
Total gastrectomy versus upper pole gastrectomy for the surgical therapy of Siewert type II adenocarcinoma of the esophagus: pathology may drive the choice
Introduction
The surgical treatment for Siewert type II adenocarcinoma of the esophagus (1) is still debated. Siewert, with others, indicated total gastrectomy due to the high frequency of perigastric and greater curvature nodal metastases (2-4).
On the basis of new data on the frequency of metastases at the gastric greater curvature (5-12), quite lower than those previously reported (2-4) (Table S1), it was concluded that the resection of the lower esophagus and the upper pole of the stomach could be as effective as total gastrectomy with regard to survival (7,10,11), eventually in association with neoadjuvant therapy (8,9,13). Recent papers support again the choice of total gastrectomy (14,15). The controversy may be produced by the Siewert’s classification, which is not precise enough to discriminate between type II and III cases (14-17), by the different modalities of lymphadenectomy performed (18) and of the pathological specimen work-up (19-22).
In 2007 to overcome biases of the Siewert classification, we adopted a new pathologic classification based on the presence/absence of intestinal metaplasia in the esophagus and/or stomach that discriminates cases in Barrett’s, cardio-pyloric and gastric types (23). Since, we started a prospective study protocol aimed to investigate biology and results of surgical therapy for esophageal adenocarcinoma. For the primary treatment of Siewert type II, we adopted the total gastrectomy associated with the esophageal resection at the azygos vein level through right thoracotomy, with an extended thoracic and abdominal (D2) lymphadenectomy; we had previously demonstrated that this technique provided a radical resection especially suited for poorly differentiated carcinoma (24). The intraoperative nodes retrieval and mapping, the pathology work-up of the surgical specimen, were aimed to investigate the oral and aboral intra mural cancer diffusion and modalities of nodal metastases spreading (24). We completed the pathology work-up with the adoption of Lauren’s classification (25), which is relevant from a prognostic, epidemiological, and pathogenic perspective for esophageal adenocarcinoma (26-30).
We indicated primary surgery for tumours up to T4 (diaphragm), N1 (peritumoural stations), with the exclusion of bulky metastases. By the time we maintained this line: at periodic analysis our results (21,22,24) were similar to those reported by others (31,32) and were competitive with survivals obtained with neoadjuvant therapy followed by surgical resection (33,34).
In the present study we considered cases classified type II according to Siewert’s, who were primarily operated upon between 2007 and 2017. We investigated the frequency of chest/abdominal nodal metastases with a particular focus on gastric greater curvature stations, the recurrence patterns, the submucosal orad cancer diffusion into the esophagus, 5-year survival according to these data and the Lauren’s classification. We present the following article in accordance with the STROBE reporting checklist (available at: http://dx.doi.org/10.21037/aoe-2020-13).
Methods
Preoperative diagnosis and clinical staging
The preoperative work-up included upper gastrointestinal tract endoscopy with multiple tumours’ and surrounding mucosa biopsies, barium swallow, thoracic and abdominal CT, PET or CT-PET. Tumours were staged according to the AJCC/UICC TNM 8th edition (35).
Surgical technique
The surgical technique we adopted (21,22,24) is extensively described in Supplementary 1 and Figure S1. Briefly, a midline or transverse laparotomy and a muscle-sparing lateral-anterior right thoracotomy were performed. The esophagus was resected at the level of the azygos vein arch, and a frozen section of the resection margin was routinely performed to achieve a proximal clean resection margin (24). The surgical specimen was comprehensive of distal esophagus, stomach and omentum. The digestive tract continuity was established with Roux-en-Y esophagojejunostomy.
Lymphadenectomy was extended to the thoracic stations R 2–4, 7–8–9, numbered according to Mountain’s classification (36) (Supplementary 2 and Figure S2), to the abdominal stations 1–12, numbered according to the Japanese Research Society for Gastric Cancer (JRSGC) 1998 classification (37) (Supplementary 2 and Figure S2).
Nodes were removed en bloc with the adjacent fat/connective tissue to achieve total lymphadenectomy (22). The surgical team labelled each lymph node when it was removed from the peripheral stations; lymph node fragments were excluded to avoid N over-count.
Mortality at 90 days and morbidity were extracted from the database.
Pathology
The pathologic work-up of the surgical specimen (21,22,24) is illustrated in detail in Supplementary 3 and Figure S3. Based on the presence (+) or absence (−) of Barrett’s intestinal metaplasia (BIM) and intestinal metaplasia in the stomach (GIM), cases were categorized as Barrett’s-like type (BIM+/GIM−), cardiopyloric-like type (BIM−/GIM−), or gastric-like type (BIM−/GIM+) (21,23).
Adenocarcinoma was classified according to Lauren as intestinal, mixed, or diffuse type (25). Lymphovascular invasion was defined as absent or present (35), perineural diffusion and the ratio of the number of metastatic lymph nodes to the total number of nodes yielded (LNR) (22) were determined. Tumour grade (G) was grouped as 1+2 and 3+4.
Follow-up and survival
After surgery, patients were followed up twice per year (clinical assessment, serum oncologic markers, at each follow-up, upper gastrointestinal tract endoscopy, chest/abdominal CT, PET, or CT-PET, once per year). Site(s), relapse modalities, and date and cause of death were registered. Cancer-specific survival (in months), calculated from the date of surgery to the date of death due to documented recurrent disease, was adopted instead of overall survival, as it may better indicate the course of a specific disease and the effects of therapy (38).
Adjuvant therapy
In the absence of general contraindications, adjuvant chemotherapy and radiation therapy were administered according to guidelines (39,40) in cases of pN+, R1 surgery, lympho-vascular invasion or after documented recurrence.
Ethics committee approval
The local institutional review board of the IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (CEAV/IRST) approved the use of the database maintained by the Division of Thoracic Surgery for research purposes (No L3P1223). The study was conducted in accordance with the Declaration of Helsinki (as revised in Edinburgh 2000).
Statistical analysis
Data are represented as median and interquartile range (IQR) for continuous variables and as n (%) for categorical variables. The χ2 test or Fisher’s test (expected number less than 5) and the Mann-Whitney test were used to analyse categorical and continuous variables. Cancer-specific survival analyses were performed using the Kaplan-Meier method and the log-rank test. Univariable and multivariable (forward stepwise conditional method) Cox regression analyses were performed to estimate the effects of clinical and pathological parameters on cancer-specific survival. Multivariable logistic regression analysis was performed to identify the predictors of lymph node metastases in the greater gastric curvature. P values <0.05 were considered significant. Data were analysed using SPSS (version 15.0) (SPSS Inc., Chicago, IL, USA).
Results
All 154 Siewert type II cases operated upon primarily in the considered period were suitable for the study. Six patients died within 90 postoperative days (6/154, 3.8%) for mediastinitis due to anastomotic fistula in 5 patients and peritonitis after dehiscence of the Roux jejunal anastomosis in 1 patient. Major morbidity included intrathoracic anastomotic leakage in 3 patients, necrosis of the jejunal loop in 2 patients, and torsion of the jejunal loop in 1 patient (6/154 patients, 3.8%). The R0 resection rate was 97.4% (150/154). Four cases showed submucosal microscopic involvement of the esophageal resection margin (R1, 2.6%). Two were of the diffuse type and two of the signet ring cells type; two patients were younger than 40 years. Sex, age, histology, grading and pathologic (p) staging data are displayed in Table 1.
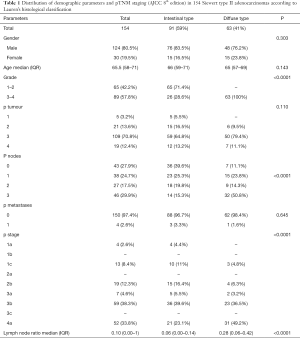
Full table
A total of 4,825 nodes were yielded (median 30.5, interquartile range per patient 22–38; minimum, 8 nodes; maximum, 61 nodes).
The clinical N staging parameter was under-estimated in 47% of cases with respect to pathological N stage, which was 2 or 3 instead of 0 or 1. Overall, 91 (59%) were adenocarcinomas of the intestinal type, 10 were mixed type (6.5%) and 53 (34.5%) were diffuse type. Mixed type cases were included in the diffuse type (diffuse + mixed 41%) for analysis. Statistically significant differences between the intestinal and diffuse histological subtypes pNodes (P<0.0001), p staging (P<0.0001) and LNR (P<0.0001) were calculated. Of 154 cases, 120 (78%) were negative for metastases at the greater curvature/pyloric lymphatic nodes, while 34 cases (22%) were positive. Of 34 cases, 24 had metastases at station 4sa (15.6%), 9 at station 4sb (5.8%), and 1 at station 4d (0.6%). Nineteen of 34 patients were positive also at station 6 (12.3%). In Table 2, cases that were negative (group 1) and positive (group 2) for greater gastric curvature and pyloric lymph node metastases are distributed according to histological intestinal/diffuse subtype, grading (G), staging parameters, LNR, lymph-vascular invasion, perineural invasion, the percentage of metastases at the thoracic and abdominal lymphatic stations and the categorization of cases according to the presence/absence of BIM and GIM.
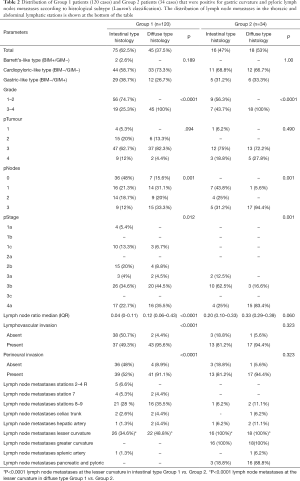
Full table
Statistically significant differences were found between groups 1 and 2 for grading (P=0.048), pT (P=0.015), pN (P<0.0001), p staging (P<0.0001), and LNR (P<0.0001) (Table 2). The median LNR (Figure 1) was higher in group 2 because both the number of positive lymph nodes (numerator) and the number of yielded lymph nodes (denominator) were greater than in group 1 (P<0.0001 and P=0.001, respectively). Logistic regression analysis identified the LNR as the only predictor of lymph node metastases in the greater curvature of the stomach (P=0.003; OR =1.045, 95% CI: 1.016–1.078). Within both groups, statistically significant differences between the intestinal and diffuse histological subtypes were found for pN, p staging, and LNR (Table 2). Siewert type II category was cardiopyloric-type in 100/154, 65% (BIM−/GIM−); gastric-type in 52/154, 33.7% (BIM−/GIM+); and Barrett’s-type in 2/154, 1.3% (BIM+/GIM−). Statistically significant differences with regards to age between gastric-like (median 67.5, IQR: 60.5–72.2 years) and cardiopyloric-like (median 63, IQR: 57.2–69 years) types were found (P=0.023). No significant differences between two types with regards to sex, histology (intestinal and diffuse type), grading, pT, pN, pM, p staging, and LNR were detected. In Table 3, cases are distributed according to BIM/GIM and the Lauren’s classification classes. The number of cases for each sub-group, with and without gastric greater curvature and pyloric nodal metastases, the number of lymph nodes harvested and the number of lymph nodes positive for metastases at the greater curvature stations are reported.
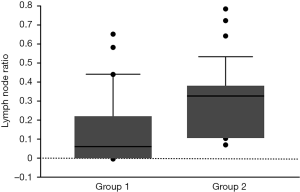

Full table
Of 148 patients who survived surgery, cancer recurred in 87 (58.7%) [27/88 (30.6%) intestinal type; 60/60 (100%) diffuse type]. Recurrence occurred at one site in 67 patients (liver, 21; lumbar-aortic nodes, 14; anastomotic level, 10; mediastinum, 10; lung, 6; brain, 5; cervical nodes, 1) and at multiple sites in 9 patients (liver and lumbar-aortic nodes, 4; bones and peritoneal area, 2; bones and anastomotic level, 2, anastomotic level and lumbar-aortic nodes, 1); peritoneal or pleura carcinosis was observed in 11 patients. The sites and modalities of recurrence were equal between the histological subtypes. Kaplan-Meier cancer-specific survival curves for the whole series and according to histology are shown in Figure 2 (5-year survival for all cases: 40.5%; for intestinal and diffuse type cases: 59.4% and 0%, respectively; P<0.0001). Univariable Cox regression analysis showed that G (P<0.0001), pT (P=0.005), pN (P<0.0001), p staging (P<0.0001), lymphovascular invasion (P<0.0001), perineural diffusion (P<0.0001), Lauren’s histology (P<0.0001), and LNR (P=0.001) significantly correlated with cancer-specific survival. Multivariable Cox regression analysis identified Lauren’s histology (P<0.0001; HR 2.9, 95% CI: 1.6–5.4) and lymphovascular invasion (P=0.028; HR 2.7, 95% CI: 1.1–6.6) as independent prognostic factors that significantly influenced cancer-specific survival.
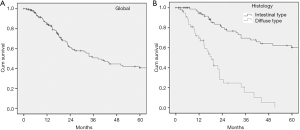
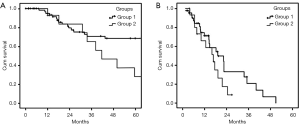
Figure 3 shows cancer-specific survival curves for the intestinal and diffuse histological types in patients without (Group 1) and with (Group 2) greater gastric curvature/pyloric metastases (intestinal type Group 1 vs. Group 2, P=0.038; diffuse type Group 1 vs. Group 2, P=0.158). Patients without greater gastric curvature/pyloric metastases had significantly longer cancer-specific survival regardless of histological subtype.
Discussion
In the past, it was generally presumed that station 4 metastases were more frequent in Siewert type III than in type II (1,2,10,11); station 4 high metastases rates were related to the higher percentage of cases of type III erroneously classified as type II (2,41,42).
In this series, in cardiopyloric-like type cases, metastases at the greater curvature region were detected in 20.4% of lymph nodes harvested, while in the gastric-like type, they occurred in 4.8% (P<0.0001); according to Lauren (25), cases were 59% intestinal type and 41% diffuse + mixed type. Histology, pTNM, and LNR parameters showed that the diffuse type is clearly more aggressive than the intestinal type. Lauren’s histological subtype was an independent prognostic factor.
Greater curvature and pyloric lymph node metastases were detected in 34 of 154 cases (22%), in stage IIIa–IV only, 47% in intestinal-type and 53% in diffuse-type. Four of 154 cases (2.59%) had positive esophageal resection margins at the azygos vein level. Two cases were of the diffuse type, and 2 were of the signet ring cell type (a 37-year-old man “a posteriori” resulted to have a CDH1 hereditary disease).
In 154 cases, the 5-year cancer-specific survival was 40.5%, with 59.4% for intestinal type and 0% for diffuse type. According to the absence/presence of gastric greater curvature metastases, the 5-year cancer-specific survival rates were 48.7% vs. 14.9%, respectively; for intestinal type, they were 67.4% vs. 27.9%, and for diffuse type, it was 0% independently from the nodal status.
For “esophageal adenocarcinoma”, pathology may: (I) discriminate cases originated from Barrett’s, cardio-pyloric and gastric mucosa, which have different patterns of nodal metastases and aggressiveness (21,22); (II) offer interesting biological/prognostic indications.
With total gastrectomy, D2 abdominal lymphadenectomy, transthoracic resection of the esophagus and of mediastinal nodal stations, we achieved 5-year cancer specific survival in 59.4% for intestinal type adenocarcinomas, of whom 64.8% were 3a–4 stage. With the same operation, none of the diffuse type cases survived over 3 years.
Greater curvature lymph nodes metastases were present in p stages III and IV only, mostly close to short gastric vessels (station 4a): these metastases possibly occurred in aggressive tumours (cardiopyloric and diffuse types), or in intestinal type cases that were diagnosed late. These data suggest that the resection of gastric greater curvature nodal stations is effective in cases of intestinal type adenocarcinoma, but not for the diffuse type.
For diffuse type adenocarcinoma, which includes signet ring cell carcinoma, in our opinion neoadjuvant therapy should be mandatory, eventually with new drug combinations (43). The same concept, to trim the extension of the surgical resection according to the aggressiveness of cancer, is probably valid for sizing the esophageal resection.
In case of intestinal type adenocarcinoma, it is not necessary to resect the esophagus at the azygos vein level; the higher the level of the esophageal-jejunal anastomosis, the higher the risk of jejunal loop ischemia-dependent complications, as demonstrated by the mortality-morbidity data displayed in this series.
In conclusion, the present study indicates that total gastrectomy associated with extended abdominal and thoracic station lymphadenectomy, with resection of the esophagus 5 centimetres above the tumour’s macroscopic upper margin (44,45), may be the right choice for intestinal type adenocarcinoma as an alternative to neoadjuvant therapy followed by the Ivor Lewis operation.
This proposal needs to be verified and confirmed by retrospective pathology analyses of data collected with cooperative studies comparing the efficacies of different surgery/chemo-radiotherapy sequences (15,39) and with new research protocols. The treatment’s modalities for diffuse type adenocarcinoma are also worth extensive investigation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Riccardo Rosati) for the series “Current issues on GEJ adenocarcinoma” published in Annals of Esophagus. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist, available at http://dx.doi.org/10.21037/aoe-2020-13
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoe-2020-13
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2020-13). The series “Current issues on GEJ adenocarcinoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The local institutional review board of the IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (CEAV/IRST) approved the use of the database maintained by the Division of Thoracic Surgery for research purposes (No L3P1223). The study was conducted in accordance with the Declaration of Helsinki (as revised in Edinburgh 2000).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457-9. [Crossref] [PubMed]
- Rüdiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000;232:353-61. [Crossref] [PubMed]
- Wang LS, Wu CW, Hsieh MJ, et al. Lymph node metastasis in patients with adenocarcinoma of gastric cardia. Cancer 1993;71:1948-53. [Crossref] [PubMed]
- Akiyama H. Adenocarcinoma of the gastroesophageal junction. In: Pearson FG, Deslauriers J, Hiebert CA, et al. editors. Esophageal surgery. New York, NY: Churchill Livingstone; 1995: 587-593.
- Fang WL, Wu CW, Chen JH, et al. Esophagogastric junction adenocarcinoma according to Siewert classification in Taiwan. Ann Surg Oncol 2009;16:3237-44. [Crossref] [PubMed]
- Fukuchi M, Mochiki E, Suzuki O, et al. Factors predicting recurrence in patients with Siewert type II carcinoma treated with curative resection. Anticancer Res 2015;35:505-9. [PubMed]
- Goto H, Tokunaga M, Sugisawa N, et al. Value of splenectomy in patients with Siewert type II adenocarcinoma of the esophagogastric junction. Gastric Cancer 2013;16:590-5. [Crossref] [PubMed]
- Mine S, Kurokawa Y, Takeuchi H, et al. Distribution of involved abdominal lymph nodes is correlated with the distance from the esophagogastric junction to the distal end of the tumor in Siewert type II tumors. Eur J Surg Oncol 2015;41:1348-53. [Crossref] [PubMed]
- Yamashita H, Katai H, Morita S, et al. Optimal extent of lymph node dissection for Siewert type II esophagogastric junction carcinoma. Ann Surg 2011;254:274-80. [Crossref] [PubMed]
- Hasegawa S, Yoshikawa T, Rino Y, et al. Priority of lymph node dissection for Siewert type II/III adenocarcinoma of the esophagogastric junction. Ann Surg Oncol 2013;20:4252-9. [Crossref] [PubMed]
- Fujitani K, Miyashiro I, Mikata S, et al. Pattern of abdominal nodal spread and optimal abdominal lymphadenectomy for advanced Siewert type II adenocarcinoma of the cardia: results of a multicenter study. Gastric Cancer 2013;16:301-8. [Crossref] [PubMed]
- Matsuda T, Takeuchi H, Tsuwano S, et al. Optimal surgical management for esophagogastric junction carcinoma. Gen Thorac Cardiovasc Surg 2014;62:560-6. [Crossref] [PubMed]
- Blank S, Schmidt T, Heger P, et al. Surgical strategies in true adenocarcinoma of the esophagogastric junction (AEG II): thoracoabdominal or abdominal approach? Gastric Cancer. 2018;21:303-14. [Crossref] [PubMed]
- Voron T, Gronnier C, Pasquer A, et al. Adenocarcinoma of the oesophagogastric junction Siewert II: An oesophageal cancer better cured with total gastrectomy. Eur J Surg Oncol 2019;45:2473-81. [Crossref] [PubMed]
- Haverkamp L, Seesing MFJ, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Dresner SM, Lamb PJ, Bennett MK, et al. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery 2001;129:103-9. [Crossref] [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. Japan Clinical Oncology Group (JCOG9502). Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 2006;7:644-51. [Crossref] [PubMed]
- Reeh M, Mina S, Bockhorn M, et al. Staging and outcome depending on surgical treatment in adenocarcinomas of the oesophagogastric junction. Br J Surg 2012;99:1406e14.
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161-81. [Crossref] [PubMed]
- Piazuelo MB, Haque S, Delgado A, et al. Phenotypic differences between esophageal and gastric intestinal metaplasia. Mod Pathol 2004;17:62-74. [Crossref] [PubMed]
- Ruffato A, Mattioli S, Perrone O, et al. Esophagogastric metaplasia relates to nodal metastases in adenocarcinoma of esophagus and cardia. Ann Thorac Surg 2013;95:1147-53. [Crossref] [PubMed]
- Ruffato A, Lugaresi M, Mattioli B, et al. Total lymphadenectomy and nodes-based prognostic factors in surgical intervention for esophageal adenocarcinoma. Ann Thorac Surg 2016;101:1915-20. [Crossref] [PubMed]
- Mattioli S, Ruffato A, Di Simone MP, et al. Immunopathological patterns of the stomach in adenocarcinoma of the esophagus, cardia, and gastric antrum: gastric profiles in Siewert type I and II tumors. Ann Thorac Surg 2007;83:1814-9. [Crossref] [PubMed]
- Mattioli S, Di Simone MP, Ferruzzi L, et al. Surgical therapy for adenocarcinoma of the cardia: modalities of recurrence and extension of resection. Dis Esophagus 2001;14:104-9. [Crossref] [PubMed]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [Crossref] [PubMed]
- van der Kaaij RT, Snaebjornsson P, Voncken FE, et al. The prognostic and potentially predictive value of the Laurén classification in oesophageal adenocarcinoma. Eur J Cancer 2017;76:27-35. [Crossref] [PubMed]
- Jiménez Fonseca P, Carmona-Bayonas A, Hernández R, et al. Lauren subtypes of advanced gastric cancer influence survival and response to chemotherapy: real-world data from the AGAMENON National Cancer Registry. Br J Cancer 2017;117:775-82. [Crossref] [PubMed]
- Pattison S, Mitchell C, Lade S, et al. Early relapses after adjuvant chemotherapy suggests primary chemoresistance in diffuse gastric cancer. PLoS One 2017;12:e0183891 [Crossref] [PubMed]
- Ma J, Shen H, Kapesa L, et al. Lauren classification and individualized chemotherapy in gastric cancer. Oncol Lett 2016;11:2959-64. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Lagarde SM, Reitsma JB, Ten Kate FJW, et al. Predicting individual survival after potentially curative esophagectomy for adenocarcinoma of the esophagus or gastroesophageal junction. Ann Surg 2008;248:1006-13. [Crossref] [PubMed]
- Lerut T, Coosemans W, Decker G, et al. Surgical techniques. J Surg Oncol 2005;92:218-29. [Crossref] [PubMed]
- van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJB, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [Crossref] [PubMed]
- Brierley J, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours - 8th edition. Hoboken. 2016.
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718-23. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma – 2nd English Edition. Gastric Cancer 1998;1:10-24. [Crossref] [PubMed]
- Howlader N, Ries LAG, Mariotto AB, et al. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 2010;102:1584-98. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]
- Kleinberg L, Brock M, Gibson M. Management of locally advanced adenocarcinoma of the esophagus and gastroesophageal junction: finally a consensus. Curr Treat Options Oncol 2015;16:35. [Crossref] [PubMed]
- Pedrazzani C, de Manzoni G, Marrelli D, et al. Lymph node involvement in advanced gastroesophageal junction adenocarcinoma. J Thorac Cardiovasc Surg 2007;134:378-85. [Crossref] [PubMed]
- de Manzoni G, Pedrazzani C, Di Leo A, et al. Metastases to the para-aortic lymph nodes in adenocarcinoma of the cardia. Eur J Surg 2001;167:413-8. [Crossref] [PubMed]
- Bekkar S, Gronnier C, Messager M, et al. The impact of preoperative radiochemotherapy on survival in advanced esophagogastric junction signet ring cell adenocarcinoma. Ann Thorac Surg 2014;97:303-10. [Crossref] [PubMed]
- Barbour AP, Rizk NP, Gonen M, et al. Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg 2007;246:1-8. [Crossref] [PubMed]
- Mine S, Sano T, Hiki N, et al. Proximal margin length with transhiatal gastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg 2013;100:1050-4. [Crossref] [PubMed]
Cite this article as: Lugaresi M, Mattioli B, Daddi N, Pilotti V, Ferruzzi L, Raulli G, Malvi D, D’Errico A, Fiocca R, Mattioli S. Total gastrectomy versus upper pole gastrectomy for the surgical therapy of Siewert type II adenocarcinoma of the esophagus: pathology may drive the choice. Ann Esophagus 2021;4:15.


