
Laparoscopic cardiomyotomy: historical overview and current operative approach
Introduction
Achalasia is a rare but debilitating disease of the esophagus characterized by aperistalsis associated with abnormal relaxation of the lower esophageal sphincter (LES). In the United States, the prevalence of this disorder is thought to be increasing and is currently estimated at 7–10 per 100,000 persons (1-3). At the time of surgical consultation, patients may present with progressive dysphagia, regurgitation, pain, aspiration, and weight loss. In many cases, patients may present with recurrence of these symptoms after limited response to medical therapy and endoscopic intervention. Laparoscopic cardiomyotomy (LCM) is the definitive operation for achalasia, with durable symptom improvement in approximately 90% of patients. The addition of an anterior or posterior partial fundoplication to cardiomyotomy is the current standard of care (4,5).
Careful patient selection is critical in determining which patients will most benefit from operative intervention. In addition to detailed history, endoscopy, and static or dynamic esophagogram, both high-resolution manometry and trial of non-operative treatment can help identify patients for whom cardiomyotomy would be most appropriate. The Chicago classification groups achalasia into three categories based on manometry patterns. Type I achalasia is characterized by aperistalsis, incomplete LES relaxation, and absent esophageal pressurization. The esophagus may be of normal caliber or may be dilated, occasionally attaining a “sigmoid” appearance on esophagram. The corresponding esophagogram reveals a non-dilated esophagus with a “bird’s beak” appearance at the gastroesophageal junction. Type II achalasia is characterized by aperistalsis with panesophageal pressurization due to unpredictable, preserved skeletal muscle contractile capacity working against the incomplete relaxation of the LES. In practice, the difference between types I and II can be ephemeral, and the same patient may have evidence of both types on manometric studies depending on the volume of liquid pressurizing the esophagus during the study. Type III achalasia patients have uncoordinated, premature, high-pressure contractions combined with incomplete relaxation of the LES. Contrastingly, diffuse esophageal spasm (DES) patients have uncoordinated and premature esophageal contractions with preserved LES function. The hallmark of DES is the preservation of normal antegrade peristalsis in greater than 30% of wet swallows. In contrast, aperistalsis is present in all three type of achalasia. Recent data suggest that approximately 90% of patients of achalasia patients fall into the types I and II categorization. These patients generally respond well to surgical intervention, with some sources citing a greater than 95% success rate after LCM (1,6,7). With esophageal dilation over 5 cm or sigmoid esophagus, LCM is recommended as first line therapy by most surgeons, but a significant proportion (20–30%) of individuals with this degree of esophageal damage will ultimately require esophagectomy (8).
Broadly, treatment of achalasia focusses on disruption of diseased muscle fibers of the LES. The past several decades has seen the simultaneous advance in endoscopic and surgical approaches to achalasia. Endoscopically, mechanical pneumatic balloon dilation, with disruption of lower esophageal muscle fibers, represents an initial treatment modality that can produce durable results in certain patients. Secondarily, endoscopic Botox injection serves as treatment reserved for non-operative candidates or, rarely, as a temporizing measure before surgery. In patients whom the diagnosis of achalasia is ambiguous, response to these non-operative treatments may be a predictor of success for surgical myotomy, either via laparoscopic or natural orifice approach. Repeated endoscopic interventions expose the patient to higher risk of perforation, and in younger patients, cardiomyotomy is preferred as first-line therapy (2,9-11).
In this chapter, we discuss our approach to LCM, also referred to in the literature as Heller myotomy. From open thoracotomy to now robotic-assisted surgery, the approach to cardiomyotomy has evolved rapidly within the last three decades. With these advances, unexpected nuances are encountered and discussed, along with the approach to perioperative care.
Historical perspective
Treatment of dysphagia and disordered esophageal function has historically been dominated by endoluminal dilation. In the 17th century, rigid instruments such as whale bones were used to mechanically dilate the esophagus. Three hundred years later in the 20th century, both endoluminal dilation techniques and surgical approaches evolved simultaneously. Ernest Heller performed the first cardiomyotomy in 1913, initially via laparotomy (12). Heller’s original technique, which involved anterior and posterior myotomy of the lower esophagus and gastric cardia, was adapted to an anterior myotomy via transthoracic, rather than trans-abdominal, approach (13,14).
Pneumatic dilation remained the preferred treatment for achalasia into the early 1990s, with surgery still reserved for failure of the less-invasive endoluminal approach. One survey of patients from 1994 showed that although open surgery produced better long-term success, three out of four patients still elected to have pneumatic dilation as primary therapy (15). The first thoracoscopic Heller myotomy was performed in 1991 (16). As surgeons continued to refine their operative skill and gain comfort with this technology, increasing numbers of achalasia cases were referred for surgery. It is notable that while in North America, the open thoracic approach was popular from the mid-20th century into the 1990s, the trans-abdominal approach described by Heller remained popular in South America and Europe (17). These parallel surgical preferences perhaps accounts for the regional differences in initial minimally-invasive approaches; as Pellegrini and team performed and described the first thoracoscopic operations for achalasia in 1991, Cuschieri and team performed the first LCM in the United Kingdom that same year (18). By the late 1990s, success in laparoscopic fundoplication and associated mediastinal dissection prompted many North American leaders in minimally-invasive foregut surgery to transition away from the thoracoscopic approach. Notably, problems with thoracoscopic cardiomyotomy included (i) the distorted “perpendicular” orientation of the esophagus and associated difficulty maintaining the necessary submucosal dissection plane, (ii) the need for simultaneous endoscopy to determine the appropriate distal length of myotomy, and (iii) relatively worse pain with thoracoscopy compared to laparoscopy. Furthermore, the laparoscopic approach was technically simpler, with easier patient positioning (supine, with or without split-leg), elimination of dual-lumen intubation for single-lung ventilation, and more reliable visualization of the gastroesophageal junction for reproducible myotomy length (1,4,19).
Another major difference between the thoracic and abdominal approach was the utilization of fundoplication to prevent postoperative reflux. In the thoracoscopic approach, Ellis’ modified Heller myotomy was utilized, with a 6 cm myotomy of the distal esophagus extending approximately 5 mm onto the gastric cardia, without fundoplication (13,19). At that time, there was some concern that laparoscopic approach, which required mobilization of bilateral phrenoesophageal ligaments, would in fact worsen reflux as compared to the limited gastroesophageal mobilization afforded by the thoracoscopic approach. However, postoperative pH studies in the thoracoscopic population revealed a high rate of silent reflux, with abnormal acid exposure seen in 60% of patients in a small case series. Furthermore, the limited thoracoscopic dissection onto the cardia sometimes resulted in inadequate myotomy and recurrent dysphagia (19). Therefore, through the early 21st century, most surgeons have transitioned to LCM, extending between 4–7 cm proximally from the gastroesophageal junction and at least 2 cm onto the gastric cardia, with partial anterior or posterior fundoplication. Transthoracic myotomy was still reserved as part of the treatment algorithm for perforation after pneumatic balloon dilation (2,20).
The addition of fundoplication to the modified Heller’s procedure has been an area of ongoing interest. In 1962, the French surgeon Vincent Dor proposed the addition of an anterior fundoplication in trans-abdominal anterior myotomy to reduce reflux rates (21). In 2006, a randomized control trial by Richards et al. suggested that the addition of Dor fundoplication to Heller myotomy was more effective in long-term prevention of postoperative reflux; while the addition of the fundoplication added operative time and immediate cost, patients without fundoplication incurred more costs over ten years due to the need for prolonged proton pump inhibitor therapy (22). In 2011, a multicenter prospective randomized-control trial comparing Dor to Toupet fundoplication in 60 patients found no significant differences in postoperative reflux or other esophageal symptoms between groups. The Dor group trended towards more abnormal pH testing, but this difference was not statistically significant (5). There has also been one randomized control trial looking at 144 patients undergoing LCM comparing Dor to Nissen or total fundoplication. The authors found that 5 years after surgery, dysphagia was statistically more frequent in the Nissen group (15%) compared to the Dor group (3%) (23). The most recent guidelines by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) suggest that a partial fundoplication should be performed, but there is no consensus as to whether an anterior or posterior wrap is superior (24).
The recent history of cardiomyotomy for achalasia has been largely characterized by the rising popularity and accessibility of robotic surgical platforms. The most widely-used robotic platforms in North America offer fully articulating wristed instruments as well as high-definition 3D visualization. Experience with robotic surgery since approximately 2005 suggest that although operative times are longer compared to LCM, robotic-assisted cardiomyotomy results in a lower rate of intraoperative esophageal perforation with little apparent learning curve for experienced laparoscopic surgeons (25,26).
Laparoscopic cardiomyotomy: technique
Patient selection
As reviewed above, careful patient selection is key to favorable and durable resolution of dysphagia after surgical intervention. The typical workup includes detailed history including attention to Eckardt score, which surveys dysphagia, regurgitation, retrosternal pain, and weight loss. The severity of each symptom is scored based on frequency (none, occasional, daily, with every meal) and amount of weight loss in kilograms (0, <5, 5–10, >10) (Table 1). The final score may range from zero to 12, and a score greater than or equal to four implies more severe disease warranting intervention. Other associated comorbidities such as malnutrition, aspiration, recurrent pneumonia, or chronic lung disease are also noted carefully. Syndromes that may confound the diagnosis of achalasia include connective tissue diseases that mimic aperistalsis, particularly systemic sclerosis and CREST syndrome (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia), autoimmune disease such as amyloidosis or sarcoidosis, and Chagas’ disease. Pseudoachalasia should also be considered but is difficult to distinguish from primary achalasia; history and physical may reveal a long history of reflux disease and rapid symptom onset, more consistent with primary esophageal malignancy. Other causes of pseudoachalasia include extrinsic compression from intrathoracic masses such as lymphoma, cardiac tumors, bronchial carcinomas, or even benign causes such as pancreatic pseudocysts, mesenchymal tumors, or mediastinal fibrosis (27). The patient’s response to any prior endoscopic interventions is assessed, including balloon dilation, Botox, and occasionally, peroral endoscopic myotomy (POEM).
Table 1
| Symptom | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Dysphagia | None | Occasional | Daily | Every meal |
| Regurgitation | None | Occasional | Daily | Every meal |
| Chest pain | None | Occasional | Daily | Multiple times per day |
| Weight loss (kg) | 0 | <5 | 5–10 | >10 |
Final score is the sum of each symptom, ranging from 0 to 12.
Preoperative studies include barium swallow, upper endoscopy, and high-resolution manometry. Occasionally, symptoms may be confusing and imaging atypical for achalasia. In these settings, 48-hour pH study can be used to rule out GERD. In pseudoachalasia, manometry may reveal an abnormally low, “wide-open” LES, while in other cases, findings may be indistinguishable from primary achalasia. Endoscopy may reveal an intraluminal mass or evidence of extrinsic compression. If suspicion of pseudoachalasia remains high, CT scan of the chest and abdomen (or comparable cross-sectional imaging), is also completed. Appropriate lab work and preoperative cardiac testing is completed based on the age and comorbidities of the patient (28).
Generally, young males with type II achalasia without prior endoscopic interventions are thought to have the best long-term resolution of symptoms after LCM. However, this disease can affect males and females equally between 30–60 years old (17). Therefore, at our institution, we pay particular attention to patient frailty as characterized by their ability to live independently, walk unaided, sustain physical activity, climb stairs, as well as their general cognitive function. Currently, multiple online frailty calculators are available for free or for purchase. The ACS Risk calculator, based on NSQIP data, is also an excellent free, online tool to estimate operative risk, particularly in elderly foregut surgery patients. Patients are encouraged to engage in “prehabilitation”, paying particular attention to daily gentle physical activity (walking 1–2 miles per day) as well as increasing protein intake with liquid supplements if tolerated.
Positioning and trocar placement
The patient is positioned in the supine split-leg (modified French) position to allow the surgeon to operate from between the legs. The legs are abducted to 30- to 60-degree on leg boards and foot boards are employed to ensure neutral foot flexion. Arms may either be padded and tucked or abducted and secured to arm boards at 80 degrees. Alternatively, a non-split leg operating table with a foot board may be used, with the surgeon usually operating from the patient’s right side. In either position, steep reverse Trendelenburg positioning is utilized to allow gravity-assisted downward retraction of the intestine, enabling optimal access to the hiatus.
Most commonly, a closed Veress technique is used to gain abdominal access, either at Palmer’s point or at the umbilicus, to insufflate the abdomen to 15 mmHg. Trocars are placed as follows: 11 mm camera port 15 cm from the xyphoid, to the left of midline, 12 mm working port in the left upper quadrant 12 cm from the xyphoid along the costal margin, 5 mm working port in the right upper quadrant 7–11 cm from the xyphoid along the costal margin, and 5 mm assistant port in the left lateral abdomen. A Nathanson liver retractor is placed at the level of the xyphoid (Figure 1). Of note, the placement of the camera port just to the left of midline reduces the risk of postoperative port-site hernia, reduces visual interference from the falciform ligament and left lobe of liver, and aligns more favorably with the natural trajectory of the esophagus as it exits the hiatus (9).
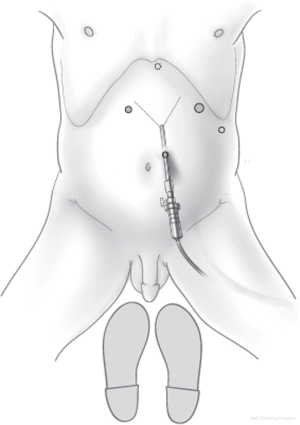
Operative technique—key steps
Dissection begins by entering the gastrohepatic ligament, taking care to preserve the hepatic branch of the vagus nerve. The interface of the right phrenoesophageal ligament and right crus is divided, allowing entry into the mediastinum and subsequent continuation of the dissection along the anterior aspect of the crus. Energy devices are used sparingly during this part of the procedure, with most of the right-to-left dissection over the anterior aspect of the esophagus and mediastinum performed bluntly using two atraumatic Hunter graspers.
After both right and left phrenoesophageal ligaments are divided, attention is turned to mobilizing the fundus of the stomach in preparation for the antireflux portion of the procedure. This is accomplished by dividing the short gastric vessels starting at the level of the inferior pole of the spleen. A circumferential esophageal dissection is completed, making a posterior window at the base of the crura and passing a Penrose drain, which is secured around the gastroesophageal junction.
Next, the gastroesophageal fat pad is divided and reflected to the left of the anterior vagus nerve to allow proper exposure and visualization of the longitudinal muscle fibers of the LES. Care is taken throughout the procedure to identify and preserve the anterior vagus nerve. The Penrose drain is used to provide downward countertraction and adequate exposure of the anterior surface of the esophagus. Mediastinal dissection is completed to the extent to which it is necessary to visualize the anterior surface of the esophagus.
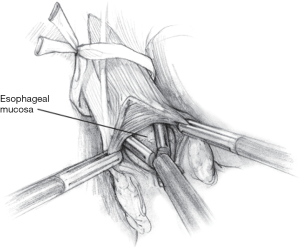
The myotomy is initiated on the esophagus just proximal to the gastroesophageal junction. The medial edge of the esophagus is retracted by the surgeon as the assistant uses the fat pad to provide countertraction retract laterally, flattening the anterior surface of the gastroesophageal junction. This maneuver allows the surgeon to identify and sharply split the longitudinal muscle fibers, exposing the circular muscle layer beneath. The circular muscle fibers are then gradually elevated away from the underlying submucosal plane using a combination of sharp and blunt dissection (Figure 2).
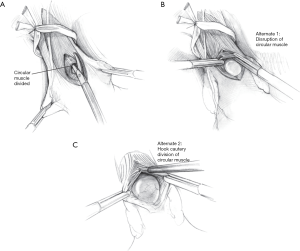
A combination of three techniques are used to safely and efficaciously divide the circular muscle fibers. The first technique employs the laparoscopic Metzenbaum scissors to sharply divide the muscle fibers (Figure 3A). The second technique is a tip-to-tip muscle-tearing method, in which the surgeon grasps the muscle fibers closely with two atraumatic graspers and fractures the tissue in a controlled manner (Figure 3B). Any resulting muscle fiber bleeding using either sharp or blunt division is controlled using a gauze sponge and direct pressure. The third technique is the use of hook electrocautery, which relies on the ability to hook and lift the muscle fibers up and away from the esophageal submucosa. Short bursts of energy are applied along with constant gentle upward tension. This technique is least preferred, as the proximity of the back of the hook to the submucosa risks thermal injury (Figure 3C).
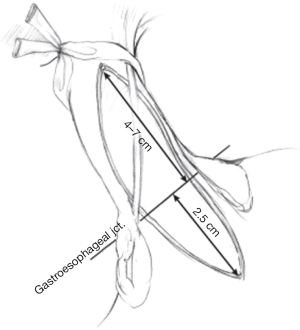
Myotomy is continued proximally to 4–7 cm from the gastroesophageal junction, and then 2–3 cm onto the gastric cardia, dividing the crossing sling fibers in a similar manner (Figure 4). A longer myotomy may be completed if there is concern for type III achalasia. The proximal extent of the dissection is guided by endoscopy, and a leak test is also performed at this time. This may be performed by insufflating the esophagus endoscopically and submerging the submucosa intraabdominally in saline irrigation. Endoscopy and transillumination may also reveal a mucosal defect. An alternative leak test employs methylene blue diluted in 250 cc of saline, delivered into the lumen of the esophagus via orogastric tube. Thinned areas of the submucosa will readily stain blue. If an esophagectomy is observed, the defect is repaired primarily using fine (3-0 or 4-0) absorbable suture. Usually, a Dor fundoplication will help buttress and protect the esophagomyotomy.
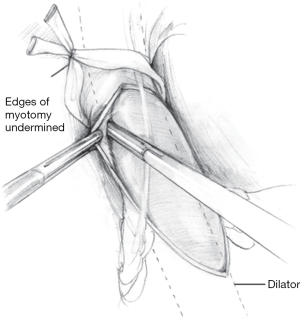
Next, a 56 French Maloney dilator is passed into the stomach either by anesthesia staff or by the co-surgeon; this instrument helps gauge the adequacy of the myotomy and serves as a guide for fundoplication. Constant open communication with between the surgeon and person passing the dilator is critical during this maneuver to prevent perforation through the esophagomyotomy. Once placed, the stretch of the bougie should reveal any remaining intact crossing muscle fibers. In a complete myotomy, the surgeon should be able to observe slack mucosa around the dilator. If the myotomized segment appears snug, the muscle fibers can be bluntly undermined to further separate it from the submucosal layer (Figure 5).
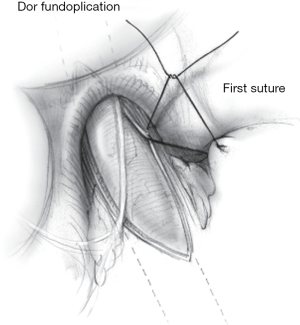
Given the propensity for the myotomy to allow for unimpeded acid reflux, we complete the cardiomyotomy with partial fundoplication, constructed around the dilator. As noted earlier, there does not appear to be a difference between Dor and Toupet fundoplication in antireflux capacity. Some surgeons will perform a Toupet fundoplication, securing the wrap to the cut edges of the myotomy, possibly helping hold the myotomy open. However, this configuration may anteriorly displace the esophagus and can result in low-grade residual dysphagia. Conversely, an anterior Dor fundoplication protects the exposed submucosa and does not require posterior esophageal dissection. The first, lateral stitch of the Dor to the left crus should also incorporate the lateral cut edge of the myotomy if possible (Figure 6). This approach is generally preferred at our institution (15). In either scenario, 0-gauge non-absorbable braided sutures (polyester or silk) are used to fashion the fundoplication (4,9,24).
Postoperative care
After extubation and immediate post-anesthetic recovery, patients are generally transferred to the ward for routine postoperative management. Water-soluble contrast radiographs are done on postoperative day one, until which time the patient remains nil by mouth. Antiemetics are scheduled in the immediate perioperative period to prevent nausea and retching. A multimodal pain control strategy is also employed, with non-narcotic agents and ice packs favored over opioids. Early ambulation and aggressive incentive spirometry use is encouraged. If the esophagram is negative for leak, and if the patient is clinically well, a clear liquid diet is started. The patient is sent home on either postoperative day one or two on a thick or “full” liquid diet for about one week, followed by a modified soft, low-residue “post-Nissen” diet for three weeks. This diet is employed to overcome the normal postoperative swelling around the gastroesophageal junction, which may feel like recurrent symptoms to the patient. Ability to tolerate oral hydration and adequate non-IV pain control are major criteria for discharge.
Complications
Laparoscopic cardiomyotomy is associated with up to 7% risk of intraoperative esophagotomy but is easily repaired intraoperatively as described above (29-33). Delayed esophageal perforation is the most serious complication following cardiomyotomy. Classically, thermal injuries may present anytime up to seven days postoperatively. The typical initial worrisome sign is tachycardia, followed by fever, tachypnea, and epigastric pain. A low index of suspicion should quickly prompt water-soluble contrast study of the esophagus and upper GI tract. Simultaneously, the patient should be treated for intraabdominal sepsis, with resuscitation and initiation of IV antibiotics per latest guidelines. A Foley catheter may be placed for accurate recording of urine output as a surrogate for end-organ perfusion. If the patient remains stable, cross-sectional imaging may reveal a collection suitable for image-guided drainage. If this is not possible, or if the patient worsens after external drainage, return to the operating room for washout and wide drainage is mandatory. In rare cases, devitalization or severe disruption of the esophagus may mandate emergency esophagectomy, with spit fistula and gastrostomy, followed by reconstruction after full recovery (eight weeks or more from the index operation).
Recurrence of symptoms weeks to years after cardiomyotomy should trigger a repeat workup, including thorough history and physical, esophagogram, endoscopy, and if necessary, manometry. In the case of recurrent achalasia or incomplete myotomy, the LES pressure is usually found to be greater than 10–15 mmHg (20). Strictures may be treated postoperatively by pneumatic balloon dilation, which disrupts scar tissue over the area of prior myotomy. In rare cases, a redo myotomy, either via laparoscopy or POEM, might be discussed. However, redo LCM is rarely successful with LES pressure less than 10 mmHg. Undoing a prior fundoplication may help alleviate dysphagia. If symptoms progress to end-stage achalasia and sigmoid deformation of the esophagus, minimally invasive esophagectomy may be offered.
Role for robotic-assisted cardiomyotomy
Increased availability and surgeon comfort with the robotic platform in the United States has led to the increase in robotic-assisted cardiomyotomy. The patient is positioned supine with arms tucked and feet neutrally flexed on a footboard. Four trocars are placed in a straight line or lazy smile across the abdomen, between 15–18 cm from the xyphoid. A Nathanson liver retractor is still generally used, although an additional robot arm and instrument may be used to lift the left lobe instead. A bedside assistant utilizes the same left lateral assistant port as in the laparoscopic approach and is able to pass sutures through any of the 8 mm robotic trocars. High-definition 3D visualization of the longitudinal and circular esophageal muscle fibers offers superior dissection and hypothetically mitigates the lack of haptic feedback. While sharp and tearing techniques as described above can still be used, the robotic hook offers wristed articulation, resulting in facile dissection of the circular muscle-submucosal plane. The hook is versatile as it may be used for both blunt “backhand” dissection as well as for electrocautery division of individual muscle bundles. Fundoplication is performed in a similar manner to the laparoscopic method (30).
Conclusions
Cardiomyotomy is a technically challenging and gratifying case for the foregut surgeon. As minimally-invasive techniques continue to improve, patients are referred for surgery earlier in their disease course. This should not completely diminish the role for non-operative management, especially balloon dilation. In 1989, Csendes et al. compared 5-year symptom relief in patients undergoing pneumatic balloon dilation to those who underwent open myotomy; 65% of dilation patients had continued symptom resolution, versus 85% in the myotomy cohort (34). Years later, in 2011, Boeckxstaens et al. performed a randomized trial for 201 patients with newly-diagnosed achalasia, comparing pneumatic balloon dilation (30 mm) to laparoscopic Heller with Dor fundoplication. At 2-year follow-up, there were no significant differences in symptoms, LES pressure, esophageal emptying, or quality of life (35).
Operatively, we have not found that prior pneumatic balloon dilation increases the difficulty of dissection. Conversely, Botox injection causes a significant inflammatory response that can increase the risk of mucosal injury during dissection. Therefore, Botox injection should be reserved for either older or infirm patients who might not tolerate general anesthesia or, rarely, to confirm the diagnosis of achalasia if the diagnosis is ambiguous.
Since 2010, POEM has become an increasingly important tool in the treatment of achalasia. In 2019, Werner et al. published their 2-year results examining clinical success in 221 patients randomly assigned to undergo either POEM or LCM with Dor fundoplication. While they found that POEM was noninferior in controlling the symptoms of achalasia, reflux appeared to be more common in the POEM group compared to the surgical group (36). A meta-analysis by Andolfi and Fisichella examining a total of 1,575 patients drawn from twenty studies (583 LCM, 449 POEM, 58 Botulinum toxin, 485 pneumatic dilation) suggested that LCM had a high success rate in type II achalasia, while POEM appeared to have a better success rate in III achalasia as the endoscopic approach allows for a longer myotomy. Treatment success was generally defined by postoperative symptom severity/resolution, though each study followed slightly different quantitative and qualitative metrics. The authors used a weighted mean to determine that POEM appeared to be more successful in type I achalasia (95% POEM vs. 81% LCM) (7). However, as noted earlier, since the distinction between type I and II achalasia is less clear-cut than with type III achalasia, LCM should remain an acceptable intervention, especially in settings without access to skilled interventional endoscopists.
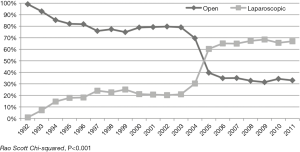
Taken together, continued advancements in interventional approaches, from robotic-assisted to natural orifice surgery, have added tools to the armamentarium that can help personalize treatment for achalasia. A 2017 study by Haisely et al. queried the Nationwide Inpatient Sample database for trends in cardiomyotomy. This dataset includes over seven million hospital admissions in the United States, as reported by 1,000 hospitals across 38 states every year. Between 1992 to 2011, the authors found a shift in open to laparoscopic approach to cardiomyotomy, with an inflection point in the year 2005 (Figure 7). This also correlated with a general increase in the number of operations performed for achalasia, as well as a gradual shifting of these cases away from rural or non-university hospitals. The consolidation of operative achalasia cases at high-volume urban teaching hospitals mirrors the regionalization of other complex operations, such as pancreaticoduodenectomy and esophagectomy, to high-volume centers (37). Indeed, similar to other complex operations, Haisley et al. found that metrics such as length of stay and mortality significantly improved as the urban teaching center was increasingly favored (3). At our institution, we discuss achalasia patients at a multidisciplinary conference including diagnostic and interventional gastroenterologists as well as surgeons. This strategy has helped optimize targeted treatment of achalasia subtypes. We also discuss therapeutic options for treatment failures, including operative myotomy after POEM or vice versa, as well as esophagectomy for end-stage disease. Indeed, these kinds of multidisciplinary conferences go hand-in-hand with the trend towards specialty care at urban teaching hospitals.
As surgical treatment continues to become more highly specialized, surgeons and gastroenterologists in the community must decide when to refer achalasia patients to tertiary care centers for treatment. Should patients be referred for index therapy, or should they only be referred as treatment failures? Furthermore, should general surgeons in the community be trained to do these operations? At our institution, patients in the region (Pacific Northwestern United States) travel tremendous distances for surgical care. There is ongoing tension between the scarcity of rural surgical care with the breadth of surgical knowledge that is expected of the community surgeon that will continue to evolve as our healthcare system changes.
Finally, as the robotic platform becomes more readily available, robotic-assisted cardiomyotomy should be considered a powerful additional tool in the minimally-invasive approach to achalasia. Multiple recent reviews of experiences over the last decade suggest that improved visualization and dexterity proffered by robotic instrumentation contribute to reduced rates of esophageal perforation (reported as zero in many data sets) and, in one study group, a trend toward lower recurrent achalasia symptoms (38). This latter finding is perhaps owed to the finding that the robotic cohort received a slightly longer myotomy on average. Differences in operative time were not statistically significant. Notably, there seemed to be no correlation between complication rate and the number of cases previously performed by the surgeon (30,31,38-41). In spite of this, there is a learning curve inherent to the robotic platform for the entire surgical team, as well as higher overall operating costs owing to instrumentation and equipment maintenance. The ergonomic benefits for the surgeon are difficult to quantify, but most surgeons agree that the seated, neutral-head/neck/shoulder console position can provide increased comfort throughout this challenging, mentally taxing case. It is still recommended that surgeons feel comfortable with converting to laparoscopic or open approach at any time prior to attempting this highly specialized procedure.
Acknowledgments
The authors would like to thank Tionna Foglio-Reed for her outstanding administrative support as well as Cory Sandonne for her excellent illustrations.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sarah Thompson) for the series “Achalasia” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2019-ach-14). The series “Achalasia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schlottmann F, Patti MG. Esophageal achalasia: current diagnosis and treatment. Expert Rev Gastroenterol Hepatol 2018;12:711-21. [Crossref] [PubMed]
- Stavropoulos SN, Friedel D, Parkman HP, et al. Diagnosis and management of esophageal achalasia. BMJ 2016;354:i2785. [Crossref] [PubMed]
- Haisley KR, Preston JF, Hunter JG, et al. Twenty-year trends in the utilization of Heller myotomy for achalasia in the United States. Am J Surg 2017;214:299-302. [Crossref] [PubMed]
- Hunter JG, Trus TL, Waring P, et al. Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg 1997;225:655-64. [Crossref] [PubMed]
- Rawlings A, Soper NJ, Oelschlager B, et al. Laparoscopic Dor versus Toupet fundoplication following Heller myotomy for achalasia: results of a multicenter, prospective, randomized-controlled trial. Surg Endosc 2012;26:18-26. [Crossref] [PubMed]
- Hamer PW, Holloway RH, Heddle R, et al. Evaluation of outcome after cardiomyotomy for achalasia using the Chicago classification. Br J Surg 2016;103:1847-54. [Crossref] [PubMed]
- Andolfi C, Fisichella PM. Meta-analysis of clinical outcome after treatment for achalasia based on manometric subtypes. Br J Surg 2019;106:332-41. [Crossref] [PubMed]
- Herbella FA, Patti MG. Laparoscopic Heller myotomy and fundoplication in patients with end-stage achalasia. World J Surg 2015;39:1631-3. [Crossref] [PubMed]
- Hunter JG, Spight DH, Sandone C, et al. Atlas of Minimally Invasive Surgical Operations. Available online: https://accesssurgery.mhmedical.com/content.aspx?bookid=2403§ionid=187824009
- Hung YC, Westfal ML, Kelleher CM, et al. Heller myotomy is the optimal index procedure for esophageal achalasia in adolescents and young adults. Surg Endosc 2019;33:3355-60. [Crossref] [PubMed]
- Bresadola V, Feo CV. Minimally invasive myotomy for the treatment of esophageal achalasia: evolution of the surgical procedure and the therapeutic algorithm. Surg Laparosc Endosc Percutan Tech 2012;22:83-7. [Crossref] [PubMed]
- Heller E. Extramucose Kardiaplastik beim chronischen Kardiospasmus mit Dilatation des Esophagus. Mitt Grenzgeb Med Chir 1914;27:141-9.
- McVey JL, Schlegel JF, Ellis FH Jr. Gastroesophageal sphincteric function after the Heller myotomy and its modifications. An experimental study. Bull Soc Int Chir 1963;22:419-23. [PubMed]
- Zaaijer JH. Cardiospasm in the aged. Ann Surg 1923;77:615-7. [Crossref] [PubMed]
- Abid S, Champion G, Koehler RE, et al. Treatment of achalasia: the best of both worlds. Am J Gastroenterol 1994;89:979-85. [PubMed]
- Pellegrini C, Wetter LA, Patti M, et al. Thoracoscopic esophagomyotomy. Initial experience with a new approach for the treatment of achalasia. Ann Surg 1992;216:291-6. [Crossref] [PubMed]
- Allaix ME, Patti MG. Endoscopic dilatation, Heller myotomy, and peroral endoscopic myotomy: treatment modalities for achalasia. Surg Clin North Am 2015;95:567-78. [Crossref] [PubMed]
- Shimi S, Nathanson LK, Cuschieri A. Laparoscopic cardiomyotomy for achalasia. J R Coll Surg Edinb 1991;36:152-4. [PubMed]
- Patti MG, Tamburini A, Pellegrini CA. Cardiomyotomy. Semin Laparosc Surg 1999;6:186-93. [PubMed]
- Richter JE. Update on the management of achalasia: balloons, surgery and drugs. Expert Rev Gastroenterol Hepatol 2008;2:435-45. [Crossref] [PubMed]
- Dor J, Humbert P, Dor V, et al. L’interet de la technique de Nissen modifiee dans la prevention de reflux apres cardiomyotomie extramuqueuse de Heller. Mem Acad Chir (Paris) 1962;88:877-83.
- Torquati A, Lutfi R, Richards WO, et al. Heller myotomy vs Heller myotomy plus Dor fundoplication: cost-utility analysis of a randomized trial. Surg Endosc 2006;20:389-93. [Crossref] [PubMed]
- Rebecchi F, Allaix ME, Morino M, et al. Laparoscopic Heller Myotomy and Fundoplication: What Is the Evidence? Am Surg 2018;84:481-8. [Crossref] [PubMed]
- Stefanidis D, Richardson W, Farrell TM, et al. SAGES guidelines for the surgical treatment of esophageal achalasia. Surg Endosc 2012;26:296-311. [Crossref] [PubMed]
- Afaneh C, Finnerty B, Zarnegar R, et al. Robotic-assisted Heller myotomy: a modern technique and review of outcomes. J Robot Surg 2015;9:101-8. [Crossref] [PubMed]
- Maeso S, Reza M, Mayol JA, et al. Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 2010;252:254-62. [Crossref] [PubMed]
- Gockel I, Eckardt VF, Junginger T, et al. Pseudoachalasia: A case series and analysis of the literature. Scand J Gastroenterol 2005;40:378-85. [Crossref] [PubMed]
- Jobe BA, Hunter JG, Watson DI. Esophagus and Diaphragmatic Hernia. In: Brunicardi F, Andersen DK, Billiar TR, et al. editors. Schwartz's Principles of Surgery. 11 edition. New York, NY: McGraw-Hill, 2019.
- Litle VR. Laparoscopic Heller myotomy for achalasia: a review of the controversies. Ann Thorac Surg 2008;85:S743-6. [Crossref] [PubMed]
- Rebecchi F, Allaix ME, Morino M. Robotic technological aids in esophageal surgery. J Vis Surg 2017;3:7. [Crossref] [PubMed]
- Huffmanm LC, Pandalai PK, Boulton BJ, et al. Robotic Heller myotomy: a safe operation with higher postoperative quality-of-life indices. Surgery 2007;142:613-8. [Crossref] [PubMed]
- Cowgill SM, Villadolid D, Boyle R, et al. Laparoscopic Heller myotomy for achalasia: results after 10 years. Surg Endosc 2009;23:2644-9. [Crossref] [PubMed]
- Rosemurgy AS, Morton CA, Rosas M, et al. A single institution’s experience with more than 500 laparoscopic Heller myotomies for achalasia. J Am Coll Surg 2010;210:637-45, 645-7.
- Csendes A, Braghetto I, Henríquez A, et al. Late results of a prospective randomized study comparing forceful dilatation and oesophagomyotomy in patients with achalasia. Gut 1989;30:299-304. [Crossref] [PubMed]
- Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med 2011;364:1807-16. [Crossref] [PubMed]
- Werner YB, Hakanson B, Martinek J, et al. Endoscopic or Surgical Myotomy in Patients with Idiopathic Achalasia. N Engl J Med 2019;381:2219-29. [Crossref] [PubMed]
- Merath K, Mehta R, Tsilimigras DI, et al. Quality of Care Among Medicare Patients Undergoing Pancreatic Surgery: Safety Grade, Magnet Recognition, and Leapfrog Minimum Volume Standards-Which Quality Benchmark Matters? J Gastrointest Surg 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Kim SS, Guillen-Rodriguez J, Little AG. Optimal surgical intervention for achalasia: laparoscopic or robotic approach. J Robot Surg 2019;13:397-400. [Crossref] [PubMed]
- Shaligram A, Unnirevi J, Oleynikov D, et al. How does the robot affect outcomes? A retrospective review of open, laparoscopic, and robotic Heller myotomy for achalasia. Surg Endosc 2012;26:1047-50. [Crossref] [PubMed]
- Milone M, Manigrasso M, Vertaldi S, et al. Robotic versus laparoscopic approach to treat symptomatic achalasia: systematic review with meta-analysis. Dis Esophagus 2019;32:1-8. [Crossref] [PubMed]
- Horgan S, Galvani C, Gorodner MV, et al. Robotic-assisted Heller myotomy versus laparoscopic Heller myotomy for the treatment of esophageal achalasia: multicenter study. J Gastrointest Surg 2005;9:1020-9. [Crossref] [PubMed]
Cite this article as: Rajdev PA, Hunter JG. Laparoscopic cardiomyotomy: historical overview and current operative approach. Ann Esophagus 2020;3:34.


