
Robotic-assisted minimally invasive esophagectomy
Introduction
Esophagectomy is a core component of multimodal therapy for advanced refractory benign esophageal disease and locally advanced esophageal malignancy (1,2). Given the perioperative morbidity and intraoperative complexity of this operation, surgeons continue to refine the best approach that would optimize patient safety, while yielding optimal results. The best approach to surgical resection remains widely debated, but modifications continue to develop with growing acceptance of surgical technology.
Open transthoracic esophagectomy (OE), video-assisted minimally invasive esophagectomy (MIE) and robotic-assisted minimally invasive esophagectomy (RAMIE) are commonly accepted approaches to esophageal resection for advanced benign and malignant disease (3-5).
The OE approach has been associated with higher peri-operative morbidity in comparison to the MIE and RAMIE approaches (6). Reduced surgical trauma (blood loss, surgical site and pulmonary infections), hospital duration, and overall morbidity, are documented as advantageous benefits of the MIE approach in comparison to the OE approach (7,8). However, limited high definition and two-dimensional views, suboptimal optical stability, and limited degrees of freedom are inherent limitations of the MIE approach (9).
Early studies of the RAMIE approach confirmed feasibility of the operation, while providing outcomes that compared favorably to the MIE approach (9-14). Technological developments of the RAMIE approach overcame the intrinsic technical limitations in MIE through translation of movements to more precise dissection and provision of self-assisting capabilities (8,9). RAMIE has gained increasing popularity likely due to these improvements while also maintaining safety, uncompromising oncological results, and improved perioperative outcomes (9-15).
The initial experience with robotic assisted esophagectomy was introduced at the turn of the millennium. In 2003, Horgan et al. reported one of the earliest robotic assisted transhiatal esophagectomies (16). Shortly after, Kernstine et al. described the first RAMIE series using a McKeown approach. This study included 14 patients with premalignant (n=2) and malignant (n=12) lesions of the esophagus. Patients (n=3) underwent robotic thoracic-only portion of the esophagectomy, with the abdominal portion performed via laparotomy. Another subgroup (n=3), underwent a similar approach, with the inclusion of thoracic duct ligation and video-assisted laparoscopy. The last subgroup (n=8) underwent a complete RAMIE (n=8). Median operative time (11.2 hours) and console time (4.9 versus 4.2 hours for open transhiatal approach), which highlighted the need for improved intraoperative efficiency at the time of the study (17). van der Sluis et al. described a prospective study of their robotic experience in 108 patients, in which the median operative time was 381 minutes. Pulmonary complications (37%) were among the more common post-operative complications, and the median intraoperative blood loss was 340 cc. The rate of anastomotic leakage and chylothorax were 19% and 18%, respectively. These results were in concordance with existing RAMIE experiences at that time (11).
Sarkaria et al. conducted a prospective trial that compared RAMIE (n=64) to open transthoracic approaches (n=164). Most of the patients underwent an Ivor-Lewis approach (RAMIE, n=62, Open, n=103), and received induction chemoradiation treatment (80.2% and 73.4%). R0 resection was equivalent between two the groups (97.2% vs. 96.9%), but there were less ICU admissions (P=0.03), reduced pulmonary (14% vs. 34%, P=0.014) and infection complications (17.2% vs. 38%, P=0.029) in the RAMIE cohort. No difference in major complications, mortality at 30 or 90 days between the two groups, but the RAMIE cohort experienced less blood loss (250 vs. 350 cc, P<0.001) and less pain severity (P<0.05) (18).
The ROBOT trial was the first randomized study to compare RAMIE (n=56) to the open approach (n=56), in order to evaluate postoperative complications and quality of life (19). RAMIE was associated with lower postoperative morbidity, decreased blood loss (400 vs. 569 cc, P<0.001) and lower duration of postoperative pain, in comparison to the open approach. Another multicenter prospective trial, the robot-assisted esophagectomy (RAE) versus conventional minimally invasive esophagectomy (MIE) for resectable esophageal squamous cell carcinoma (RAMIE) trial, is ongoing and will compare outcome differences between RAMIE to the MIE approach (20). This study will compare the safety profile and efficacy of RAMIE (n=180) and MIE (n=180) in patients with resectable esophageal squamous cell carcinoma. The main end points include 5-year overall survival, 3-year overall survival, 5-year disease free survival, quality of life and short-term outcomes (20).
Furthermore, improved lymph node harvesting is an advantage of the RAMIE, in comparison to the MIE approach (21-23). Deng et al. conducted a propensity score matched analysis based on outcomes between RAMIE versus MIE and reported higher yield of recurrent laryngeal nerve lymph nodes (mean: 1.0±1.8 vs. 0.4±0.8; P=0.033) and total lymph nodes (20.6 vs. 17.9; P=0.048) in the RAMIE cohort versus the MIE cohort, without increasing the risk of recurrent laryngeal nerve paralysis (21).
Based on the existing retrospective studies and RAMIE trial, there is accruing data to suggest RAMIE can provide superior post-operative results and favorable technical advantages in comparison to OE and MIE approaches, respectively (11,17,19).
We look to highlight our technical approach and management for patients undergoing RAMIE in the setting of esophageal cancer.
Pre-operative assessment
Pre-operative assessment does not vary from patients who undergo MIE or OE. Patients undergo thorough pre-operative investigation that include physical examination, lab testing, diagnostic, and staging imaging. Nutritional status is assessed, particularly in patients that have undergone induction chemoradiation or experienced limited oral alimentation. Pulmonary function testing and selected cardiac stress testing further assess cardiopulmonary reserve. Furthermore, surgeons should optimize pre-operative physical functionality and engage patients in pre-habilitation to improve physiological reserve and post-operative recovery.
18-fluorodeoxyglucose positron emission tomography and computed tomography of the chest, abdomen, and pelvis are used to detect the presence of distant metastatic disease as well as assess the response to induction treatment. An esophagogastroduodenoscopy (EGD) and endoscopic ultrasound staging (EUS) are performed to locally stage malignancy by assessing tumor depth and regional lymph node involvement. Bronchoscopic evaluation is utilized in the event of an upper to mid-thoracic esophageal lesion to assess for tracheobronchial involvement.
Operative technique
Abdominal portion
Perioperative considerations and patient positioning
All patients receive a prophylactic dose of subcutaneously administered enoxaparin or heparin prior to anesthetic induction. After the patient has undergone general anesthesia induction and orotracheal intubation, the patient is placed in the supine posture. At our institution, we routinely use an arterial line and large bore peripheral intravenous lines for hemodynamic monitoring and resuscitation.
An EGD is routinely performed by the operating surgeon primarily to assess the proximal and distal extent of tumor. It is important to assess involvement of the stomach, extension of disease onto the cardia that may compromise distal margins and prohibit use of the stomach as conduit. Minimal insufflation during this endoscopy is paramount to prevent undue distention of the bowel prior to laparoscopy. The patient is positioned to the right side of the bed, to facilitate use of the liver retractor (DiamondFlex, Snowden Pencer, USA) and stabilization system (MediFlex, USA). A footboard is placed to support the patient’s position during reverse Trendelenburg positioning. The patient’s left arm is tucked and secured, while the right arm remains abducted.
The operative table is positioned to allow safe entry for the robotic cart apparatus and arms (da Vinci Xi Surgical Robot, Intuitive Surgical Inc., Sunnyvale, CA, USA) over the patient’s midline.
Port placement
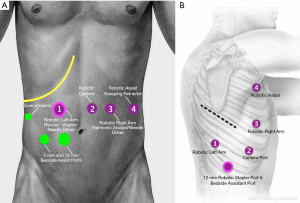
Abdominal entry is performed at the discretion of the surgeon. Generally, we utilize a standard 5 mm optical separator to gain entry to the abdomen under direct vision in either the midline camera site, or “right hand” (left upper mid-abdominal) working site. This port is later exchanged for a robotic 8 mm port and the 5 mm optical separator conserved and used at the right lateral subcostal site for introduction of the liver retractor. Alternatively, the robotic 8 mm port can be inserted with a direct Hassan technique using a direct open cut down. CO2 insufflation at a pressure of 15 mmHg is achieved, and a 30-degree robotic camera is utilized to visualize safe placement of subsequent ports. Approximately 9 cm between robotic ports is advised to minimize collision and optimize bedside assistance, although tolerance for closer distances on the newer robotic platforms (da Vinci Xi, Intuitive Surgical, USA) is substantial. Eight mm ports are inserted at the left subcostal margin and left mid clavicular line in alignment with the mid-abdomen, and a 12 mm robotic port in the right mid-clavicular mid-abdomen to later accommodate the robotic stapler. An 8 mm reducer is used at this site to accommodate the “left hand” instrument during much of the dissection. A 5 mm port is also positioned at the right subcostal margin, with care to avoid inadvertent injury to the ascending colon. The liver retractor is placed in the 5 mm port and a robotic atraumatic grasper is placed in the 8 mm left subcostal port (Small Grasping Retractor, Intuitive Surgical, USA), bipolar forcep (Force Bipolar or Fenestrate Bipolar, Intuitive Surgical, USA) in the 12 mm right midclavicular port using an 8 mm reducer, and robotic ultrasonic shears (Harmonic Scalpel; Ethicon Inc and Intuitive Surgical, USA) in the 8 mm left midclavicular port.
A 12-mm standard laparoscopic port is inserted at the right para-umbilical level, between the 12 mm right midclavicular and 8 mm umbilical ports. This port is used for bedside assistance, additional retraction, and subsequent staple use if not utilizing robotic stapling. Secondly, this port provides an alternative entry site for camera use during gastroepiploic arcade and omental mobilization.
RAMIE port placements are illustrated in Figure 1.
Gastric mobilization
After reverse Trendelenburg is achieved, we begin the dissection by exposing the lesser sac and dividing the lesser omentum. As an initial assessment of extent of disease and resectability, limited dissection to determine involvement of the hiatal crura, aorta, pancreatic body and tail, and the celiac axis is performed. During this dissection, a replaced left hepatic artery may be encountered and is often sacrificed if there is no evidence of vascular insult to the left lobe of the liver after temporary arterial ligation. In approximately 5% of cases, a significant replaced artery must be spared to avoid significant liver ischemia. Retrogastric, peri-hepatic and peri-splenic lymph node basins are dissected and lifted above the division line of the left gastric artery and vein. This lymphatic tissue will be removed en bloc with the surgical specimen. Anterior retraction of the stomach with the robotic assist arm allows optimal visualization and skeletonization of the left gastric vascular pedicle and provides optimal exposure of the celiac axis. An endovascular robotic stapler is inserted through the 12 mm “left hand” port and a vascular staple load is used to divide the left gastric vascular pedicle.
While maintaining anterior gastric retraction, retrogastric mobilization is further achieved through adhesiolysis and ultrasonic shears are used to divide the short gastric arteries from a posterior approach. The gastrosplenic vessels are dissected while mobilizing the left crus, and the lesser sac is re-entered to further mobilize the stomach up to the pylorus.
Intraoperative fluorescence angiography using near-infrared imaging (NIFI) is a useful adjunctive tool for intra-operative visualization of conduit perfusion (24,25). IV administration of 10 milligrams of ICG is given, and the conduit and vascular supply are visualized via near infrared fluorescence imaging built in to the robotic platform optical system (Firefly, Intuitive Surgical, Sunnyvale, CA, USA). In our previous experience, NIFI angiography located the gastroepiploic arcade in all cases after IV ICG was administered (median time: 37 seconds) (24,26). Ladak et al. described the role of intraoperative ICG in visualizing microvascular perfusion and conduit site selection, resulting in reduced anastomotic leaks following esophagectomy (27). Initial reports of innovative use of NIFI, include its application in improved visualization of lymph nodes intraoperatively during robot-assisted laparoscopic gastrectomy (28).
The gastroepiploic arcade is clearly exposed with medial and superior retraction of the stomach’s greater curve, with use of the left lateral robotic assistant arm. Care is taken to fully preserve the gastroepiploic arcade along the stomach’s greater curvature, and the operating surgeon must adhere to the “no touch” technique in order to reduce trauma to the portion of the stomach that would be used for the conduit.
Pyloroplasty
The antrum is retracted laterally leftward with left lateral robotic assistant arm, to optimize the view of the pylorus. The vein of Mayo may be used as a landmark to identify the location for the pyloroplasty. Non-absorbable retraction sutures (2-0 Ethibond, Covidien, USA) are placed at the lateral aspect of the pylorus. Ultrasonic shears are used to open the pylorus and a pyloroplasty is performed in a Heineke-Mikulicz fashion. The pyloroplasty is transversely closed using 4–6 interrupted sutures.
Construction of the gastric tube
The left subcostal robotic assistant arm retracts the mobile tip of the fundus towards the left upper quadrant and an additional robotic grasper retracts the antrum inferiorly. After removing the nasogastric tube, the robotic endo-gastrointestinal stapler is inserted in the 12 mm “left-hand” assistant port. Serial stapling applications are applied to create a conduit with a diameter measurement of 3–4 cm. Care is taken to keep the conduit in proper orientation during sequential stapling. The gastric conduit is secured to the surgical specimen to allow for appropriate orientation upon entry into the thoracic cavity during the thoracoscopic portion of the operation.
Jejunostomy tube placement
A 12 French feeding jejunostomy tube is placed using a standard laparoscopic approach.
Thoracic portion
Port placement
The patient is placed in the left lateral decubitus position. The right arm should be in neutral position and sterile preparation and drape is applied. The robotic 5 mm port with an optical separator can be used to enter the chest under direct vision in the superior most anterior port site in the posterior axillary line (Small Grasping Retractor, Intuitive Surgical, USA). CO2 insufflation at 8 mmHg is utilized, and the remaining ports are placed. The 8 mm camera port is introduced into the eighth intercostal space at the posterior mid-axillary margin under direct visualization, and an additional 8 mm placed in the 3rd and 5th space. An 8 mm robotic port is placed in approximately the eighth or ninth intercostal space roughly in line with the scapular tip and over the hiatus (Force Bipolar Grasper or Fenestrated Biopolar Grasper, Intuitive Surgical, USA). A 12 mm robotic assistant port is inserted at the level of diaphragm between the “left hand” port and the camera port. This is used as an assist port as well as robotic stapling port. The robotic cart is maneuvered over the ports and docked.
RAMIE port placements for thoracic portion in Figure 1.
Mobilization of the esophagus
The robotic grasping retractor is used to retract the right lower lobe superiorly and harmonic scalpel is used to divide the inferior ligament to the level of the inferior pulmonary vein. Subsequently, the right lung is retracted anteriorly and the posterior mediastinal pleura overlying the esophagus is dissected open using ultrasonic shears and mobilized to the level of the azygous vein. All node bearing tissues are harvested with the esophagus and the subcarinal lymph node dissection should be carefully dissected to avoid injury to the posterior membranous portion of the tracheobronchial tree. Blunt dissection and precise use of thermal energy are key maneuvers in preventing thermal injury to the airway. The robotic bipolar forceps may be another alternative for dissection. After successfully dissecting the posterior mediastinal to the level of the azygous vein, the vein is divided using endovascular stapler. The vagus nerve is also divided to reduce traction injury to the recurrent nerve and maybe spared or divided caudal of its pulmonary branches, therefore reducing risk of aspiration and pulmonary morbidity.
The robotic approach allows for a better dissection in the limited domain at the upper mediastinum, were thoracoscopic instrument dissection maybe more challenging. Chao et al. demonstrated higher lymph node yield along the left recurrent laryngeal nerve while limiting injury and subsequent morbidity, following the RAMIE approach (29). The REVATE trial is an ongoing randomized control trial, that will prospectively compare RAMIE and MIE, to assess outcomes following radical lymph node dissection along the left recurrent laryngeal nerve (23).
The dissection should terminate roughly 3–4 cm above the azygous bed. Dissection may be accomplished well into the thoracic inlet, if necessary. All thoracic duct tributaries and aortoesophageal perforating branches may be ligated with clips. While not performed routinely in our practice, thoracic duct resection has been suggested by others. While it is unclear if en bloc resection of the thoracic duct significantly impacts prognosis versus adding potential morbidity, it can be readily accomplished according to the practice pattern and technique of the surgeon. Some studies suggest advantages in yielding higher lymph node sampling and more accurate oncologic staging with thoracic duct resection (30). Other well-matched studies suggest no difference in overall or disease free survival in cases with or without the thoracic duct routinely removed (31).
After the hiatal dissection is completed, the conduit and specimen are introduced into the thoracic cavity while maintaining the appropriate orientation; the staple line should face the lateral position. It is important to handle the conduit with caution to reduce traumatic injury to the conduit and adjacent gastroepiploic arcades. The suture securing the conduit and specimen is divided and the conduit is secured to the diaphragm to prevent retraction into the abdominal cavity. The specimen is laterally retracted and mobilized superiorly to the level of the thoracic inlet.
During the dissection and harvest of nodal bearing tissue along the contralateral pleura, peri-aortic region, and the left main bronchus and pericardium, the surgeon must be aware of the airway’s proximity.
The nasogastric tube is retracted proximally, and the esophagus is divided approximately 3–4 cm above the azygous vein. An access incision is created by extending the fifth intercostal port site and a wound protector is inserted. The specimen is removed en bloc and sent for pathologic study of the margins.
Creation of the esophagogastric anastomosis
The esophageal orifice is held with the robotic assistant grasper, and a 28 mm anvil inserted into the distal esophageal opening. A “baseball” purse string suture is robotically sewn prior to insertion to secure the anvil in place, along with an additional purse string for reinforcement after initially securing the anvil. The conduit is separated from the diaphragm, a proximal gastrotomy made in the conduit, and the extra-long end-to-end anastomotic stapler (DST XL EEA Stapler, Covidien, USA) is introduced into the access incision and placed into the proximal portion of the conduit. The stapler spike is deployed along the greater curve of the conduit, just above the origin of the vascular arcade. The anvil and stapler are docked, and tissue apposition is achieved in the correct orientation. The anastomosis is created and the gastrotomy along with the redundant portion of the conduit is sealed and removed with the robotic endo-gastrointestinal stapler. The anastomosis may be secured with omental flap coverage (if harvested during the abdominal portion). Studies have shown potential for reduced anastomotic leakage and stricture rate after wrapping the anastomosis with omental flap (32-34). The nasogastric tube is re-advanced under direct visualization.
A 28 French chest tube is placed in the apicoposterior position and a Jackson-Pratt drain placed posteriorly adjacent to the anastomosis.
Postoperative management
Generally patients are extubated in the operating room and admitted to the intensive care unit. Judicious fluid administration is advised, and we encourage early ambulation on post-operative day one. Patients are discharged to the step-down unit on the second postoperative day and tube feeding (via jejunostomy tube) is initiated at an initial slow rate. On the fifth postoperative day, the nasogastric tube is removed and barium esophagogram is conducted to assess for anastomotic integrity. In the absence of a leak a liquid diet is initiated, and the chest tube is removed prior to discharge. Patients report to follow up clinic visit one to two weeks following the operation. The anastomotic drain is evaluated and removed during the clinic visit, and the jejunostomy tube is removed depending on the patient’s ability to tolerate solid food.
Discussion
Despite reduced surgical morbidity with adoption of the MIE approach, there are limited studies that compare RAMIE to MIE or OE. The disadvantages of the MIE are largely focused on technical limitations: limited degrees of freedom, long learning curve, two-dimensional view and coordination with a manual assistant (35,36). The technological advancements of the RAMIE have allowed surgeons to overcome the visual limitations, while providing precise atraumatic dissections of mediastinal lymph nodes, peribronchial and periesophageal planes (9). Challenging portions of the MIE (creation of the esophagogastric anastomosis—anvil reinforcement, pyloroplasty, hiatal dissection) are facilitated with robotic visualization and instrument dexterity. General dissection is performed with greater precision due to the superior optics and degrees of freedom provided by robotic capabilities. Additionally, superior three-dimensional view, improved magnification, ambidexterity, and self-assisting capabilities are examples of favorable technical features in robotic-assisted surgery.
The adaptation of the robotic approach allows surgeons to perform the thoracoscopic anastomosis in a multitude of ways: robotic hand-sewn, circular stapling or linear stapling and robotic hand-sewn closure of the stapler defect. Plat et al. published a review that discussed the advantages and limitations of the multiple anastomotic techniques (37). Circular stapling is uniformly adopted, particularly with surgeons transitioning to the robotic platform. The linear stapling and hand-sewn technique are more technically challenging but does not require bedside assistance for performance (37). To our knowledge, there are no prospective studies that delineate which technique is superior.
Our institutional results have been favorable with utilizing RAMIE in patients with esophageal cancer. Okusanya et al. described our initial experience with RAMIE in twenty-five patients (14). The median operative time was 661 minutes and median blood loss was 250 cc. There was an 8% conversion rate (n=4) but no deaths within 30 or 90 days following surgery. This study showed an equivalent mortality (0% vs. 2.8%), to our previous institutional MIE series (n=1,011). R0 resection rate (96% vs. 98%), lymph node harvest (26 vs. 19) and anastomotic leak rate (4% vs. 5%) were also comparable (38). Cerfolio et al. performed a retrospective study of twenty-two patients that underwent RAMIE. Median blood loss was 75 cc and 17 lymph nodes were harvested. No patients underwent thoracoscopic to thoracotomy conversion, but one abdominal conversion was performed. R0 resection was achieved in all patients (13). Weksler et al. demonstrated equivalent outcomes between RAMIE (n=11) and thoracoscopic MIE (n=26). Operative times (439 vs. 483 minutes), blood loss (20 vs. 26), nodal harvest (23 vs. 23), and ICU LOS (8.7 vs. 10) were equivalent between the two approaches (39).
Given the growing interest and applicability of the RAMIE approach, surgical training in the technique is increasing and the learning curve is becoming better defined to provide guidance for surgical trainees and surgeons. Hernandez et al. reported surgical proficiency in the RAMIE approach following 20 cases performed by experienced esophageal surgeons, and Zhang et al. reported decreased operative duration after the 26th RAMIE case (40,41). Sarkaria et al. highlighted the learning curve to achieve proficiency based on a cohort of 100 patients that underwent RAMIE at Memorial Sloan Kettering Cancer Center with a 90-day mortality of 1%, and suggested that 40–45 cases are needed to achieve proficiency and minimize complications (42). The learning curve has also been assessed under the implementation of a proctored program, resulting in proficiency following 24 cases versus 70 non-proctored cases (43). Proficiency in the MIE approach may be acquired after 30 to 50 cases, according to Association of Upper Gastrointestinal Surgeons and the Association of Laparoscopic Surgeons of Great Britain and Ireland (44). Other retrospective studies endorsed at least 35–40 cases are needed to gain proficiency in the MIE approach (45).
Early proficiency in the RAMIE cohorts, may be attributed to prior experience in non-robotic esophagectomies and foregut cases. Thus, the learning curve of RAMIE may be reduced with the implementation of proctoring/training programs for surgical trainees and surgeons.
Innovative steps to improving operative dissection and reducing morbidity following the RAMIE approach, remains ongoing. To date, the use of transcervical robotic-assisted esophagectomy via single port has been performed in pre-clinical cadaver studies (n=3) using the da Vinci Single Port (SP) (n=2) and da Vinci Xi (n=1) (46). Esophageal mobilization was accomplished in all 3 cadaver models, but the da Vinci SP allowed for a more extensive esophageal dissection (to the level of hiatus) in comparison to the Xi (to the level of the carina). High esophageal tumor dissection, improved lymph node dissection at the level of the superior left recurrent laryngeal nerve, and reduced pulmonary sequalae due to extra-pleural dissection are the possible benefits of this approach (46). Intraoperative diagnostic adjuncts including tumor specific fluorescence markers for intraoperative molecular imaging (IMI), similar to those utilized to detect pulmonary adenocarcinomas, may play a role in detecting esophageal cancer and assessing margins during surgery (47).
Our description details the feasibility of this procedure, in concordance with studies that reveal favorable short-term outcomes in RAMIE. Additional prospective studies are required to determine long-term differences in oncological outcomes between RAMIE and MIE vs. OE.
Acknowledgments
Funding: This review was supported by the Department of Cardiothoracic Surgery at the University of Pittsburgh and the NCI T32CA113263-11 (Ekeke).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christopher R. Morse and Uma M. Sachdev) for the series “Minimally Invasive Esophagectomy” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-34). The series “Minimally Invasive Esophagectomy” was commissioned by the editorial office without any funding or sponsorship. ISS serves as an unpaid editorial board member of Annals of Esophagus from Mar 2020 to Feb 2022. JDL reports grants from University of Texas SWMC, grants from Anpac Tech of USA, non-financial support from Covidien, other from Intuitive Surgical Inc., Proctor and Gamble, and Cigna Corp, outside the submitted work. ISS reports grants and personal fees from Intuitive Surgical, Inc., personal fees from On Target Laboratories, personal fees from Cambridge Medical Robotics, and personal fees from Auris Medical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. [Crossref] [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- Turner GG. Carcinoma of the (esophagus the question of its treatment by surgery. Lancet 1936;227:130-4. [Crossref]
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC, Wilkins EW Jr, et al. Transthoracic esophagectomy: a safe approach to carcinoma of the esophagus. Ann Thorac Surg 1988;45:137-43. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Nguyen NT, Follette DM, Wolfe BM, et al. Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg 2000;135:920-5. [Crossref] [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [PubMed]
- van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2006;20:1435-9. [Crossref] [PubMed]
- Okusanya OT, Hess NR, Luketich JD, et al. Technique of robotic assisted minimally invasive esophagectomy (RAMIE). J Vis Surg 2017;3:116. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol 2015;22 Suppl 3:S1350-6. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP. Robotic-assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin 2014;24:211-22. vii. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [Crossref] [PubMed]
- Okusanya OT, Sarkaria IS, Hess NR, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg 2017;6:179-85. [Crossref] [PubMed]
- Zhang X, Su Y, Yang Y, et al. Robot assisted esophagectomy for esophageal squamous cell carcinoma. J Thorac Dis 2018;10:3767-75. [Crossref] [PubMed]
- Horgan S, Berger RA, Elli EF, et al. Robotic-assisted minimally invasive transhiatal esophagectomy. Am Surg 2003;69:624-6. [PubMed]
- Kernstine KH, DeArmond DT, Shamoun DM, et al. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc 2007;21:2285-92. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Goldman DA, et al. Early Quality of Life Outcomes After Robotic-Assisted Minimally Invasive and Open Esophagectomy. Ann Thorac Surg 2019;108:920-8. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [Crossref] [PubMed]
- Yang Y, Zhang X, Li B, et al. Robot-assisted esophagectomy (RAE) versus conventional minimally invasive esophagectomy (MIE) for resectable esophageal squamous cell carcinoma: protocol for a multicenter prospective randomized controlled trial (RAMIE trial, robot-assisted minimally invasive Esophagectomy). BMC Cancer 2019;19:608. [Crossref] [PubMed]
- Deng HY, Luo J, Li SX, et al. Does robot-assisted minimally invasive esophagectomy really have the advantage of lymphadenectomy over video-assisted minimally invasive esophagectomy in treating esophageal squamous cell carcinoma? A propensity score-matched analysis based on short-term outcomes. Dis Esophagus 2019;32:doy110. [Crossref] [PubMed]
- Park S, Hwang Y, Lee HJ, et al. Comparison of robot-assisted esophagectomy and thoracoscopic esophagectomy in esophageal squamous cell carcinoma. J Thorac Dis 2016;8:2853-61. [Crossref] [PubMed]
- Chao YK, Li ZG, Wen YW, et al. Robotic-assisted Esophagectomy vs Video-Assisted Thoracoscopic Esophagectomy (REVATE): study protocol for a randomized controlled trial. Trials 2019;20:346. [Crossref] [PubMed]
- Okusanya O, Lu M, Luketich JD, et al. Intraoperative near infrared fluorescence imaging for the assessment of the gastric conduit. J Thorac Dis 2019;11:S750-4. [Crossref] [PubMed]
- Okusanya OT, Hess NR, Luketich JD, et al. Infrared intraoperative fluorescence imaging using indocyanine green in thoracic surgery. Eur J Cardiothorac Surg 2018;53:512-8. [Crossref] [PubMed]
- Sarkaria IS, Bains MS, Finley DJ, et al. Intraoperative near-infrared fluorescence imaging as an adjunct to robotic-assisted minimally invasive esophagectomy. Innovations (Phila) 2014;9:391-3. [Crossref] [PubMed]
- Ladak F, Dang JT, Switzer N, et al. Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 2019;33:384-94. [Crossref] [PubMed]
- Herrera-Almario G, Patane M, Sarkaria I, et al. Initial report of near-infrared fluorescence imaging as an intraoperative adjunct for lymph node harvesting during robot-assisted laparoscopic gastrectomy. J Surg Oncol 2016;113:768-70. [Crossref] [PubMed]
- Chao YK, Hsieh MJ, Liu YH, et al. Lymph Node Evaluation in Robot-Assisted Versus Video-Assisted Thoracoscopic Esophagectomy for Esophageal Squamous Cell Carcinoma: A Propensity-Matched Analysis. World J Surg 2018;42:590-8. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Clinical outcome of transthoracic esophagectomy with thoracic duct resection: Number of dissected lymph node and distribution of lymph node metastasis around the thoracic duct. Medicine 2016;95:e3839. [Crossref] [PubMed]
- Oshikiri T, Takiguchi G, Miura S, et al. Thoracic Duct Resection During Esophagectomy Does Not Contribute to Improved Prognosis in Esophageal Squamous Cell Carcinoma: A Propensity Score Matched-Cohort Study. Ann Surg Oncol 2019;26:4053-61. [Crossref] [PubMed]
- Liu K, Zhang GC, Cai ZJ. Avoiding anastomotic leakage following esophagogastrostomy. J Thorac Cardiovasc Surg 1983;86:142-5. [Crossref] [PubMed]
- Goldsmith HS, Kiely AA, Randall HT. Protection of intrathoracic esophageal anastomoses by omentum. Surgery 1968;63:464-6. [PubMed]
- Zhang K, Yang YH. Use of pedicled omentum in oesophagogastric anastomosis: analysis of 100 cases. Ann R Coll Surg Engl 1987;69:209-11. [PubMed]
- Germain A, Bresler L. Robotic-assisted surgical procedures in visceral and digestive surgery. J Visc Surg 2011;148:e40-6. [Crossref] [PubMed]
- Ruurda JP, van Vroonhoven TJ, Broeders IA. Robot-assisted surgical systems: a new era in laparoscopic surgery. Ann R Coll Surg Engl 2002;84:223-6. [Crossref] [PubMed]
- Plat VD, Stam WT, Schoonmade LJ, et al. Implementation of robot-assisted Ivor Lewis procedure: Robotic hand-sewn, linear or circular technique? Am J Surg 2020;220:62-8. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Hernandez JM, Dimou F, Weber J, et al. Defining the Learning Curve for Robotic-assisted Esophagogastrectomy. J Gastrointest Surg 2013;17:1346-51. [Crossref] [PubMed]
- Zhang H, Chen L, Wang Z, et al. The Learning Curve for Robotic McKeown Esophagectomy in Patients With Esophageal Cancer. Ann Thorac Surg 2018;105:1024-30. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Grosser R, et al. Attaining Proficiency in Robotic-Assisted Minimally Invasive Esophagectomy While Maximizing Safety During Procedure Development. Innovations (Phila) 2016;11:268-73. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Learning Curve for Robot-Assisted Minimally Invasive Thoracoscopic Esophagectomy: Results From 312 Cases. Ann Thorac Surg 2018;106:264-71. [Crossref] [PubMed]
- Hardwick RH. The Association of Upper Gastrointestinal Surgeons (AUGIS) and the Association of Laparoscopic Surgeons (ALS) of Great Britain & Ireland. A Consensus View and Recommendations on the Development and Practice of Minimally Invasive Oesophagectomy. September 2009, AUGIS, 2011. Available online: http://www.augis.org
- Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg 2014;218:1130-40. [Crossref] [PubMed]
- Grimminger PP, van der Sluis PC, Stein H, et al. Feasibility of Transcervical Robotic-Assisted Esophagectomy (TC-RAMIE) in a Cadaver Study—A Future Outlook for an Extrapleural Approach. Appl Sci 2019;9:3572. [Crossref]
- Predina JD, Okusanya O, D, Newton A, et al. Standardization and Optimization of Intraoperative Molecular Imaging for Identifying Primary Pulmonary Adenocarcinomas. Mol Imaging Biol 2018;20:131-8. [Crossref] [PubMed]
Cite this article as: Ekeke CN, Luketich JD, Sarkaria IS. Robotic-assisted minimally invasive esophagectomy. Ann Esophagus 2021;4:7.


