
Pathophysiology of achalasia
Introduction
Achalasia is a rare disorder, but its presentation is so profound and clinically unique that reports of its treatment predate the modern pathophysiologic concepts by at least a hundred years (1). The term ‘achalasia’ translates into ‘lack of relaxation’, reflecting abnormal lower esophageal sphincter (LES) relaxation. As a result, achalasia manifests as obstruction to passage of food from the esophagus into the stomach, presenting with dysphagia, regurgitation, weight loss, and less commonly, chest pain and aspiration pneumonia. The 17th century management of dysphagia from achalasia consisted of passing a curved whalebone into the esophagus. This evolved into mercury tube dilation over the next two centuries, but it was not until the early 20th century that the true pathophysiology, and resultant definition, were elucidated. However, even when Rake’s theory of incomplete sphincter relaxation from plexus destruction was corroborated by histological analysis, the longstanding dogma of LES spasm continued for decades, prompting the term ‘cardiospasm’ (2). Manometric definitions of incomplete relaxation and aperistalsis, now so heavily relied upon, did not arise until a few decades ago (3).
Normal esophageal physiology
The adult esophagus is roughly 18–26 cm in length; it can expand 2 cm in the anteroposterior plane and up to 3 cm laterally to accommodate a food bolus. The esophagus is bi-phenotypic, skeletal muscle comprising the upper third and smooth muscle making up the lower two thirds, with a transition zone of a mix of the two muscle types. The esophagus is anchored by two high pressure barriers: the upper and LES. The upper esophageal sphincter (UES), like the LES, remains tonically closed, augmented by either contraction or relaxation based upon neural input. The UES is a complex structure composed of muscular and cartilaginous tissue. In contrast, the LES is predominantly muscle, composed anatomically of intrinsic (esophageal motor fibers) and extrinsic (diaphragmatic muscle fibers) components. Circular muscle layers and so-called clasp and sling fibers have been extensively studied in both form and function, with clasp fibers demonstrating myogenic tone and sling fibers participating in vigorous response to stimuli.
Both sensory and motor innervation of the esophagus is via the vagus nerve, and can be explained by the embryonic origin of the upper esophagus from branchial arches 4, 5, and 6. Some investigations suggest rostrocaudal migration of neural crest cells and subsequent differentiation, with sufficient integrity to allow peristalsis as early as the first trimester of pregnancy (4). Sensation is carried from the dorsal root ganglia to the spinal column and nuclei gracilis and cuneatus, with subsequent routing through the thalamus into the primary sensory and insular cortices (5). Vagal efferent fibers arising in the nucleus ambiguus innervate the proximal esophagus, whereas the distal smooth muscle is supplied by nerves from the vagal dorsal motor nucleus (Figure 1). Ganglia between the circular and longitudinal smooth muscle of the distal esophagus form the Auerbach’s myenteric plexus; these mediate contraction of the outer longitudinal muscle layers and act as the relay point between the vagus and circular muscle fibers (Figure 1). They also interconnect with Meissner’s plexus, which lies within and coordinates peristalsis for the muscularis mucosae.
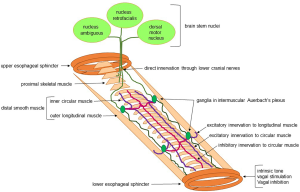
Normal esophageal motor function depends on an appropriate balance between excitatory and inhibitory signaling. Excitation, which drives contraction, is mediated by cholinergic post-ganglionic neurons, which are found in greater numbers in the mid esophagus. In fact, there is a gradient of neurotransmitter influence in the esophageal body, with dominance of excitation in the proximal smooth muscle esophagus, and higher inhibitory elements in the distal esophagus (6). The latter manifests as higher contraction latency as peristalsis progresses distally in the esophagus, which is mediated by increased numbers of inhibitory—non-adrenergic, non-cholinergic—post-ganglionic neurons. Inhibitory neurons, which are nitric oxide (NO) and vasoactive intestinal peptide (VIP) mediated, are also responsible for swallow induced LES relaxation (7,8). Pharmacologic studies have shown, accordingly, that contraction can be augmented with cholinomimetics and that blocking inhibitory function results in a peristaltic wave that arrives at the distal esophagus earlier than expected. Dysfunction in either or both of excitatory and inhibitory pathways can result in esophageal motor disorders.
Neuromuscular pathophysiology
Abnormal inhibition can be isolated to the esophageal body or LES, or it can involve both. Intact inhibition is needed for normal timing of peristalsis and dampening of contraction vigor; therefore deficient inhibition leads to premature or rapid (simultaneous) sequences (9,10). In some settings, there may be unopposed contraction producing exaggerated or vigorous contraction (11,12). Therefore, various esophageal motor syndromes can result depending on selective loss of inhibition. If inhibitory dysfunction is dominant in the esophageal body without LES involvement, distal esophageal spasm (DES) is diagnosed. In contrast, when imbalance between excitation and inhibition favors exaggerated contraction, hypercontractile peristalsis develops. Abnormal inhibitory control of the LES results in inadequate relaxation with swallows-and esophageal outflow obstruction; these findings in conjunction with premature sequences in the esophageal body fulfill criteria for type 3 achalasia (13). Esophageal body contraction can also be hypercontractile with abnormal LES relaxation, termed esophagogastric junction outflow obstruction (EGJOO) in the Chicago Classification v 3.0, although a more appropriate term may be hypercontractility with obstruction, or jackhammer esophagus with obstruction (12).
Genetic predisposition
The first cases of affected siblings were reported in the 1960s and an early case report of an affected parent and offspring exists from 1972 (14). Since that time at least two genetic achalasia syndromes have been described. Allgrove (AAA) syndrome presents in childhood with gastrointestinal manifestations of achalasia and gastric atonia in addition to glossitis, Addison disease and alacrima. This syndrome is the result of a missense or truncation mutation on chromosome 12 (15). Chromosome 2 aberrations are part of another genetic syndrome with achalasia and intellectual dysfunction (16).
Achalasia has been demonstrated to have associations with HLA alleles. An early study demonstrated a significant association with the HLA DQB1*0602 allele in white achalasia patients but not in blacks. However, trends were noted with the HLA DRB1*15 allele in whites and the DRB1*12 allele in blacks (17). Additionally, the frequency of a single nucleotide polymorphism in HLA-DQB1 on chromosome 6 was found to be higher in type 1 achalasia than the other manometric subtypes (18), suggesting that genetics could also play a role in severity of disease and clinical manifestations. Finally, interesting new links to Parkinson’s disease and Down’s syndrome have recently been reported and raise new questions regarding genetic origins (19).
Neuronal pathophysiology
The primary neuronal abnormality in achalasia is a selective loss of inhibitory neurons in the myenteric plexus of the distal esophagus and LES, with consequent imbalance of excitatory and inhibitory activity. A localized decrease of inhibitory neurotransmitters (VIP and NO) in concert with unopposed excitatory activity results in abnormal LES relaxation and absence of orderly esophageal peristalsis (20,21). Two pathways have been proposed for neuronal dysfunction in achalasia (Figure 2) (22,23). In the traditional pathway associated with ‘classic’ achalasia, there is immune mediated destruction of myenteric neurons resulting in aperistalsis and abnormal LES relaxation. Thus, the esophageal smooth muscle initially maintains tone despite loss of peristalsis, manifesting as pressure compartmentalization in the esophageal body, and a type 2 achalasia pattern. Over time, this decays into a dilated esophageal body with loss of muscle tone, resulting in a type 1 pattern with aperistalsis, no esophageal pressure compartmentalization, and abnormal LES relaxation (24).
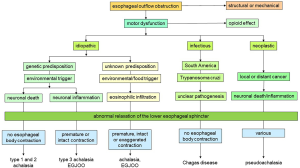
The second pathway consists of immune mediated inflammatory injury that damages but does not kill the myenteric neurons, resulting in an imbalance between excitatory and inhibitory influences (Figure 2). This myenteric plexitis leads to exaggerated, premature and rapid esophageal body contraction, with or without esophageal outflow obstruction. This accounts for type 3 achalasia, and other patterns with intact esophageal body peristalsis, and possibly, other esophageal body hypercontractile or spastic disorders with or without abnormal LES relaxation.
Evidence exists supporting an autoimmune basis for achalasia, where an antibody response to a common antigen, perhaps a virus, selectively knocks out esophageal autonomic control mechanisms at the myenteric plexus ganglia and neurons (25,26). Herpes simplex virus type I (HSV-1) has been proposed as an infectious trigger; other viruses implicated include measles and human papilloma virus (22). Both LES and esophageal body are profoundly impacted in type 1 and type 2, with ganglion cell death; little inflammatory response has been found both these subtypes (27). This could imply that inflammation could have burnt out by the time achalasia presents, or that inflammation is patchy. The higher magnitude of aganglionosis in type 1 achalasia compared to type 2 achalasia has led to speculation that type 1 achalasia is a later stage in the progression of the disease (27); however, it remains possible that the gradient of ganglion cell death differentiates type 1 from type 2 achalasia. In contrast, an imbalance between excitatory and inhibitory influence from inflammation of the myenteric plexus, but without profound ganglion cell death is likely in type 3 achalasia (27). Limited histopathologic data available suggests a plexitis with CD3+/CD8+ lymphocytes, but with mostly intact ganglion cells (27).
In addition to traditional inflammatory pathways, eosinophilic inflammation has been identified in esophageal smooth muscle in some phenotypes of type 3 achalasia and EGJOO. While eosinophilic infiltration has not been identified in the esophageal mucosa or submucosa in these achalasia cases, pathogenic eosinophilic proteins have been demonstrated. Post-surgical analysis of esophageal tissue from myotomy specimens has also demonstrated high levels of eosinophilia (28). Further, a case report exists of an individual with eosinophilic esophagitis and achalasia in whom symptoms disappeared following steroid therapy (29). This raises the question as to whether an allergic or hypersensitivity based mechanism underlies the pathophysiology of some achalasia phenotypes.
Spastic esophageal disorders manometrically identical to type 3 achalasia, EGJOO, DES and hypercontractile esophagus have been identified in the setting of opioid medication usage, at a higher frequency than in patients not on these medications (30). While the exact mechanism of opioid induced esophageal dysmotility is not fully understood, prevalent hypotheses suggest reversible impairment of inhibitory nerve function from concurrent opioid use (31).
Consequently, studying esophageal innervation and muscle function demonstrates absent/fibrosed nerves and ganglia, with atrophic muscle in type 1 and type 2 achalasia (24). In contrast, inflammatory injury with varying damage to ganglia and post ganglionic neurons has been noted in type 3 achalasia.
Pathophysiologic consequences
The most pronounced pathological phenotype from esophageal neuronal dysfunction is exhibited in classic achalasia. Although three distinct forms of the disease are now recognized, type 1, the classic variant, continues to represent the most profound and well-studied phenotype. Surgical resection and myotomy specimens have demonstrated absence or dramatic reduction in ganglionic cells, with varying degrees of inflammatory cells (24). The resultant neuromuscular dysfunction is well characterized by topographical representation on high resolution manometry (HRM) as complete absence of esophageal body peristalsis with a tonically closed LES. Type 1 achalasia is thought to be a late stage manifestation of an achalasia phenotype where no esophageal body contraction persists in the context of absent LES relaxation (Figure 1).
In contrast, type 2 achalasia manifests panesophageal pressurization, in addition to aperistalsis and abnormal LES relaxation seen in type 1 achalasia (13). The pressurization is a consequence of retained esophageal body muscle tone, such that pressure from ingested content raises intraluminal pressure throughout the esophagus, since obstruction from the non-relaxing LES prevents dispersion of this pressure into the stomach. This is believed to represent an earlier stage manifestation of a similar phenotype as type 1 achalasia. In fact, if LES obstruction is relieved mechanically or with myotomy, esophageal body peristalsis may be identified (32). It is unclear whether this represents recovery of incompletely compromised neuronal dysfunction, or uncovering of retained esophageal body peristalsis previously masked by panesophageal pressure compartmentalization.
Type 3 achalasia demonstrates retention of esophageal body contraction, albeit it in the setting of disordered inhibition, and have also been shown to have augmentable LES relaxation with tests designed to enhance deglutitive inhibition [such as multiple rapid swallows (MRS)] (33). These characteristics of type 3 achalasia overlap with some EGJOO phenotypes (34,35). However, EGJOO is extremely heterogenous, and there is currently no good single test to select out achalasia variant phenotypes within EGJOO. While dysphagia is a dominant symptom, chest pain can be a prominent symptom. Resolution of dysphagia requires myotomy of the entire contracting segment in type 3 achalasia, as can be provided by per oral endoscopic myotomy (POEM). There are reports of persisting dysphagia in type 3 achalasia when only myotomy of the LES is performed, requiring myotomy of the remnant contracting smooth muscle for dysphagia relief (36). On the other hand, chest pain may not improve with myotomy alone, and may require neuromodulator therapy or complementary approaches.
Abnormal inhibitory function
The evidence for inhibitory nerve dysfunction as the basis for spastic esophageal body disorders comes in part from studies performed by Sifrim et al. over two decades ago. When Sifrim distended a balloon with a recording sensor, he could demonstrate that the pressure recorded by the sensor declined just prior to the arrival of the contraction wave—this was the first demonstration of esophageal inhibition (37). Sifrim went on to demonstrate that this measurement of inhibition was abnormal or suboptimal in patients with contraction wave abnormalities and those with diffuse esophageal spasm, establishing that these were part of the inhibitory disorder spectrum (9).
Another method of evaluating esophageal inhibition is using MRS. MRS assess the phenomenon of deglutitive inhibition (38,39). When swallows are administered in rapid succession, there is inhibition of esophageal body peristalsis, with profound LES relaxation. Following the final swallow of the series, there is robust esophageal body contraction, with reestablishment of LES tone. When inhibition is abnormal, the deglutitive inhibition phase shows contraction fragments.
Studying deglutitive inhibition using MRS in achalasia spectrum disorders provides clues to the gradient of inhibitory dysfunction in these disorders (33). With achalasia type 1 and 2 where there is death of inhibitory neurons, there is no relaxation of the LES during deglutitive inhibition, and no esophageal body contraction. In contrast, in achalasia type 3 where there is rapid or premature contraction, or when esophageal body contraction is preserved, the LES does partially relax, and there is exaggerated contraction following MRS, suggesting imbalance between inhibition and contraction rather than complete loss of inhibition (12). A similar imbalance between inhibition and excitation could explain the pathophysiologic basis of hypercontractile disorders (11), both with and without an obstructive component (12).
Abnormal esophageal emptying
Since the cardinal symptoms of achalasia relate to abnormal esophageal emptying, demonstrating outflow obstruction is an important part of esophageal testing. The first esophageal investigation typically performed is endoscopy, and the finding of a puckered and closed LES, along with a dilated esophageal body with retained esophageal debris is suggestive of achalasia. However, these classic features are encountered less than half the time. Barium radiography may demonstrate a non-relaxing tapered LES appearance (bird’s beak) with a dilated esophageal body and poor esophageal emptying on a timed upright study, but even these features are encountered in only two thirds. Esophageal physiologic testing has a sensitivity of 98% and specificity of 96% in identification of abnormal esophageal emptying using the integrated relaxation pressure (IRP), a software tool used during HRM to define the nadir pressure achieved during post swallow LES relaxation (40). However, this metric does not differentiate between abnormal LES relaxation and structural processes at the EGJ, and a composite of clinical presentation and data from other esophageal tests are combined with HRM manifestations to make a conclusive diagnosis of achalasia.
In the context of symptoms consistent with esophageal outflow obstruction, particularly significant dysphagia and weight loss, a normal IRP does not exclude achalasia or EGJ outflow obstruction. Adjunctive tests challenge the esophagus to bring out features of esophageal outflow obstruction in these settings. One such test is the rapid drink challenge (RDC), where a 100–200 mL water load is administered during HRM (41,42)—this can result in esophageal pressurization or shortening when outflow obstruction exists, even when these findings are not evident on routine HRM water swallows (35). The IRP during RDC direct correlates with validated symptom assessment such as the Eckardt score (43). Another test that can demonstrate abnormal emptying is the standardized test meal, where the patient eats components of a meal during HRM—this has been demonstrated to augment the diagnostic yield of major motor disorders including achalasia spectrum disorders (44). Barium esophagography using a barium pill, or a timed upright barium study are additional adjunctive studies that can be utilized to demonstrate obstruction. Functional lumen imaging probe (FLIP) evaluates esophageal and EGJ cross sectional area, and can demonstrate abnormal EGJ distensibility in achalasia even when HRM parameters are inconclusive (45).
Consequences of stasis
Retention of food in the esophagus may have consequences beyond impairment of nutrition and weight loss. Food can regurgitate back into the pharynx and create an aspiration risk, leading to pulmonary changes from micro-aspiration, or even frank aspiration pneumonia (46). Esophageal stasis can also promote candida esophagitis (47). Stasis related mucosal changes, including ulcers, have been reported (48).
Over time, the esophagus can dilate and become tortuous. In extreme states, the esophagus adopts a sigmoid configuration, where esophageal emptying remains compromised despite lack of obstruction at the esophagogastric junction. Symptoms may not be predictive of the degree of stasis (49). Enteral feeding through a gastrostomy or jejunostomy tube, or esophagectomy are the only viable treatment options.
There is a small risk of esophageal squamous cell cancer in patients with long standing achalasia, particularly in the setting of esophageal dilation and stasis (50,51).
Conditions that mimic achalasia
The term achalasia has traditionally been applied only for idiopathic achalasia. Since esophageal outflow obstruction can occur from mechanisms other than achalasia, and since manometry may demonstrate patterns and metrics identical to achalasia, these conditions are termed ‘pseudoachalasia’ (52,53).
Mechanical etiologies of esophageal outflow obstruction include iatrogenic causes (tight fundoplication, laparoscopic band placement), masses impinging on the EGJ (intraluminal, intramural and extraluminal), tight strictures, and even hiatus hernias, especially those with a paraesophageal component (34,35,54). The pressure effect of these structural processes results in IRP elevation. In the setting of EGJ obstruction, upstream motor function may initially augment in an attempt to overcome the obstruction (55), but subsequently diminishes, and hypomotility or aperistalsis may ensue (54).
Local and distal cancer can induce an achalasia like motor disorder. Antigenic mimicry could exist between certain cancers and esophageal neurons, such that antibodies formed against cancer cells could in turn result in an idiopathic achalasia like picture (22). This may be particularly true when pseudoachalasia occurs in the setting of a distant cancer, such as small cell lung cancer. Alternatively, local infiltration from an EGJ cancer could impact innervation of the distal esophagus and LES, resulting in both mechanical and neural mechanisms for esophageal outflow obstruction.
Finally, Chagas’ disease, also called trypanosomiasis, is an infectious disease prevalent in South America (56,57). It is caused by trypanosome cruzi, a parasite that is transmitted by an infected triatomine bug or the ‘kissing bug’. In 10–20% of patients who survive the acute phase of the disease, gastrointestinal involvement can manifest, primarily as achalasia or slow colonic transit (57). Chronic persistence of the infection is a prerequisite to esophageal involvement, which raises the possibility of a similar antigen mimicry mechanism as idiopathic achalasia (58). Cardiac involvement may manifest as cardiomyopathy.
Conclusions
Achalasia is an esophageal motor disorder where esophageal inhibitory function is irreversibly compromised, leading to abnormal LES relaxation and lack of effective esophageal body peristalsis. Current understanding of achalasia pathophysiology implicates an environmental insult, potentially viral, in a genetically predisposed individual, resulting in an antibody response that targets esophageal ganglia and neurons because of a shared antigenic structure with the insulting agent. The consequence is essentially an obstructed esophageal outflow, from unbalanced LES contraction and lack of relaxation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sarah Thompson) for the series “Achalasia” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2019-ach-07). The series “Achalasia” was commissioned by the editorial office without any funding or sponsorship. CPG reports personal fees from Medtronic, Diversatek, Ironwood, Isothrive, and Quintiles, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spiess AE, Kahrilas PJ. Treating achalasia: from whalebone to laparoscope. JAMA 1998;280:638-42. [Crossref] [PubMed]
- Rake AT. Achalasia and Degeneration of Auerbach's Plexus. Proc R Soc Med 1928;21:1775-7. [Crossref] [PubMed]
- Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut 2001;49:145-51. [Crossref] [PubMed]
- Bowie JD, Clair MR. Fetal swallowing and regurgitation: observation of normal and abnormal activity. Radiology 1982;144:877-8. [Crossref] [PubMed]
- Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology 1998;114:559-78. [Crossref] [PubMed]
- Gidda JS, Goyal RK. Regional gradient of initial inhibition and refractoriness in esophageal smooth muscle. Gastroenterology 1985;89:843-51. [Crossref] [PubMed]
- Akbarali HI, Hatakeyama N, Wang Q, et al. Transient outward current in opossum esophageal circular muscle. Am J Physiol 1995;268:G979-87. [PubMed]
- Murthy KS, Zhang KM, Jin JG, et al. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am J Physiol 1993;265:G660-71. [PubMed]
- Sifrim D, Janssens J, Vantrappen G. Failing deglutitive inhibition in primary esophageal motility disorders. Gastroenterology 1994;106:875-82. [Crossref] [PubMed]
- Behar J, Biancani P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology 1993;105:111-8. [Crossref] [PubMed]
- Mauro A, Quader F, Tolone S, et al. Provocative testing in patients with jackhammer esophagus: evidence for altered neural control. Am J Physiol Gastrointest Liver Physiol 2019;316:G397-G403. [Crossref] [PubMed]
- Quader F, Mauro A, Savarino E, et al. Jackhammer esophagus with and without esophagogastric junction outflow obstruction demonstrates altered neural control resembling type 3 achalasia. Neurogastroenterol Motil 2019;31:e13678 [Crossref] [PubMed]
- Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 2008;135:1526-33. [Crossref] [PubMed]
- Kilpatrick ZM, Milles SS. Achalasia in mother and daughter. Gastroenterology 1972;62:1042-6. [Crossref] [PubMed]
- Brooks BP, Kleta R, Stuart C, et al. Genotypic heterogeneity and clinical phenotype in triple A syndrome: a review of the NIH experience 2000-2005. Clin Genet 2005;68:215-21. [Crossref] [PubMed]
- Koehler K, Malik M, Mahmood S, et al. Mutations in GMPPA cause a glycosylation disorder characterized by intellectual disability and autonomic dysfunction. Am J Hum Genet 2013;93:727-34. [Crossref] [PubMed]
- Verne GN, Hahn AB, Pineau BC, et al. Association of HLA-DR and -DQ alleles with idiopathic achalasia. Gastroenterology 1999;117:26-31. [Crossref] [PubMed]
- Vackova Z, Niebisch S, Triantafyllou T, et al. First genotype-phenotype study reveals HLA-DQbeta1 insertion heterogeneity in high-resolution manometry achalasia subtypes. United European Gastroenterol J 2019;7:45-51. [Crossref] [PubMed]
- Johnston BT, Colcher A, Li Q, et al. Repetitive proximal esophageal contractions: a new manometric finding and a possible further link between Parkinson's disease and achalasia. Dysphagia 2001;16:186-9. [Crossref] [PubMed]
- Park W, Vaezi MF. Etiology and pathogenesis of achalasia: the current understanding. Am J Gastroenterol 2005;100:1404-14. [Crossref] [PubMed]
- Mearin F, Mourelle M, Guarner F, et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest 1993;23:724-8. [Crossref] [PubMed]
- Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology 2013;145:954-65. [Crossref] [PubMed]
- Gyawali CP. Achalasia: new perspectives on an old disease. Neurogastroenterol Motil 2016;28:4-11. [Crossref] [PubMed]
- Sodikoff JB, Lo AA, Shetuni BB, et al. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil 2016;28:139-45. [Crossref] [PubMed]
- Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology 1996;111:648-54. [Crossref] [PubMed]
- Villanacci V, Annese V, Cuttitta A, et al. An immunohistochemical study of the myenteric plexus in idiopathic achalasia. J Clin Gastroenterol 2010;44:407-10. [PubMed]
- Sodikoff JB, Lo AA, Shetuni BB, et al. Histopathologic patterns across achalasia subtypes. Neurogastroenterol Motil 2016;28:139-45. [PubMed]
- Cools-Lartigue J, Chang SY, McKendy K, et al. Pattern of esophageal eosinophilic infiltration in patients with achalasia and response to Heller myotomy and Dor fundoplication. Dis Esophagus 2013;26:766-75. [Crossref] [PubMed]
- Savarino E, Gemignani L, Zentilin P, et al. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin Gastroenterol Hepatol 2011;9:1104-6. [Crossref] [PubMed]
- Ratuapli SK, Crowell MD, DiBaise JK, et al. Opioid-Induced Esophageal Dysfunction (OIED) in Patients on Chronic Opioids. Am J Gastroenterol 2015;110:979-84. [Crossref] [PubMed]
- Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs 2003;63:649-71. [Crossref] [PubMed]
- Roman S, Kahrilas PJ, Mion F, et al. Partial recovery of peristalsis after myotomy for achalasia: more the rule than the exception. JAMA Surg 2013;148:157-64. [Crossref] [PubMed]
- Kushnir V, Sayuk GS, Gyawali CP. Multiple rapid swallow responses segregate achalasia subtypes on high-resolution manometry. Neurogastroenterol Motil 2012;24:1069-e561. [Crossref] [PubMed]
- van Hoeij FB, Smout AJ, Bredenoord AJ. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil 2015;27:1310-6. [Crossref] [PubMed]
- Biasutto D, Mion F, Garros A, et al. Rapid drink challenge test during esophageal high resolution manometry in patients with esophago-gastric junction outflow obstruction. Neurogastroenterol Motil 2018;30:e13293 [Crossref] [PubMed]
- Weche M, Saad AR, Richter JE, et al. Revisional Procedures for Recurrent Symptoms After Heller Myotomy and Per-Oral Endoscopic Myotomy. J Laparoendosc Adv Surg Tech A 2020;30:110-6. [Crossref] [PubMed]
- Sifrim D, Janssens J, Vantrappen G. A wave of inhibition precedes primary peristaltic contractions in the human esophagus. Gastroenterology 1992;103:876-82. [Crossref] [PubMed]
- Shaker A, Stoikes N, Drapekin J, et al. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol 2013;108:1706-12. [Crossref] [PubMed]
- Fornari F, Bravi I, Penagini R, et al. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil 2009;21:718-e41. [Crossref] [PubMed]
- Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol 2007;293:G878-85. [Crossref] [PubMed]
- Marin I, Serra J. Patterns of esophageal pressure responses to a rapid drink challenge test in patients with esophageal motility disorders. Neurogastroenterol Motil 2016;28:543-53. [Crossref] [PubMed]
- Ang D, Hollenstein M, Misselwitz B, et al. Rapid Drink Challenge in high-resolution manometry: an adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil 2017; [Crossref] [PubMed]
- Woodland P, Gabieta-Sonmez S, Arguero J, et al. 200 mL Rapid Drink Challenge During High-resolution Manometry Best Predicts Objective Esophagogastric Junction Obstruction and Correlates With Symptom Severity. J Neurogastroenterol Motil 2018;24:410-4. [Crossref] [PubMed]
- Ang D, Misselwitz B, Hollenstein M, et al. Diagnostic yield of high-resolution manometry with a solid test meal for clinically relevant, symptomatic oesophageal motility disorders: serial diagnostic study. Lancet Gastroenterol Hepatol 2017;2:654-61. [Crossref] [PubMed]
- Ponds FA, Bredenoord AJ, Kessing BF, et al. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil 2017; [Crossref] [PubMed]
- Makharia GK, Seith A, Sharma SK, et al. Structural and functional abnormalities in lungs in patients with achalasia. Neurogastroenterol Motil 2009;21:603-8, e20.
- Hoversten P, Otaki F, Katzka DA. Course of Esophageal Candidiasis and Outcomes of Patients at a Single Center. Clin Gastroenterol Hepatol 2019;17:200-2.e1. [Crossref] [PubMed]
- Kjellin AP, Ost AE, Pope CE 2nd. Histology of esophageal mucosa from patients with achalasia. Dis Esophagus 2005;18:257-61. [Crossref] [PubMed]
- van Hoeij FB, Smout A, Bredenoord AJ. Esophageal stasis in achalasia patients without symptoms after treatment does not predict symptom recurrence. Neurogastroenterol Motil 2017; [Crossref] [PubMed]
- Leeuwenburgh I, Haringsma J, Van Dekken H, et al. Long-term risk of oesophagitis, Barrett's oesophagus and oesophageal cancer in achalasia patients. Scand J Gastroenterol Suppl 2006;7-10. [Crossref] [PubMed]
- Kim H, Park H, Choi H, et al. Retention Esophagitis as a Significant Clinical Predictor of Progression to Esophageal Cancer in Achalasia. Clin Endosc 2018;51:161-6. [Crossref] [PubMed]
- Liu W, Fackler W, Rice TW, et al. The pathogenesis of pseudoachalasia: a clinicopathologic study of 13 cases of a rare entity. Am J Surg Pathol 2002;26:784-8. [Crossref] [PubMed]
- Gockel I, Eckardt VF, Schmitt T, et al. Pseudoachalasia: a case series and analysis of the literature. Scand J Gastroenterol 2005;40:378-85. [Crossref] [PubMed]
- O'Rourke RW, Seltman AK, Chang EY, et al. A model for gastric banding in the treatment of morbid obesity: the effect of chronic partial gastric outlet obstruction on esophageal physiology. Ann Surg 2006;244:723-33. [Crossref] [PubMed]
- Gyawali CP, Kushnir VM. High-resolution manometric characteristics help differentiate types of distal esophageal obstruction in patients with peristalsis. Neurogastroenterol Motil 2011;23:502-e197. [Crossref] [PubMed]
- Bern C. Chagas' Disease. N Engl J Med 2015;373:456-66. [Crossref] [PubMed]
- Pérez-Molina JA, Molina I. Chagas disease. Lancet 2018;391:82-94. [Crossref] [PubMed]
- de Oliveira RB, Troncon LE, Dantas RO, et al. Gastrointestinal manifestations of Chagas' disease. Am J Gastroenterol 1998;93:884-9. [Crossref] [PubMed]
Cite this article as: Rogers AB, Rogers BD, Gyawali CP. Pathophysiology of achalasia. Ann Esophagus 2020;3:27.


