOG9907 trial (28,33,34).
In order to develop more curative surgical strategies, clinical trials evaluating the benefits of intensification in neoadjuvant settings are needed. In Japan, we are currently running a three-arm phase III trial to evaluate the superiority of neoadjuvant triplet chemotherapy using docetaxel, cisplatin, and fluorouracil (DCF) over CF as well as the superiority of neoadjuvant CF radiation over CF (35). In addition, docetaxel, oxaliplatin, leucovorin, and 5-fluorouracil (FLOT) therapy may be less toxic than DCF, which is administered in outpatient settings. We are close to commencing the multicenter phase II trial to evaluate the efficacy of FLOT for ESCC. For AC, in order to compare the efficacy of FLOT and CROSS regimens, the ESOPEC trial is currently in progress (36).
Another strategy to improve outcomes is the use of adjuvant therapy in combination with neoadjuvant chemotherapy–a strategy that was already adopted for perioperative FLOT. Mokdad et al. conducted a network meta-analysis where patients who received neoadjuvant therapy were reviewed, and those who received adjuvant therapy were compared with those without postoperative treatment (37). Following a propensity score matching to eliminate the difference in patient backgrounds, which could affect the tolerability of chemotherapy, the survival benefit of adjuvant therapy was significantly confirmed. Therefore, incorporating postoperative treatment could be a promising strategy for the improvement of survival. For ESCC patients, Zhao et al. conducted randomized control trial comparing perioperative chemotherapy with preoperative therapy. Results demonstrated that perioperative, in which adjuvant chemotherapy was provided after surgery, showed a significantly longer overall survival. The efficacy of adding adjuvant even after neoadjuvant treatment was also confirmed in a phase II trial from another cohort (38).
Necessity to develop the individualization of treatment
Tolerability is a potential concern while administering adjuvant therapy. As mentioned, esophagectomy is highly invasive and comes with high morbidity, which could delay postoperative recovery. Accordingly, 40% of study participants could not be subjected to adjuvant FLOT therapy in the FLOT4 trial, despite the fact that 44% of patients presented with gastric and not esophageal cancer (34). For ESCC, the JCOG9907 trial reported that 25% of patients who were intended to receive adjuvant CF therapy were not able complete the entire treatment (28). Therefore, it is unlikely that all patients after surgery are capable of receiving neoadjuvant and adjuvant therapies. In the next section, as a future perspective, we describe treatment individualization based on the risk stratification of postoperative recurrence utilizing upgraded technology (Figure 1).
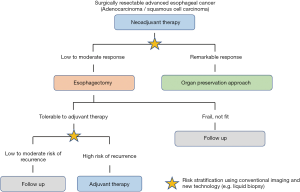
Moreover, another disadvantage of adding adjuvant chemotherapy is that all most half of patients will not experience postoperative recurrence even without adjuvant therapy. As suggested by Kim et al., the advantage of adjuvant therapy was observed in patients who had residual disease after neoadjuvant chemotherapy, whereas no difference was observed in those who manifested pathological complete response, indicating that the upgraded method identifying patients who have high risk of recurrence need to be selected as future perspective (39).
Future perspective
The improvement of drug efficacy in multimodal treatment could be demanding in esophageal cancer. Nowadays, immune checkpoint inhibitors, such as nivolumab and pembrolizumab, are approved worldwide for ESCC (40,41). When incorporating combination therapy with other cytotoxic drugs, the novel treatment with higher response and lower toxicities are expected. A promising feasibility trial of nivolumab with neoadjuvant CF or DCF therapy for locally advanced ESCC is currently ongoing (42).
Risk stratification of postoperative recurrence is also needed. Currently, pathological findings represented by TNM staging are the gold standard. However, a wide range of variation in terms of survival exists, even within the same TNM stage. As additional indicators which help to predict postoperative recurrence, the histological response in resected specimens has been shown to be strongly correlated to postoperative survival (43,44). Furthermore, independent of the local tumor, liquid biopsy, including blood and urine, began to be recognized as the new standard for monitoring tumor burden (45). We have been focusing on the inflammation and coagulation marker as a blood biomarker. One of the coagulation markers, fibrinogen, was shown to be a useful biomarker for predicting postoperative survival in patients with esophageal cancer (46). Furthermore, we developed a simple prognostic score, the FA score, combining fibrinogen and albumin levels, which was validated in a multicenter prospective trial (47,48). The direct detection of tumor derivatives such as cell-free tumor DNA and circulating tumor cell has been widely validated in various types of cancer (49). In colorectal cancer, patients with positive circulating tumor DNA (ctDNA) demonstrated significantly worse relapse-free survival after surgery, indicating that ctDNA can be used to detect minimal residual disease and select patients who can attain a survival advantage through adjuvant chemotherapy (50), as well as in breast and lung cancer (51,52). In esophageal cancer, Azad et al. reported that ctDNA monitoring after chemoradiotherapy was useful for monitoring tumor recurrence (53). Although further validation and interventional study are required, liquid biopsy could be a promising indicator for guiding multidisciplinary treatments for esophageal cancer.
Conclusions
For surgically resectable esophageal cancer, esophagectomy has been a mainstay of multidisciplinary treatment. While neoadjuvant chemotherapy developed as a standard treatment worldwide for both AC and ES, survival outcomes remain unsatisfactory. Upgrading modalities, such as through use of liquid biopsies, could be informative for selecting patients at high risk of postoperative recurrence. Individualized therapies, in which adjuvant therapy is administered only to select patients, could become the next standard for esophageal cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sjoerd Lagarde, Bas Wijnhoven, and Florian Lordick) for the series “Novel Developments in the Multimodality Treatment of Esophageal Cancer” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-2020-41). The series “Novel Developments in the Multimodality Treatment of Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. YK reports grants and personal fees from AsahiKASEI Co., Ltd., grants from Taiho Pharmaceutical Co., Ltd, grants from Chugai Pharmaceutical Co., Ltd., grants from Daiichi Sankyo Company, Limited, grants from Merck Serono Co., Ltd., grants from AsahiKASEI Co., Ltd., grants from EA Pharma Co., Ltd., grants from Yakult Honsha Co. Ltd., grants from Otsuka Pharmaceutical Co., Ltd., grants from Takeda Pharmaceutical Co., Ltd., grants from Otsuka Pharmaceutical Factory Inc., grants from Shionogi & Co., Ltd., grants from Kaken Pharmaceutical Co., Ltd., grants from Kowa Pharmaceutical Co., Ltd., grants from Astellas Pharma Inc., grants from Medicon Inc., grants from Dainippon Sumitomo Pharma Co., Ltd., grants from Taisho Toyama Pharmaceutical Co., Ltd., grants from Kyouwa Hakkou Kirin Co., Ltd., grants from Pfizer Japan Inc., grants from Ono Pharmaceutical Co., Ltd., grants from Nihon Pharmaceutical Co., Ltd., grants from Japan Blood Products Organization, grants from Medtronic Japan Co., Ltd., grants from Sanofi K.K., grants from Eisai Co., Ltd., grants from Tsumura & Co., grants from KCI Licensing, Inc., grants from Abbott Japan Co., Ltd., grants from FUJIFILM Toyama Chemical Co., Ltd., outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Aggelis V, Cunningham D, Lordick F, et al. Peri-operative therapy for operable gastroesophageal adenocarcinoma: past, present and future. Ann Oncol 2018;29:1377-85. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Current Advancement in Multidisciplinary Treatment for Resectable cStage II/III Esophageal Squamous Cell Carcinoma in Japan. Ann Thorac Cardiovasc Surg 2016;22:275-83. [Crossref] [PubMed]
- Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today 2020;50:12-20. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Kurokawa Y, Takeuchi H, Doki Y, et al. Mapping of Lymph Node Metastasis From Esophagogastric Junction Tumors: A Prospective Nationwide Multicenter Study. Ann Surg 2019; Epub ahead of print. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: 5-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1001. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus 2019;16:221-45. [Crossref] [PubMed]
- Akutsu Y, Kato K, Igaki H, et al. The Prevalence of Overall and Initial Lymph Node Metastases in Clinical T1N0 Thoracic Esophageal Cancer: From the Results of JCOG0502, a Prospective Multicenter Study. Ann Surg 2016;264:1009-15. [Crossref] [PubMed]
- Takeuchi H, Fujii H, Ando N, et al. Validation study of radio-guided sentinel lymph node navigation in esophageal cancer. Ann Surg 2009;249:757-63. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72; discussion 372-3. [Crossref] [PubMed]
- Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg 2000;232:225-32. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Three-field lymph node dissection in esophageal cancer surgery. J Thorac Dis 2017;9:S731-40. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Gotoh M, et al. A Risk Model for Esophagectomy Using Data of 5354 Patients Included in a Japanese Nationwide Web-Based Database. Ann Surg 2014;260:259-66. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Ozawa S, et al. Comparison of Short-Term Outcomes Between Open and Minimally Invasive Esophagectomy for Esophageal Cancer Using a Nationwide Database in Japan. Ann Surg Oncol 2017;24:1821-7. [Crossref] [PubMed]
- Kataoka K, Takeuchi H, Mizusawa J, et al. A randomized Phase III trial of thoracoscopic versus open esophagectomy for thoracic esophageal cancer: Japan Clinical Oncology Group Study JCOG1409. Jpn J Clin Oncol 2016;46:174-7. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Fuchs CS, Niedzwiecki D, Mamon HJ, et al. Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy With Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer: Results From CALGB 80101 (Alliance). J Clin Oncol 2017;35:3671-7. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Booka E, Takeuchi H, Nishi T, et al. The Impact of Postoperative Complications on Survivals After Esophagectomy for Esophageal Cancer. Medicine (Baltimore) 2015;94:e1369 [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Correlation Between Intense Postoperative Inflammatory Response and Survival of Esophageal Cancer Patients Who Underwent Transthoracic Esophagectomy. Ann Surg Oncol 2015;22:4453-60. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus 2019;16:25-43.
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 2019;16:1-24.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 2013;43:752-5. [Crossref] [PubMed]
- Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016;16:503. [Crossref] [PubMed]
- Mokdad AA, Yopp AC, Polanco PM, et al. Adjuvant Chemotherapy vs Postoperative Observation Following Preoperative Chemoradiotherapy and Resection in Gastroesophageal Cancer: A Propensity Score-Matched Analysis. JAMA Oncol 2018;4:31-8. [Crossref] [PubMed]
- Ardalan B, Spector SA, Livingstone AS, et al. Neoadjuvant, surgery and adjuvant chemotherapy without radiation for esophageal cancer. Jpn J Clin Oncol 2007;37:590-6. [Crossref] [PubMed]
- Kim GJ, Koshy M, Hanlon AL, et al. The Benefit of Chemotherapy in Esophageal Cancer Patients With Residual Disease After Trimodality Therapy. Am J Clin Oncol 2016;39:136-41. [Crossref] [PubMed]
- Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506-17. [Crossref] [PubMed]
- Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631-9. [Crossref] [PubMed]
- Yamamoto S, Kato K, Daiko H, et al. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol 2020;16:1351-7. [Crossref] [PubMed]
- Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. [Crossref] [PubMed]
- Kurokawa Y, Shibata T, Ando N, et al. Which is the optimal response criteria for evaluating preoperative treatment in esophageal cancer: RECIST or histology? Ann Surg Oncol 2013;20:3009-14. [Crossref] [PubMed]
- Kilgour E, Rothwell DG, Brady G, et al. Liquid Biopsy-Based Biomarkers of Treatment Response and Resistance. Cancer Cell 2020;37:485-95. [Crossref] [PubMed]
- Takeuchi H, Ikeuchi S, Kitagawa Y, et al. Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol 2007;22:2222-7. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Prognostic Impact of Change in the Fibrinogen and Albumin Score During Preoperative Treatment in Esophageal Cancer Patients. World J Surg 2017;41:2788-95. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Cumulative Prognostic Scores Based on Plasma Fibrinogen and Serum Albumin Levels in Esophageal Cancer Patients Treated with Transthoracic Esophagectomy: Comparison with the Glasgow Prognostic Score. Ann Surg Oncol 2015;22:302-10. [Crossref] [PubMed]
- Corcoran RB, Chabner BA. Application of Cell-free DNA Analysis to Cancer Treatment. N Engl J Med 2018;379:1754-65. [Crossref] [PubMed]
- Reinert T, Henriksen TV, Christensen E, et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol 2019;5:1124-31. [Crossref] [PubMed]
- Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017;545:446-51. [Crossref] [PubMed]
- Coombes RC, Page K, Salari R, et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clin Cancer Res 2019;25:4255-63. [Crossref] [PubMed]
- Azad TD, Chaudhuri AA, Fang P, et al. Circulating Tumor DNA Analysis for Detection of Minimal Residual Disease After Chemoradiotherapy for Localized Esophageal Cancer. Gastroenterology 2020;158:494-505.e6. [Crossref] [PubMed]
Cite this article as: Matsuda S, Kawakubo H, Mayanagi S, Irino T, Kitagawa Y. Surgery and adjuvant therapy after esophagectomy. Ann Esophagus 2021;4:17.


