
Adenocarcinoma of the gastro-esophageal junction: is centralization policy always a good idea?
Introduction
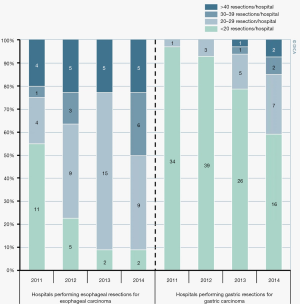
Esophageal carcinoma is the seventh most common and the sixth most lethal malignancy worldwide (1). Curative treatment usually consists of neoadjuvant chemoradiotherapy followed by esophagectomy, an invasive procedure with considerable morbidity. Postoperative complication rates of up to 65% have been reported after esophageal cancer surgery in the Netherlands (2). Numerous studies suggest that procedural, hospital and surgeon volumes are important determinants of outcomes after high-risk, low-incidence oncologic surgery like esophagectomy (3-6). These studies led to the introduction of surgical volume standards for esophageal cancer in the Netherlands in 2011. According to the Dutch guideline, the current hospital minimum is 20 esophageal resections annually (7). Ongoing centralization for upper-gastrointestinal cancer surgery in the Netherlands was analyzed by the Dutch Upper gastrointestinal Cancer Audit in 2014, showing that the number of low-volume hospitals (<20 annual esophagectomies) decreased from 11 in 2011 to 2 in 2014 (Figure 1) (8). Worldwide, a similar trend towards centralization is visible (9). A survey among upper gastro-intestinal surgeons published in 2017 compared the proportion of high-volume surgeons (>21 annual esophagectomies) in 2014 with that in the previous survey of 2007 (10). Among the 478 respondents from 49 countries, the proportion of high-volume surgeons rose from 45% to 54%. However, literature on centralization is not unambiguous. Several authors investigating the relationship between procedural volume and surgical outcomes found contrasting results (11,12). A population-based study by Gillison et al. found no association between improvement in 30-day mortality, or long-term survival, and increasing surgeon workload (11). In addition, most of the evidence on centralization focuses on esophageal cancer in general. Literature on the volume-outcome relationship for gastro-esophageal junction (GEJ) carcinoma is scarce. The optimal staging strategy and surgical treatment of GEJ tumors is even more difficult to establish, as both esophagectomy and gastrectomy is a viable treatment option (13,14). The eighth edition of the TNM-classification classifies Siewert III junction tumors as gastric cancer, therewith they reversed an earlier change that classified Siewert III as esophageal cancer (15-17). This controversy indicates that the staging and treatment of junctional tumors should be disease- and patient-tailored. Therefore, junctional tumors might require more clinician experience or volume.
In the scope of the controversies described above, this study aims to review the available literature on centralization policy for several aspects of multidisciplinary GEJ cancer care.
Diagnostics
In the Netherlands, regional hospitals often perform preoperative work-up before referral to an expert surgical center. After referral, the imaging is re-evaluated in the expert center, but not always repeated. In addition, false-negative patients will not be referred to expert clinics. Referral to an expert clinic might also be withheld from patients with false-positive results for metastases (18). Little evidence is available on the impact of staging in low-volume or non-expert centers for GEJ cancer. However, some studies investigated the volume-outcome relationship for diagnostics for esophageal cancer in general. An Asian study found inter-observer reliability was not associated with hospital endoscopy volume or endoscopist experience (19). The study included endoscopists from 7 Asian centers with a minimum endoscopy experience of 5 years and a minimum of 1,000 endoscopies. After video-based training, inter-observer reliability of assessment of location of the GEJ or grading of suspected Barret’s esophagus was high, irrespective of hospital endoscopy volume or endoscopist volume. A Dutch study published in 2006 compared the quality of preoperative metastases detection between 61 regional centers of esophageal cancer diagnosis, and a specialized referral center treating approximately 120 patients with esophageal cancer annually (20). The specialized hospital did not always repeat the imaging, but always re-evaluated the results. The study included 1,088 patients of whom 41% had a GEJ carcinoma, but no separate results for junction tumors were reported. Overall, the sensitivity for detection of regional lymph node metastases and distant metastases by CT-scan was 26% and 44% in regional centers, and 52% and 84% in the specialized hospital (P<0.01). The sensitivity for detecting lymph node metastases by ultrasound of the neck was also higher in the referral center (26% versus 84%). Specificity of imaging was comparable between regional centers and the referral center. Combining all diagnostic modalities, metastases missed in the referring center were detected by the specialized center in 13% of patients. This has impact on treatment planning. The results of this study indicate that specialized, experienced radiologists are of great importance. However, better CT-scanners in specialized hospitals are also imperative (21). A different study by the same group concluded that results of endoscopic ultrasonography (EUS) performed by low-volume endoscopists (<50 EUS/year) compared unfavorably with EUS performed in high-volume EUS centers (22). This indicates that EUS should be centralized and be performed by dedicated, specialized endoscopists to optimize esophageal cancer staging. Another study, from England, including junctional tumors (results not reported separately) as well as esophageal and gastric cancer, found patients from high-volume medical specialists underwent more diagnostic imaging compared to patients treated by low-volume doctors (23). This led, after correction for possible confounders, to significantly lower avoidable surgery (open-close) rates for high-volume surgeons indicating more adequate staging.
The results from the studies described above suggest staging of esophageal carcinoma should be performed in high-volume centers by dedicated, specialized radiologists and endoscopists. This applies especially to GEJ tumors since slight differences in staging (e.g., Siewert II versus Siewert III) might greatly impact treatment decision-making.
Endoscopic treatment of Barret’s esophagus or early cancer
The Dutch guideline advocates centralization of endoscopic treatment of Barret’s esophagus with dysplasia or early cancer (24). The guideline specifies Barret’s expert centers as centers in which: (I) a minimum of 10 patients with dysplasia or early cancer are treated endoscopically by one dedicated endoscopist per year; (II) all histological preparations are assessed by a maximum of two dedicated pathologists; (III) all Barret’s related carcinomas are discussed in a multi-disciplinary team (MDT) including upper gastro-intestinal surgeons and oncologists; (IV) state-of-the-art, high-resolution endoscopes are available, and (V) facilities to treat complications (e.g., perforation or bleeding) are available. The national guideline from the American Gastroenterological Association (AGA) also sets the annual minimum volume endoscopic treatments at 10 (25). The British guideline, from the British Society of Gastroenterology, advocates a volume standard of 15 yearly endoscopic treatments (26). These recommendations are based on a Dutch study published in 2012 and on a recent Asian study (27,28). The Dutch study evaluated the results of the first 120 endoscopic resections by 6 endoscopists after a structured and intense hands-on training program. They found perforation rates of 5% in the first 20 resections performed by each endoscopist, which reflects the complexity of the procedure. In addition, the peak of the learning curve was not yet reached after 20 resections (27). The second study included 430 patients undergoing radio-frequent ablation of their Barret’s esophagus. It found an annual ablation volume of 10 or higher was associated with a significantly lower recurrence rate compared to low-volume hospitals (<3 annual ablations) (28).
Since early neoplastic signs are subtle, state of the art endoscopes and sufficient endoscopist and pathologist expertise are necessary. In addition, given the possible complications of endoscopic resections, an expert center having surgical and endoscopic experience in treating complications is advisable. Therefore, concentrating Barret’s esophagus and early cancer care in experienced esophagectomy centers seems logical and efficient.
Surgical treatment—hospital volume
Short and long-term surgical outcomes
There is much literature available on the association between hospital volume and surgical outcomes after esophagectomy. A meta-analysis including 16 high-quality studies (multicenter studies reporting case-mix corrected outcomes using multivariable logistic regression) found significantly higher postoperative mortality rates (odds ratio 2.30, 95% confidence interval: 1.89–2.80) in low-volume hospitals (29). In addition, they found shorter long-term survival in low-volume hospitals. As this meta-analysis pooled all high-volume and low-volume hospitals of the included studies, the number of procedures performed in high-volume hospitals differed. The definition of a high-volume hospital ranged from 2.33 to as much as 87 annual esophagectomies. Another meta-analysis, including 13 studies, found that an annual hospital volume of 20 esophagectomies or higher was associated with lower postoperative mortality rates compared to medium and low-volume hospitals (11–20 and ≤10 esophagectomies respectively) (5). They also found lower complication rates and better long-term survival in high-volume hospitals. A third meta-analysis endorsed these conclusions (30). In this meta-analysis high-volume hospitals were defined as hospitals performing 18 or more annual esophagectomies. It concluded that 10 patients had to be referred from a low-volume to a high-volume hospital to prevent one postoperative death. This meta-analysis also found a significant inverse relationship between hospital volume and short and long-term survival for gastrectomy. Another study using data from the CRITICS (ChemoRadiotherapy after Induction chemotherapy In Cancer of the Stomach) trial included 494 patients (31). It concluded better overall survival and disease-free survival in high-volume gastrectomy hospitals (>21 annual gastrectomies).
Better long-term survival in high-volume hospitals might be related to more extensive oncological resections in these centers. Several studies found better lymph node yield in high-volume hospitals for both esophagectomy and gastrectomy (32-35). The percentage of patients with >15 examined lymph nodes after esophagectomy ranged from 29% to 47% in low-volume hospitals and from 44% to 76% in high-volume hospitals. For gastrectomy, a Dutch study analyzing data from the CRITICS found significantly more accurate lymph node dissection in high-volume hospitals (31 or more annual gastrectomies). Another reason for the better long-term survival for both esophagectomy and gastrectomy in high-volume hospitals might be the reported higher radicality rates (36-38). Another hypothesis is that lower postoperative mortality rates or better long-term survival after esophagectomy in high-volume hospitals is due to less “failure to rescue”. High-volume hospitals are more able to quickly recognize and effectively manage postoperative morbidity (39-41). In these studies, failure to rescue rates ranged from 20% to 30% in the lowest-volume hospitals whereas this ranged from 12% to 13% in the highest-volume hospitals.
Several studies use composite annual hospital volumes rather than annual esophagectomy volume (42-44). A well-conducted Dutch study including 4,837 gastric cancer patients found association between annual complex upper-gastrointestinal surgery (esophagectomy, gastrectomy, and pancreatic surgery) and postoperative mortality (42). High-volume hospitals performed 40 or more complex upper-gastrointestinal resections annually. Another interesting finding was that elderly (75 years and older) benefitted the most from surgery in high-volume centers suggesting referral to high-volume centers is especially necessary for vulnerable patients. An English study by Coupland et al. also used composite volume-standards (annual numbers of esophagectomies and gastrectomies). Hospitals performing more than 80 surgeries were considered high-volume. After correction for confounders they found lower short-term mortality rates and better 1-year overall survival in high-volume centers (43).
How far should centralization go?
The Leapfrog Group was one of the first movements to establish volume standards in surgery (45). They suggested an esophagectomy volume standard of 7 annual esophagectomies. Another study including 1,634 esophagectomy patients tried to verify the Leapfrog volume standards (46). They did not find a strong relationship between the Leapfrog threshold and postoperative mortality. However, they did find a threshold of 22 annual esophagectomies to be a predictor of postoperative mortality. Another well-conducted Dutch study using data from the National Cancer Registry (NCR) tried to define a meaningful cutoff point for annual esophagectomy hospital volume (47). It included 10,025 patients with esophageal or GEJ cancer undergoing resection. The authors found rising hospital volume was associated with lower 6-month mortality rates. However, the hazard ratios of 6-month mortality reached a plateau when hospital volume exceeded 60 esophagectomies per year. A similar trend was seen for 2-year overall survival: this was significantly better in high-volume hospitals compared to lower-volume hospitals but reached a plateau at 50 annual resections. The results of these studies suggest centralization is necessary up to a certain threshold but not infinitely. This conclusion is endorsed by a third study that found postoperative mortality rates fell in line with increasing hospital volume, but rose again once the annual volume exceeded 100 cases (48).
Costs and centralization
Several authors investigated the relationship between hospital volume and hospital costs in patients undergoing esophageal cancer surgery. One study conducted in the United States included 1,561 patients treated for esophageal cancer from 1990 through 1994 (49). It found higher total hospital costs in high-volume hospitals (>30 operations in 5 years) compared to low-volume hospitals (1–5 operations in 5 years). However, significantly more patients were discharged home in high-volume hospitals whereas patients were more often discharged to intermediate-care facilities after resection in low-volume centers. This has a significant economic impact, which the study did not quantify. A study by Swisher et al. including 340 esophagectomy patients found contradictory results (50). After correction for confounders hospital costs in high-volume hospitals (>5 annual esophagectomies) were significantly lower. This difference might be attributed to the lower complication rates and shorter length of hospital stay Swisher et al. found in high-volume hospitals. Another American study found comparable hospital costs in high and low-volume hospitals (<6 or >6 annual esophagectomies, respectively) (51). Next to lower mortality rates in high-volume hospitals they found shorter length of hospital stay and shorter length of intensive care unit (ICU) admission in high-volume hospitals. This did however not result in lower hospital costs. In addition, they found more home discharge in high-volume hospitals, but they did not report on its economic impact.
Literature focusing on GEJ tumors
The studies discussed earlier did not report separate results for GEJ carcinomas. A spin-off study from the Japanese randomized controlled trial JCOG9502 included only GEJ carcinomas and investigated the volume-outcome relationship (52). The JCOG9502 included 157 patients from 21 hospitals and randomized between total gastrectomy via a left thoracoabdominal approach and a transhiatal abdominal approach (53). The authors found higher hospital volume was associated with less intra-operative blood loss. They found no association between hospital volume and the number of dissected lymph nodes, operative time, number of patients with postoperative complications, and overall survival rate. However, the annual gastrectomy volume in all the included hospitals could be considered high, since it ranged from 44 to 511. A German study included 544 patients with GEJ tumors in 108 hospitals (54). Hospitals with a case-load of 11–20 junction tumors in 3 years were associated with significantly higher neoadjuvant treatment rates compared to the other volume groups (<11 and >20). They did not report on the association of postoperative outcomes with hospital volume. In addition, they reported only on GEJ carcinoma volume, not on total esophagectomy or gastrectomy volume. Therefore, the proportion of patients undergoing treatment in high-volume hospitals was low. A third, Scottish, study also reported separate outcomes for GEJ tumors (55). It included 206 patients with junction tumors. The postoperative mortality rate in high-volume hospitals (>35 annual resections) was 0% whereas this was 8.1% in lower-volume hospitals (<35). However, they found no association between hospital volume and long-term survival. In a Dutch study including 555 patients 82.2% had a GEJ or distal esophageal carcinoma (36). It found patients in high-volume hospitals (>10 annual resections) had more comorbidities. Despite these differences in patient population, high-volume hospitals had lower postoperative mortality rates (6.3% versus 2.9%). In addition, high-volume hospitals had significantly less postoperative (surgical) complications and a significantly shorter hospital stay (median of 22 days in low-volume hospitals versus 14 days in high-volume hospitals). No association was found between volume and long-term survival.
The results of these studies on GEJ tumors are not unambiguously in favor of centralization. However, most studies found some positive effect of hospital volume on surgical outcomes. No inferior outcomes for high-volume hospitals were reported. In addition, no literature is available on the association of failure to rescue and hospital volume in GEJ carcinomas even though this might be a key element in the volume-outcome relationship.
Surgical treatment—surgeon volume
Although literature on surgeon volume is not as abundant as literature on hospital volume, several authors investigated the association between surgeon volume and surgical outcomes after esophagectomy. As early as 1997 the surgeon volume-outcome relationship was investigated (56). A total of 74 esophagectomy patients from the Hamilton Regional Cancer Center in Ontario, Canada, were included. Esophagectomy was performed by 20 different surgeons of whom 3 were high-volume surgeons (>6 annual esophagectomies). High-volume surgeons had lower postoperative morbidity rates (0% versus 22%) and lower anastomotic leakage rates (7% versus 22%). A more recent study by Sundelöf et al. included 232 patients (including 118 cardia carcinomas) and investigated the surgeon volume-outcome relationship (57). In their study, a high-volume surgeon performed a minimum of 10 annual esophagectomies. They found better long-term survival after surgery by a high-volume surgeon. They also found non-significant trends towards better short-term surgical outcomes in high-volume surgeons. In addition, they showed large correlation between surgeon and hospital volume. A 2016 population-based study in England sought a minimum surgeon volume for optimum operative mortality (58). After proper statistical correction for surgeon experience, hospital type, and patient-related confounders, higher surgeon volume was a significant predictor of lower postoperative mortality. The risk-adjusted mortality rate for high-volume surgeons (>12 annual esophagectomies) was 2.96% whereas this was 5.19% for low-volume surgeons (<8 annual esophagectomies). Using CUSUM-analyses no reliable estimation of the optimal surgeon volume could be made. In addition, the study found clear overlap between surgeon and hospital volume. For gastrectomy, similar positive surgeon volume-outcome trends have been described (58).
Several studies found contrasting results. For example, the previously discussed JCOG9502 trial, including only junction tumors, found no association between overall survival and surgeon volume (52). It only found a non-significant trend towards a higher number of dissected lymph nodes in surgery by high-volume surgeons. In addition, a study by Gillison et al. including 1,125 patients in England found no association between long-term survival and postoperative mortality and surgeon volume (high-volume surgeons of 12 or more annual resections versus low-volume surgeons of 4 or less annual esophagectomies) (11).
Volume aspect in palliative cancer treatment
To our knowledge three studies reported on esophagectomy hospital volume in the palliative setting. The addition of trastuzumab to systemic therapy in metastasized gastro-esophageal adenocarcinomas with HER2 overexpression leads to prolonged overall survival and better quality of life (59,60). Therefore, a nationwide cohort study investigated the relationship between HER2 testing and hospital volume (61). This study included 2,846 patients of whom 19.1% had GEJ carcinomas. The authors divided hospitals into four volume categories, based on the total number of patients treated with gastro-esophageal cancer in 2015 and 2016 (<13, 13–31, 32–76, and >76). High-volume hospitals tested significantly more often for HER2 overexpression than low-volume hospitals (88% versus 68%; P<0.001). In addition, after dichotomizing hospital volume around the national median, overall survival was significantly better in high-volume hospitals compared to low-volume hospitals. This conclusion is supported by another nationwide Dutch cohort study that also found a survival advantage of higher hospital surgical and hospital treatment volume in metastatic esophageal cancer patients after propensity score matching (62). They found a median survival difference of 10 weeks and 6 weeks respectively between high and low-volume surgical and treatment hospitals. The authors claim high-volume centers have the appropriate, well-developed infrastructure that is necessary for the complex treatment of metastatic esophageal cancer. Similar conclusions were drawn by a third study (63). It included 1,433 stage IV gastric cancer patients of whom 27% had GEJ tumors. High-volume specialist consultation was associated with a significant survival benefit.
Conclusions
Although most literature suggests a positive effect of centralization, international consensus on how far centralization should go is difficult to reach. As this review demonstrates, literature on the volume-outcome relationship is heterogeneous. The altering definition of high-volume hospitals in literature makes proper meta-analyses difficult. Some studies consider an annual volume of 6 or 10 esophagectomies to be high, whereas for other studies, or countries, this would be a low-volume hospital. Few studies sought cut-off values for optimal hospital volume. However, one well conducted study found better results with rising volume up to 60 annual esophagectomies suggesting this is a meaningful hospital volume threshold (47). There is even heterogeneity in literature on which surgical procedures should be included in hospital volume; only the surgical procedure of interest or a more composite surgical volume? The role of surgeon volume is also not unequivocal. In order to reach international consensus, literature should be standardized. A similar initiative from the Esophagectomy Complications Consensus Group (ECCG) standardized data collection on complications after esophagectomy in order to facilitate international comparative studies (64). Pre-set volume standards would also significantly reduce confirmation bias in which researchers adjust volume cut-offs in order to find statistically significant results.
In conclusion, this review of literature underlines the importance of centralization of all aspects of the multimodal treatment of gastro-esophageal cancer. Literature showing better outcomes in high-volume hospitals is available for all aspects of the gastro-esophageal cancer treatment, from diagnostics to palliative treatment. Especially for GEJ carcinomas highly-specialized medical personnel is necessary, given the difficulties and controversies surrounding its treatment. Clear international volume thresholds are however difficult to establish since literature on the volume-outcome relationship is heterogeneous.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Riccardo Rosati) for the series “Current issues on GEJ adenocarcinoma” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2020-geja-01). The series “Current issues on GEJ adenocarcinoma” was commissioned by the editorial office without any funding or sponsorship. MIvBH serves as an unpaid editorial board member of Annals of Esophagus from May 2020 to Apr. 2022. RvH is proctoring surgeon for Intuitive Surgical Inc. and trains other surgeons in robot-assisted minimally invasive esophagectomy. MIvBH reports grants from Olympus, grants from Stryker, outside the submitted work; and MIvBH is consultant for Mylan and Medtronic and Johnson & Johnson. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- van der Werf LR, Busweiler LAD, van Sandick JW, et al. Reporting National Outcomes After Esophagectomy and Gastrectomy According to the Esophageal Complications Consensus Group (ECCG). Ann Surg 2020;271:1095-101. [Crossref] [PubMed]
- Birkmeyer JD, Sun Y, Wong SL, et al. Hospital volume and late survival after cancer surgery. Ann Surg 2007;245:777-83. [Crossref] [PubMed]
- van Lanschot JJ, Hulscher JB, Buskens CJ, et al. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574-8. [Crossref] [PubMed]
- Metzger R, Bollschweiler E, Vallböhmer D, et al. High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus 2004;17:310-4. [Crossref] [PubMed]
- Vonlanthen R, Lodge P, Barkun JS, et al. Toward a consensus on centralization in surgery. Ann Surg 2018;268:712-24. [Crossref] [PubMed]
- Integraal Kankercentrum Nederland. Oesophaguscarcinoom. Landelijke richtlijn, versie 3.1, 2015. consulted on: 16-3-2020. Available online: https://www.oncoline.nl/oesofaguscarcinoom
- Dutch Institute for Clinical Auditing. Jaarrapport Dutch Upper gastrointestinal Cancer Audit (DUCA) 2014. consulted on: 16-3-2020. Available online: https://dica.nl/jaarrapportage-2014/duca
- Messager M, de Steur W, Boelens PG, et al. Description and analysis of clinical pathways for oesophago-gastric adenocarcinoma, in 10 European countries (the EURECCA upper gastro intestinal group - European Registration of Cancer Care). Eur J Surg Oncol 2016;42:1432-47. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Gillison EW, Powell J, McConkey CC, et al. Surgical workload and outcome after resection for carcinoma of the oesophagus and cardia. Br J Surg 2002;89:344-8. [Crossref] [PubMed]
- Rodgers M, Jobe BA, O’Rourke RW, et al. Case volume as a predictor of inpatient mortality after esophagectomy. Arch Surg 2007;142:829-39. [Crossref] [PubMed]
- Nobel T, Molena D. Surgical principles for optimal treatment of esophagogastric junction adenocarcinoma. Ann Gastroenterol Surg 2019;3:390-5. [PubMed]
- Zhang S, Orita H, Fukunaga T. Current surgical treatment of esophagogastric junction adenocarcinoma. World J Gastrointest Oncol 2019;11:567. [PubMed]
- Zanoni A, Verlato G, Baiocchi GL, et al. Siewert III esophagogastric junction adenocarcinoma: does TNM 8th save us? Updates Surg 2018;70:241-9. [Crossref] [PubMed]
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457-9. [Crossref] [PubMed]
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th edition. John Wiley & Sons, 2017.
- van Putten M, Koëter M, van Laarhoven HW, et al. Hospital of diagnosis influences the probability of receiving curative treatment for esophageal cancer. Ann Surg 2018;267:303-10. [Crossref] [PubMed]
- Lee YC, Cook MB, Bhatia S, et al. Interobserver reliability in the endoscopic diagnosis and grading of Barrett’s esophagus: an Asian multinational study. Endoscopy 2010;42:699-704. [Crossref] [PubMed]
- van Vliet EP, Eijkemans MJ, Kuipers EJ, et al. A comparison between low-volume referring regional centers and a high-volume referral center in quality of preoperative metastasis detection in esophageal carcinoma. Am J Gastroenterol 2006;101:234-42. [Crossref] [PubMed]
- van Vliet EP, Hermans JJ, De Wever W, et al. Radiologist experience and CT examination quality determine metastasis detection in patients with esophageal or gastric cardia cancer. Eur Radiol 2008;18:2475-84. [Crossref] [PubMed]
- van Vliet EPM, Eijkemans MJ, Poley JW, et al. Staging of esophageal carcinoma in a low-volume EUS center compared with reported results from high-volume centers. Gastrointest Endosc 2006;63:938-47. [Crossref] [PubMed]
- Bachmann MO, Alderson D, Edwards D, et al. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg 2002;89:914-22. [Crossref] [PubMed]
- Nederlandse Vereniging van Maag-Darm-Leverartsen. Richtlijn Barrett-oesofagus. consulted on: 2-3-2020. Available online: https://www.mdl.nl/sites/www.mdl.nl/files/richlijnen/Richtlijnen%20Barrett%20oesofagus%20-%20jan%202018%20-%20tbv%20website.pdf
- Sharma P, Shaheen NJ, Katzka D, et al. AGA Clinical Practice Update on Endoscopic Treatment of Barrett’s Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020;158:760-9. [Crossref] [PubMed]
- Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7-42. [Crossref] [PubMed]
- van Vilsteren FG, Pouw RE, Herrero LA, et al. Learning to perform endoscopic resection of esophageal neoplasia is associated with significant complications even within a structured training program. Endoscopy 2012;44:4-12. [Crossref] [PubMed]
- Tan MC, Kanthasamy KA, Yeh AG, et al. Factors Associated With Recurrence of Barrett's Esophagus After Radiofrequency Ablation. Clin Gastroenterol Hepatol 2019;17:65-72.e5. [Crossref] [PubMed]
- Wouters MW, Gooiker GA, van Sandick JW, et al. The volume-outcome relation in the surgical treatment of esophageal cancer: a systematic review and meta-analysis. Cancer 2012;118:1754-63. [Crossref] [PubMed]
- Gruen RL, Pitt V, Green S, et al. The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin 2009;59:192-211. [Crossref] [PubMed]
- Claassen YHM, van Amelsfoort RM, Hartgrink HH, et al. Effect of hospital volume with respect to performing gastric cancer resection on recurrence and survival: results from the CRITICS trial. Ann Surg 2019;270:1096-102. [Crossref] [PubMed]
- Merkow RP, Bilimoria KY, Chow WB, et al. Variation in lymph node examination after esophagectomy for cancer in the United States. Arch Surg 2012;147:505-11. [Crossref] [PubMed]
- Dikken JL, Dassen AE, Lemmens VE, et al. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in the Netherlands between 1989 and 2009. Eur J Cancer 2012;48:1004-13. [Crossref] [PubMed]
- van der Werf LR, Dikken JL, van Berge Henegouwen MI, et al. A Population-based Study on Lymph Node Retrieval in Patients with Esophageal Cancer: Results from the Dutch Upper Gastrointestinal Cancer Audit. Ann Surg Oncol 2018;25:1211-20. [Crossref] [PubMed]
- Claassen YHM, van Sandick JW, Hartgrink HH, et al. Association between hospital volume and quality of gastric cancer surgery in the CRITICS trial. Br J Surg 2018;105:728-35. [Crossref] [PubMed]
- Wouters MW, Karim-Kos HE, le Cessie S, et al. Centralization of esophageal cancer surgery: does it improve clinical outcome? Ann Surg Oncol 2009;16:1789-98. [Crossref] [PubMed]
- Javidfar J, Speicher PJ, Hartwig MG, et al. Impact of Positive Margins on Survival in Patients Undergoing Esophagogastrectomy for Esophageal Cancer. Ann Thorac Surg 2016;101:1060-7. [Crossref] [PubMed]
- van der Werf LR, Cords C, Arntz I, et al. Population-based study on risk factors for tumor-positive resection margins in patients with gastric cancer. Ann Surg Oncol 2019;26:2222-33. [Crossref] [PubMed]
- Nimptsch U, Haist T, Krautz C, et al. Hospital Volume, In-Hospital Mortality, and Failure to Rescue in Esophageal Surgery. Dtsch Arztebl Int 2018;115:793-800. [PubMed]
- Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care 2011;49:1076-81. [Crossref] [PubMed]
- Busweiler LA, Henneman D, Dikken JL, et al. Failure-to-rescue in patients undergoing surgery for esophageal or gastric cancer. Eur J Surg Oncol 2017;43:1962-9. [Crossref] [PubMed]
- Busweiler LA, Dikken JL, Henneman D, et al. The influence of a composite hospital volume on outcomes for gastric cancer surgery: A Dutch population-based study. J Surg Oncol 2017;115:738-45. [Crossref] [PubMed]
- Coupland VH, Lagergren J, Lüchtenborg M, et al. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004–2008. Gut 2013;62:961-6. [Crossref] [PubMed]
- Gordon TA, Bowman HM, Bass EB, et al. Complex gastrointestinal surgery: impact of provider experience on clinical and economic outcomes. J Am Coll Surg 1999;189:46-56. [Crossref] [PubMed]
- Milstein A, Galvin RS, Delbanco SF, et al. Improving the safety of health care: the leapfrog initiative. Eff Clin Pract 2000;3:313-6. Erratum in: Eff Clin Pract 2001 Mar-Apr;4(2):94. [PubMed]
- Christian CK, Gustafson ML, Betensky RA, et al. The Leapfrog volume criteria may fall short in identifying high-quality surgical centers. Ann Surg 2003;238:447-55; discussion 455-7. [PubMed]
- Henneman D, Dikken JL, Putter H, et al. Centralization of esophagectomy: how far should we go? Ann Surg Oncol 2014;21:4068-74. [Crossref] [PubMed]
- Kohn GP, Galanko JA, Meyers MO, et al. National trends in esophageal surgery—are outcomes as good as we believe? J Gastrointest Surg 2009;13:1900-10; discussion 1910-2.
- Patti MG, Comera CU, Glasgow RE, et al. A hospital’s annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg 1998;2:186-92. [Crossref] [PubMed]
- Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg 2000;119:1126-32. [Crossref] [PubMed]
- Kuo EY, Chang Y, Wright CD. Impact of hospital volume on clinical and economic outcomes for esophagectomy. Ann Thorac Surg 2001;72:1118-24. [Crossref] [PubMed]
- Kurokawa Y, Yamaguchi T, Sasako M, et al. Institutional variation in short-and long-term outcomes after surgery for gastric or esophagogastric junction adenocarcinoma: correlative study of two randomized phase III trials (JCOG9501 and JCOG9502). Gastric Cancer 2017;20:508-16. [Crossref] [PubMed]
- Kurokawa Y, Sasako M, Sano T, et al. Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br J Surg 2015;102:341-8. [Crossref] [PubMed]
- Steinert R, Gastinger I, Ridwelski K, et al. Surgical treatment of carcinomas of the oesophagogastric junction - results achieved in multicentre studies. Zentralbl Chir 2013;138:403-9. [PubMed]
- Thompson AM, Rapson T, Gilbert FJ, et al. Hospital volume does not influence long-term survival of patients undergoing surgery for oesophageal or gastric cancer. Br J Surg 2007;94:578-84. [Crossref] [PubMed]
- Miller JD, Jain MK, De Gara CJ, et al. Effect of surgical experience on results of esophagectomy for esophageal carcinoma. J Surg Oncol 1997;65:20-1. [Crossref] [PubMed]
- Sundelöf M, Lagergren J, Ye W. Surgical factors influencing outcomes in patients resected for cancer of the esophagus or gastric cardia. World J Surg 2008;32:2357-65. [Crossref] [PubMed]
- Mamidanna R, Ni Z, Anderson O, et al. Surgeon volume and Cancer Esophagectomy, gastrectomy, and pancreatectomy. Ann Surg 2016;263:727-32. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Satoh T, Bang YJ, Gotovkin EA, et al. Quality of life in the trastuzumab for gastric cancer trial. Oncologist 2014;19:712-9. [Crossref] [PubMed]
- Dijksterhuis WPM, Verhoeven RHA, Meijer SL, et al. Increased assessment of HER2 in metastatic gastroesophageal cancer patients: a nationwide population-based cohort study. Gastric Cancer 2020; Epub ahead of print. [Crossref] [PubMed]
- Haj Mohammad N, Bernards N, van Putten M, et al. Volume-outcome relation in palliative systemic treatment of metastatic oesophagogastric cancer. Eur J Cancer 2017;78:28-36. [Crossref] [PubMed]
- Dixon M, Mahar AL, Helyer LK, et al. Prognostic factors in metastatic gastric cancer: results of a population-based, retrospective cohort study in Ontario. Gastric Cancer 2016;19:150-9. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy. Ann Surg 2015;262:286-94. [Crossref] [PubMed]
Cite this article as: Voeten DM, van Hillegersberg R, van Berge Henegouwen MI, Gisbertz SS. Adenocarcinoma of the gastro-esophageal junction: is centralization policy always a good idea? Ann Esophagus 2020;3:29.


