
Achalasia subtypes are front and center of the Chicago classification—strategies to overcome limitations in clinical application
Introduction
Esophageal high-resolution manometry (HRM) is the gold standard test for diagnosis of achalasia (1). The dominant symptom raising suspicion of achalasia is long standing dysphagia (2). Initial evaluation of dysphagia includes endoscopy and fluoroscopy (3), however many patients with achalasia, particularly in early stage of disease onset, are reported to have a normal endoscopy and barium swallow (4-6). Thus diagnosis of achalasia, a rare yet widely known motility disorder with no disease specific biomarker, is based on abnormal manometric pressures (7).
The hallmark manometric features of achalasia, absence of normal peristalsis and incomplete esophagogastric junction (EGJ) relaxation on swallowing, are best recorded with HRM (8). Over the past decade, wide-spread uptake of HRM in clinical practice reflects the advantages of HRM technology over previous manometry systems (9,10). Accompanying the advancements in pressure sensor technology, computerization and analysis algorithms at the heart of HRM, is the development of a relevant classification system of esophageal motility disorders (11). Now in its third iteration, the Chicago classification guides interpretation of HRM findings (12,13). Front and center is recognition of manometric subtypes of achalasia that are clinically relevant for treatment outcomes.
The utility of HRM and the Chicago classification v3.0, 2015 (CCv3.0) (13) for the diagnosis of achalasia is ironically both simpler and more complex than ever before! HRM makes it easier to acquire good quality manometric studies (9,10). However, recent debate regarding training and competency in motility testing highlights the complexity of interpreting HRM findings (14,15). An additional layer is discovery and evolution. Five years have passed since the release of CCv3.0 and the International Working Group for Disorders of GI Motility & Function are in the process of formulating another update, with the release of Chicago classification v4.0 expected in 2020.
For a review of the literature, electronic databases MEDLINE (PubMed), EMBASE (Ovid), and Cochrane Library (Cochrane Central Register of Controlled Trials) were searched from January 2008 to November 2019, subsequently updated in February 2020, to identify all relevant articles published. The search strategy was limited to (‘esophagus spasm/or esophagus achalasia’ OR achalasia’) AND (‘high resolution manometry’) AND/OR (subtype I or subtype II or subtype III or classical or pressurization or spastic or type I or type II or type III or Chicago classification). Non relevant and duplicate papers were removed. Original full manuscripts of articles were reviewed. Bibliographies of relevant key-papers, review articles, meta-analyses and original articles were manually searched to identify additional publications.
This article reviews HRM and the current Chicago classification v3.0 for achalasia, with a focus on how the classification works, recent advances in HRM, and its limitations.
History
The recognition of disorder of motility related to dysphagia has a varied history. Achalasia was first described over 300 years ago, in 1674, by Sir Thomas Willis (16,17) and subsequently referred to as ‘cardiospasm’ until Dr. Arthur Hertz challenged this in 1915. Following post-mortem examinations, Hertz proposed the problem was not cardiac sphincter spasm, but a lack of sphincter relaxation. As a direct consequence, the term ‘achalasia’ (fail to relax) was coined by Sir Cooper Perry (18). The recognition of achalasia long ago contrasts with more recent discovery of other motility disorders related to dysphagia. Epiphrenic diverticulum was recognised 90 years ago by Mondiere in 1933 (19). While eosinophilic esophagitis noted by Landres et al. in 1978, appeared on endoscopy reports from 1990 (20).
Intriguingly, descriptions of achalasia or ‘cardiospasm’ formed part of the medical literature long before acceptance of a lower esophageal sphincter (LES) (18,21). The first published manometry study recording LES pressure was in 1956 (22). Shortly thereafter the first guide to motility disorders, a book published in 1958, described just three disorders of esophageal motility: achalasia, diffuse spasm, and scleroderma esophagus (23). This pictorial atlas is a fascinating read of manometry from another era yet was published only 60 years ago!
The first motility classification system for water-perfused manometry, using external pressure transducers, was proposed by Spechler & Castell in 2001, after review and analysis of the literature (24). Motility patterns were described by standardised criteria to distinguish six motility disorders, namely: achalasia; atypical disorders of LES relaxation; diffuse esophageal spasm; nutcracker esophagus; isolated hypertensive LES, and ineffective esophageal motility. Independently, a similar classification was devised and published a couple of months later by Richter (25).
The advancement from low-resolution to HRM, with an increase in the number and proximity of closely spaced intraluminal pressure sensors, has revolutionised visualization and interpretation of luminal esophageal pressures (26). Visualization enhanced by the innovation of spatiotemporal contour plots, whereby pressure amplitudes are color coded and interpolation of pressure values between recording sensors yields smooth topographic displays to create pressure maps (i.e., esophageal pressure topography or color contour plots) (27,28). Interpretation advanced in two-ways by (I) software development, with sophisticated algorithms for semi-automated data analysis plus new standardized topographic metrics for consistent analysis (26,29), and (II) classification of pressure analysis findings to aid diagnosis of major and minor motility disorders (11). Now called the ‘Chicago classification’ (9), this system was originally designed to categorize distal esophageal motor disorders focused on dysphagia (30), and continues to evolve with the intention for 3-yearly updates (13). The CCv3.0 uses a hierarchical approach to firstly categorize disorder of the EGJ and then disorder of esophageal peristalsis (13). Use of HRM is expanding, reflected by an increase in publications (of the top 10 esophageal motility disorder papers, CCv3.0 has the highest citation rate) (31,32). Research and development, through a consensus process, will inform an update, Chicago classification 4.0.
Clinical presentation
Dysphagia, the perceived difficulty in swallowing with possible impaired bolus passage (33), is the main presenting symptom of several very different pathophysiological conditions. While the etiology of dysphagia cannot be diagnosed from symptoms, the circumstances of symptom onset can be informative. Sudden onset progressive dysphagia, particularly in middle-aged Caucasian men, is an alarm signal for carcinoma (34). Intermittent dysphagia to solids implies a benign structural disorder. Troublesome constant dysphagia to solids and liquids is more common with motility disorders (35). Motility disorders are more common in patients with dysphagia (53%) than patients with non-cardiac chest pain (28%) (36). Dysphagia is experienced by 90–100% patients with achalasia, less so regurgitation, chest pain and atypical symptoms: heartburn, cough and epigastric pain (2,37).
A direct consequence of dysphagia in achalasia is altered bolus transport and food stasis (17,38). Some but not all patients report weight loss (4,6,39). Patients with heartburn are more likely to experience weight loss (39) and many are at risk of malnutrition (40). Other factors may be denial of weight loss associated with longer duration of symptoms, and achalasia subtype (see later) (41).
Questionnaires for patient self-reporting of symptoms bring to the fore dysphagia symptom severity and/or quality of life. The Eckardt score combines scores for dysphagia, regurgitation, chest pain and weight loss, all highly relevant in achalasia (42,43). In-depth dysphagia assessments include eating capacity assessment, such as: Dakkak and Bennett composite dysphagia score (44); the Mayo Dysphagia Questionnaire for scoring dysphagia in the preceding 30-day (45); and a disease specific tool, the Achalasia Severity Questionnaire (46). These are all validated instruments for clinical use.
An appraisal of the aforementioned symptoms and signs, and noting any history of prior surgery around the hiatus is required, as primary idiopathic achalasia and secondary pseudoachalasia (of benign, malignant or surgical cause) (47,48) are conditions with similar symptoms and manometric findings (see article on pseudoachalasia in this issue).
Initial investigations
Endoscopy is a necessary first step for evaluation of dysphagia, to exclude obstruction associated with a stricture, tumour or inflammation (3,49). Features raising suspicion of achalasia such as a dilated esophagus, undigested food, or mild difficulty in passing the scope through the EGJ, are often but not consistently present (4). Studies reveal 50–70% of patients with achalasia reportedly have a normal endoscopy (4,5). Knowledge of EGJ resistance or a dilated esophagus is helpful prior to manometry.
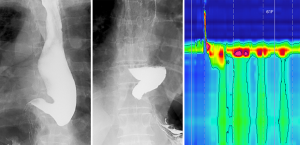
A radiographic barium swallow utilizing video fluoroscopy may reveal typical findings of achalasia, namely: aperistalsis, a dilated esophagus (varying from mild to sigmoid shape), distal esophageal tapering with a ‘bird-beak’ shaped EGJ, and retention of contrast with poor esophageal emptying (5,50). These features may not be present in early stages of achalasia (4,51,52).
Endoscopy and fluoroscopy ideally precede manometry (to exclude other causes of dysphagia) and they may provide insight into anatomical anomalies for caution and care during manometry catheter insertion, as well as complementary information aiding interpretation of HRM findings (Figure 1).
High resolution manometry
Acquisition of esophageal HRM
In brief, esophageal HRM incorporates closely spaced (at 1–2 cm) pressure sensors for measuring luminal pressures from the throat to the stomach.
Preparation
Preparation includes a 6-hr fast and cessation of medications that alter upper gastro-intestinal motility for at least 48 hours (53). For patients with suspected achalasia, consumption of a liquid diet 48-hr prior is advised, to lower the risk of aspiration or vomiting during catheter intubation (54) and to provide a cleaner motility recording by reducing pressure artifact related to the presence of food in the esophagus.
Prior to HRM, an esophageal HRM catheter is connected to a biomedical pressure recording system, requiring sensor function check and two-point calibration (54). Current multi-sensor solid-state HRM catheters consist of 32–36 pressures sensors, either unidirectional or averaged circumferential sensors, positioned at 1-cm spacing along the catheter length (53,55). The pressure sensors yield low voltage electrical signals, which are amplified, filtered and digitized for display in real-time with proprietary computer software (54).
Introducing the manometry catheter
The prospect of a nasogastric intubation while awake is a daunting thought for most people (54) and may cause some anxiety related to ‘fear of the unknown’. A high-quality motility recording is dependent on both a well-trained operator and a co-operative patient (54,56). Thus, education on the purpose and nature of the test; assurance of brief instruction prior to each step; followed by verbal or written consent prior to intubation is important (54). Topical anaesthetic nasal spray (5% lignocaine) is applied to the most patent nostril 5-min prior to intubation, performed sitting or semi-upright while lying on a barouche, aided by water swallows.
Specific to patients with suspected achalasia, great care and attention is required when attempting to pass the manometry catheter across the EGJ. The risk of a motility catheter curling in the distal esophagus or not traversing the EGJ is greater in achalasia and/or dilated esophagus (57,58) with a failure rate of 7–12% (57-59). Further, during blind nasogastric intubation, subtle tactile feedback of resistance to passage across the EGJ will be missed if the catheter is not held lightly and passed gently. Successful intubations may require modified posture (left or right lateral) (54,60), or sometimes a deep breath or a gentle cough, for momentary axial separation of the lower sphincter and crural diaphragm, to allow passage of the catheter tip into the abdominal stomach. Once the catheter traverses the EGJ, the level of the crural diaphragm is identified with a deep breath or sniff to reveal the pressure inversion point. Above the inversion point, inspiration shows as negative intra-thoracic pressure; and below the inversion point, as positive intra-abdominal pressure (61).
HRM acquisition protocol
There are 3 essential elements: (I) correct positioning of the catheter for recording motility inclusive of two sensors above the upper sphincter (to observe swallow initiation) and at least three sensors beyond the EGJ on the gastric side; recording in the supine or semi-supine position (head of bed elevated by 10–15o); (II) after taping in position, a minimum 2-minute (up to 5-minute) acclimatization period with minimal swallowing is followed by a series of ten, 5 mL water swallows at 30 s intervals i.e., spaced to ensure no interference from the previous wet or spontaneous swallow (25–30 s refractory period) (62,63); (III) the operator (scientist, technician, nurse or medical officer) requires specialist training and experience to recognise during acquisition: correct position, common artifacts, equipment failure and make ‘within study’ adjustments for technical adequacy or apply protocol variations to improve diagnostic yield (54,56).
Adjunct testing
Although not part of the HRM protocol required for CCv3.0, additional swallow challenge tests and position change can prove helpful when findings are equivocal for achalasia (64,65). These adjunctive tests are designed to increase the workload for the esophagus and create a more realistic record of swallowing function or dysfunction (58,66). Multiple rapid swallows (MRS) involve five rapid sequence swallows, each of 2 mL water bolus at <3 s intervals during supine posture (67,68), optimally performed in triplicate (69). While a normal MRS response leads to inhibition of esophageal smooth muscle contractions; stimulates long and more complete EGJ relaxation; and gives rise to a vigorous peristaltic after contraction (67,70), these are abnormal/absent in patients with achalasia (71-73). Rapid drink challenge (RDC) involves free drinking of 200 mL water to enhance detection of EGJ dysfunction and the physiological response of an obstructive EGJ pattern (74-76). Of relevance, the degree of incomplete EGJ relaxation during RDC correlates with dysphagia severity (74). Another option is the incorporation of five, 5 mL water swallows during upright posture. EGJ pressures are lower during upright posture, however sustained abnormal EGJ relaxation with upright swallows better correlate with radiological EGJ obstruction, thus useful to assess EGJ dysfunction, including achalasia (77).
Analysis of HRM pressure data
The bridge between the pressure data of manometry acquisition and a motility diagnosis using CCv3.0, is analysis of HRM data (78). Visualization of pressure data as color contour plots aids correct placement of markers for analysis algorithms (79). In brief, HRM metrics are determined for each standard wet swallow, including these key variables: (I) integrated relaxation pressure (IRP), an indication of residual EGJ pressure during swallow induced relaxation; (II) distal contractile integral (DCI), recording the contractile vigour for the distal esophagus of peristaltic or non-peristaltic contractions—an integral, a unit of measure for pressure magnitude for the period of a swallow over an axial length of contractile segment i.e., amplitude (mmHg) × duration time (s) × length (cm); (III) distal latency (DL), the time elapsed between swallow onset and arrival of the contraction in the distal esophagus. In addition to contractile vigour, the pattern of esophageal contractions is assessed for each wet swallow, for example: failed, intact, premature (rapid) or fragmented contractions. These metrics and patterns are utilised in the CCv3.0 to categorize motility disorders (13). The classification is intended to evaluate motility in patients with dysphagia without prior upper gastrointestinal surgery. The CCv3.0 includes guidelines to classify EGJ morphology, but hiatus hernia is uncommon in achalasia (4–5%) (80-82) and most patients with achalasia show complete overlap of LES and crural diaphragm pressures (EGJ type I), with or without transient separation during swallowing as a result of LES elevation (56,58).
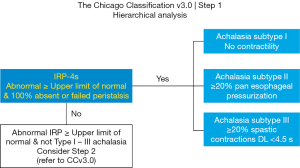
The CCv3.0 follows a hierarchical approach, beginning with evaluation of EGJ relaxation, after which an evaluation of esophageal body function follows (13).
Evaluation of EGJ relaxation
Esophageal emptying is dependent on flow through the EGJ, without which bolus transport is not achieved. Detection of an abnormally relaxing EGJ is required for an achalasia diagnosis (60). EGJ IRP or IRP-4s is a pressure topography metric, defined as the mean of 4s of maximum EGJ deglutitive relaxation occurring within a 10s timeframe from swallow onset. The 4s of EGJ relaxation may be contiguous or non-contiguous, to allow for interruptions by diaphragmatic contractions of respiration. IRP-4s (mmHg) referenced to gastric pressure, is recorded for 10 wet swallows, with initially mean and now median IRP-4s for classification (11,13,83,84).
In a landmark publication of 2007, Ghosh et al. explored a range of measures and criteria for EGJ relaxation and found the IRP-4s with a cut-off value of 15 mmHg* was optimal for separation of patients with and without achalasia (98% sensitivity; 96% specificity) (83,85). If the IRP-4s value is less than 15 mmHg, then EGJ relaxation is considered normal.
IRP-4s is however a complex metric measured across time (described above) and across axial EGJ length, between the proximal and distal axial margins i.e., several 1 cm-spaced pressure sensors (84). This means IRP quantifies deglutitive EGJ relaxation for 4 s across a 3–5 cm axial length and is unlikely to capture LES relaxation alone. The dynamic EGJ environment is influenced by LES function, crural diaphragm function, distal esophageal luminal pressure during swallowing, as well as pressure associated with bolus presence. A rigorous study of IRP values by regression tree analysis and algorithm approach found EGJ relaxation by IRP is best assessed in context of esophageal pressure patterns (86). Thus in CCv3.0, the cut-off IRP value of 15 mmHg is the upper limit of normal, with flexibility for borderline IRP when there is absence of peristalsis (i.e., for aperistalsis with borderline IRP, range, 10–15 mmHg, consider achalasia) (13). This aspect is made further complex by different (higher) cut-off levels for alternative manometry systems (85) and decreased EGJ relaxation (higher IRP) in the elderly (87-89).
Evaluation of esophageal body function
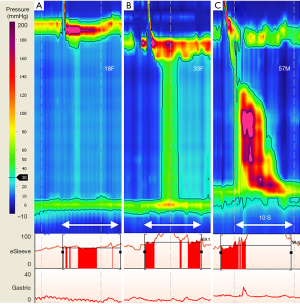
During formulation of the first version of the Chicago classification (11) an in-depth analysis of HRM data in persons with impaired EGJ relaxation (90) identified three distinct subtypes of achalasia, namely subtype I, II, III, for esophagus showing absent pressures; uniform pressures; and spastic pressures respectively (Table 1; Figure 2). All achalasia subtypes are characterized by incomplete EGJ relaxation, so the subtype reflects the different but prevalent esophageal body pressure patterns of abnormal or absent peristalsis (12,90) (Figure 3). Subtype I and II are distinguished by esophageal pressurization of <30 or >30 mmHg, respectively. Upon swallowing, esophageal body pressure pattern shows absent pressures (subtype I) or pan esophageal pressurization with uniform pressure across the entire esophagus (subtype II). Subtype III is characterized by at least 20% of wet swallows with rapid i.e., premature contractions (defined as DL <4.5 s). Most importantly, treatment outcomes were different for the different subtypes, whether treating the poorly relaxing LES by paralysing it with Botulinum toxin; or disrupting the muscle fibres by pneumatic dilatation, or laparoscopic Heller myotomy. Notably 47/49 patients with subtype II had successful treatment, with just 4% treatment failure (cf. 44%, 71% for subtype I & III respectively) (90).
Table 1
| Achalasia subtypes | Criteria |
|---|---|
| Subtype I: Classic achalasia | • Elevated median IRP-4s (>15 mmHg*)† |
| Subtype II: Achalasia with pressurization | • Elevated median IRP-4s (>15 mmHg*) |
| Subtype III: Spastic achalasia | • Elevated median IRP-4s (>15 mmHg*) |
IRP-4s, integrated relaxation pressure (4 sec); DCI, distal contractile integral; DL, distal latency; pan esophageal pressurization refers to uniform pressure, which spans from EGJ to UES (gastroesophageal junction to upper sphincter). *, cut-off value is dependent on device; CCv3.0 based on Sierra (Medtronic) [consult normative values for device in use (
Inspired, Salvador et al. undertook retrospective subtyping of predominantly conventional manometry tracings for 246 patients, finding more treatment failures with subtype III (30%), cf. subtype II (5%) and I (14%) (91). A pivotal study followed, where Rohof et al. applied achalasia subtypes to manometric data for 176 patients already enrolled in the European achalasia trial (randomized: pneumatic dilatation, PD, or laparoscopic Heller myotomy, LHM). By subtypes, achalasia treatment success rates at 2 years post treatment were best in subtype II (100% PD vs. 95% LHM), less in subtype I (86% PD vs. 81% LHM), and worst in subtype III (40% PD vs. 86% LHM) (92).
Several subsequent studies report various treatment modality outcomes by achalasia subtype (93), most confirming these findings (94-96), while others found similar treatment outcomes for subtypes (97-99). Long-term outcome for the aforementioned European achalasia trial reveals that success at 5 years post treatment was best in subtype II (96% PD vs. 88% LHM), however success rate declined between years 2 and 5 for subtype I and II, while subtype III remained stable (48% PD vs. 86% LHM) (100). Two recent meta-analyses show older patients and achalasia subtype III were strongest predictors of clinical outcome (101); with recommendations for when to offer pneumatic dilatation, POEM and LHM (with a caveat that post treatment reflux data was not consistently available for analysis) (102). The concluding statement that personalized treatment for achalasia subtype could help achieve the best outcome (102) highlights the clinical importance of subtyping.
A major shift is underway. First, application of HRM and CCv3.0 is enabling improved classification of patients with achalasia into three achalasia subtypes, which can be applied consistently across all manometric acquisition and analysis systems (103). Second, single centre, multi-centre studies, and meta-analyses of treatment outcomes are an evidence base to assess treatment efficacy for an achalasia subtype. Third, individualized patient treatment is being realized, guided by therapeutic responses for specific achalasia subtypes. Different treatments for achalasia (pneumatic balloon dilatation, Botulinum toxin, laparoscopic Heller myotomy + Dor fundoplication, and per oral endoscopic myotomy) are reviewed separately (see subsequent articles in this issue).
Subtype analysis in HRM
Achalasia subtype I
Evidence to date suggests that in achalasia subtype I, the absence of peristalsis and minimal esophageal pressurization in the distal smooth muscle of the esophagus relates to a large cross-sectional area; thin esophageal wall muscle; little or no longitudinal muscle contraction; and poor emptying. These features are compatible with a dilated esophagus that predominantly empties by gravity (104).
A dilated esophagus is most consistently observed in subtype I and also subtype II, although observations vary and do not discriminate subtypes (92,105,106). Regurgitation, a common symptom in achalasia, relates to greater lumen width rather than achalasia subtype (92,105).
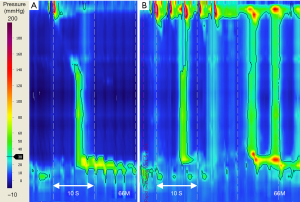
IRP is more often within the normal range in achalasia subtype I than other achalasia subtypes (86,107), requiring careful attention during classification. It is important that low amplitude incomplete EGJ relaxation of achalasia subtype I, is not confused with an adynamic or scleroderma-like esophagus, which features: absent or poor contractility, complete EGJ relaxation, and a high rate of reflux symptoms (38,108). If there is uncertainty of pressure characteristics with standard 5 mL wet swallows, several studies show adjunct swallow challenge tests may provide clarity (38,107) (Figure 4). However, swallow challenge tests may change the achalasia subtype (73,109). A MRS response may change a subtype I to subtype II, not unexpectedly as esophageal pooling may affect swallow pressurization from <30 to >30 mmHg, the distinguishing isocontour threshold. In such cases, the baseline 5 mL water swallow findings inform the diagnosis, with MRS providing supportive evidence. More studies are needed to build the evidence base for response characteristics of MRS in achalasia.
Achalasia subtype II
Established early on and confirmed by a recent meta-analysis, achalasia subtype II is the most common subtype (93,102). It features pan esophageal pressurization that spans the full length of the esophagus with at least 20% of wet swallows, followed often by EGJ contraction post swallow (110). Of the three subtypes, patients with achalasia subtype II show homogeneity of esophageal pressure pattern from swallow to swallow and elegant studies utilising HRM-impedance concurrently with intraluminal ultrasound images, suggest esophageal longitudinal muscles are responsible for esophageal emptying in subtype II (104,110). With each swallow, esophageal pressurization relates to a decrease in lumen size, increase in esophageal wall muscle thickness and an increase in luminal pressures. The observed pan esophageal pressurization most likely arises from longitudinal muscle elevating luminal cavity pressure, rather than non-lumen occlusive circular muscle contractions (111).
Esophageal body pan-pressurization is a distinctive feature of achalasia subtype II. Regression tree analysis of HRM dataset reveals that the presence of esophageal body pan-pressurization in of itself is sufficient to establish the diagnosis of achalasia subtype II, without consideration of IRP. This statement seemingly contravenes the hierarchical analysis approach (Figure 2): first EGJ IRP, then esophageal body pressures, but it actually does not! The analysis simply establishes that esophageal pan-pressurization does not occur without EGJ outflow obstruction (86).
Achalasia subtype III
When classifying achalasia into different subtypes, inter- and intra-rater agreement is reported highest for classification of subtype III (103). In contrast, a study classifying a broader range of motility disorders, for 36 participants registered with the international HRM Working Group, inter-rater agreement was substantial for aperistalsis, while moderate for achalasia subtype III (kappa 0.66; 0.56 respectively) (108). Similarly, in a multi-center study of gastroenterology trainees undertaking HRM training and competency assessment for a range of motility disorders, diagnostic accuracy for achalasia subtype III was lower (58%) than for subtype I (87%) or subtype II (77%) (14). The co-presentation of incomplete EGJ relaxation (abnormal IRP) with spastic, premature or abnormal esophageal contractions of subtype III is very different from the other achalasia subtypes. However, there is heterogeneity of this pressure pattern in subtype III (111), requiring careful attention to the HRM metrics and CCv3.0 hierarchy for diagnostic interpretation. For the untrained, the presence of spastic esophageal contractions may lead to a presumptive diagnosis of distal esophageal spasm, when in fact CCv3.0 provides guidance: if simultaneous contractions occur together with incomplete EGJ relaxation, then the diagnosis is not distal esophageal spasm but achalasia subtype III (13,108).
Two seminal papers expand our understanding of achalasia subtype III pathophysiology (111,112). A focus on esophageal contraction pattern and bolus clearance (combining HRM, impedance, and ultrasound), revealed that in subtype III esophageal contraction onset appears simultaneous, while the contraction peak and termination appear sequential. An observation compatible with esophageal contractions of short DL. The baseline esophageal wall muscle thickness (circular & longitudinal) are reported larger in subtype III compared with normal subjects, while axial shortening is similar. The authors speculate that muscle hypertrophy results in poor distensibility of the esophagus (112). Further, due to the short DL, a bolus travels much closer to the contraction wave, with elevated common cavity pressure resulting from the bolus being trapped or compressed between esophageal contractions and the incompletely relaxed EGJ. Thus in subtype III, there is delayed bolus arrival to the distal esophagus with shorter dwell time—a potential mechanism of dysphagia symptom generation and a pattern observed in patients susceptible to dysphagia post-fundoplication (111,113).
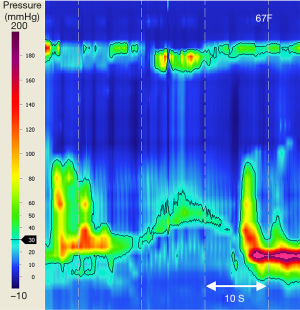
The CCv3.0 criteria for achalasia subtype III include incomplete or absent EGJ relaxation (abnormal IRP) and at least 20% of water swallows with esophageal contractions of short DL (<4.5 s). Adjunct swallow challenge test with RDC can confirm achalasia diagnosis by demonstrating EGJ dysfunction when HRM metrics for standard water swallows is inconclusive (Figure 5) (65,66,114). Doubt or debate may arise regarding diagnostic characteristics of achalasia subtype III cf. EGJ outflow obstruction (EGJOO). In EGJOO there is impaired EGJ relaxation with preserved primary peristalsis i.e., abnormal IRP, but intact peristalsis with normal DL for all standard wet swallows. A recent study proposes the addition of five, 5 mL liquid swallows during upright posture, with a 94% negative predictive value for revealing patients in whom IRP normalises during upright posture (77). EGJOO is raised as a possible early stage or variant of achalasia, an “achalasia subtype IV” (7,80). Whether EGJOO is a separate entity or whether a select subtype of EGJOO is an early or variant of achalasia is an unresolved debate (7,115,116). Very few EGJOO progress to achalasia (115,117-119). Of note, incidental discovery of EGJOO (after exclusion of pseudoachalasia) is best managed conservatively, often with spontaneous resolution of symptoms (120,121).
Limitations
Limitations of HRM and CCv3.0 specific for achalasia includes technical or procedural issues, variation in pathophysiological presentation, and interpretation of measurements (Table 2).
Table 2
| Limitations | Solutions |
|---|---|
| Technical or procedural issues | |
| Technical (equipment errors) | Systematic check of equipment prior to procedure |
| Catheter placement | |
| Inability to traverse the LES | Change patient posture to L- or R-lateral |
| Radiologic/Endoscopic catheter placement | |
| Catheter curling in the esophagus | Withdraw to 30 cm, alter posture, advance gently |
| Radiologic/Endoscopic catheter placement | |
| Procedural intolerance | Alternate tests supportive of diagnosis: sedated manometry, radiology (VFSS/TBE) or FLIP |
| MDT discussion | |
| Esophageal pooling of ingested contents with study progression (may limit study duration) | Care in interpretation. Alternate tests supportive of diagnosis: radiology (VFSS/TBE) or FLIP |
| Analysis of high-resolution manometry data | |
| Pooling of esophageal content makes interpretation of bolus presence |
Switch from color contour to line-plot mode |
| Use of esophageal pressure-impedance analysis | |
| Difficulty identifying contractile deceleration point (critical for determining DL) | Manometric training and certification |
| Switch from color contour to line-plot mode | |
| Use of esophageal pressure-impedance analysis | |
| IRP-4s (median) within normal range with high index of suspicion (may occur: subtype I achalasia) | Upright liquid swallows, for upright IRP-4s |
| Additional manometric maneuvers: MRS, RDC. Alternate tests supportive of diagnosis: radiology (VFSS/TBE) or FLIP. MDT discussion | |
| EGJ ‘pseudorelaxation’ due to esophageal shortening from longitudinal muscle contraction | Manometric training and certification |
| Careful study interpretation | |
| Incomplete transient lower esophageal sphincter relaxation (LES elevation without LES relaxation) | Manometric training and certification |
| Careful study interpretation | |
| Use of esophageal pressure-impedance analysis | |
| Inability to separate LES from crural diaphragm when aligned as EGJ | 3D HRM. Radiology: VFSS/TBE |
| Novel metrics e.g., EGJ-contractile integral | |
| Use of esophageal pressure-impedance analysis | |
| Hiatus hernia | Corroborate interpretation with alternate imaging sources: endoscopy, radiology |
| Inability to separate circular and longitudinal muscle activity | Complex issue. Currently requires endoluminal-ultrasound. Not part of diagnosis of achalasia |
| Classification using Chicago Classification v3.0 | |
| Partial expression of achalasia or variants | Upright liquid swallow IRP-4s |
| Additional manometric maneuvers: MRS, RDC. Alternate test supportive of diagnosis: radiology (VFSS/ TBE) or FLIP. MDT discussion | |
| Pseudoachalasia suspected | Review clinical presentation: symptom duration; check history for prior upper GI surgery |
| Endoscopy with EGJ biopsies to exclude pathology and/or endoscopic ultrasonography. CT chest/abdomen. MDT discussion | |
| Failure to exclude opioids prior to HRM study | Cease opioids (as possible) for 24 hr prior to manometry. Careful study interpretation |
| Additional considerations | |
| Appropriate high-quality interpretation | Manometric training and certification |
| Decreased EGJ relaxation (elevated IRP) in patients over 80 years of age | Careful study interpretation |
| Care with IRP threshold; obtain supportive evidence | |
| Use of nitrates to induce LES relaxation | Care should be taken due to small but serious risk of medication interactions or cardiac events |
HRM, high-resolution manometry; LES, lower esophageal sphincter; VFSS, video-fluoroscopic swallowing study; TBE, timed barium esophagram; FLIP, functional lumen imaging probe; MDT, multi-disciplinary team; IRP-4s, integrated relaxation pressure (4 sec); DL, distal latency (sec); MRS, multiple rapid swallows; RDC, rapid drinking challenge; EGJ, esophagogastric junction; 3D, three dimensional.
Procedural limitations
The main technical issue for HRM in achalasia is the motility catheter curling in the esophagus or not traversing the EGJ (discussed earlier). HRM analysis requires IRP of the EGJ relative to gastric baseline, so traversing the EGJ is essential for diagnosis (60,122). It is possible this limitation may not be recognized during or even following manometry, as a bent segment of the catheter coiled in the esophageal body, may lead to a distortion of pressure on the HRM recording misinterpreted as LES/EGJ pressure (58,123,124). Radiology guided catheter placement may overcome this limitation. Alternatively, the HRM catheter can be placed under endoscopic vision although this is resource intensive, carries risks associated with sedation including aspiration, plus the influence of conscious sedation agents on esophageal motility and LES pressure is controversial (57,125-127).
During acquisition, esophageal HRM pressure profiles in achalasia may show pooling of esophageal contents with successive water swallows; atypical esophageal or EGJ pressure patterns for achalasia; residual EGJ pressure on swallowing within the normal range; and longitudinal muscle pull-up of the LES impacting measurement of EGJ pressure. Awareness of these pressure variations during acquisition can prompt inclusion of adjunct swallow challenge tests or protocol variations, and need to be taken into account when applying CCv3.0 for a motility diagnosis.
HRM analysis limitations
Pooling of esophageal contents elevates esophageal basal pressures, which may create difficulty for recognizing the onset of esophageal contractions or pan-pressurization (111). Switching HRM display mode from color contour to pressure line plot or the use of impedance-based pressure-flow analysis (128) is often helpful to differentiate cavity pressure from compartmentalized esophageal pressurization; for placement of HRM analysis markers; and for identifying the contractile deceleration point, critical for determining DL (13).
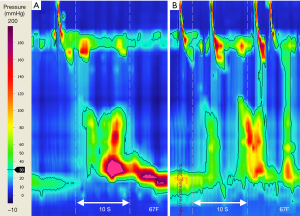
During swallowing, esophageal longitudinal muscle contraction results in proximal ‘pull-up’ of the LES component of the EGJ, sometimes by several centimeters, which if unrecognized can be mis-interpreted as sphincter relaxation (EGJ pseudorelaxation) (56). Consequently HRM metrics will fail to meet criteria for achalasia, with misinterpretation as jackhammer esophagus or distal esophageal spasm (129). During position change to upright posture, incomplete transient LES relaxation may show a similar manometric profile with LES elevation (Figure 6) (7,130). Studies are needed to assess accuracy of achalasia subtype III diagnoses, where spastic esophageal contractions may mask esophageal shortening and LES elevation.
During HRM, we are primarily recording the intraluminal pressure effects of circular muscle, not longitudinal muscle contractions, with an often-overlooked limitation of HRM: the inability to measure these separately. A few studies to date, using high-frequency intraluminal ultrasound in addition to HRM, show altered interactions of circular and longitudinal muscles in achalasia (110-112), thought to be a consequence of LES dysfunction. This provides insights into the variation in pathophysiology of achalasia subtypes, but more studies are needed to advance this further.
Similarly, in the absence of a hiatus hernia, HRM is unable to record separately LES and crural diaphragm pressure. When the LES and the crural diaphragm are aligned, EGJ pressure represents the combined synergistic pressure effects of both intrinsic LES and extrinsic crural diaphragm. Achalasia affects only LES but not crural diaphragm pressure, which means intraluminal pressure sensors measure EGJ relaxation, not LES relaxation (56). New 3D HRM study found an asymmetric EGJ pressure peak (greater curvature side) and localized the magnitude and orientation of crural diaphragm and LES pressure in achalasia, and holds great potential for future studies (131).
Chicago classification limitations
Partial expression or atypical variants of achalasia pose a diagnostic challenge and are currently not part of CCv3.0 (7,56,84). HRM does not capture all achalasia, but it does provide clues. Partial expression of achalasia includes aperistalsis where residual EGJ pressure on swallowing is within the normal range (normal IRP) and also cases of aperistalsis or abnormal esophageal contractions not meeting the criteria for subtype I, II or III (7) (Figure 4). This currently unclassified group warrant further study (132).
CCv3.0 does not classify opioid induced esophageal dysfunction, manifesting as elevated resting & reduced EGJ relaxation pressure, and LES or esophageal hyper-contractility, including short DL (i.e., mimicking achalasia subtype III) (133). A large retrospective study of 66 patients studied while taking opioids (98% short-acting), found 83% showed dysmotility: 45% distal esophageal spasm; 27% EGJOO; and 11% achalasia subtype III (134). Use of nitrates to induce LES relaxation may provide clarity (135).
With each iteration of the Chicago classification, the diagnosis of achalasia has been refined and yet with increasing complexity! The main issues identified for inter-observer disagreement are: multiple abnormalities in a single study; failure to apply the hierarchical classification scheme; lack of HRM metrics that defined pressure thresholds (i.e., IRP), and recognition of the frequency of dysmotility required for objective diagnosis (e.g., least 20% of esophageal contractions of short DL <4.5 s) (108). As competency in motility testing is not easy to obtain, particularly for interpreting complex HRM studies (14,15), more attention should be given to include supplementary educational material with the next version of the Chicago classification (v4.0).
Novel use of adjunct manometric testing in equivocal cases of achalasia
In certain circumstances (described above) the diagnosis of achalasia may be equivocal, yet a correct diagnosis is essential for correct treatment. Furthermore, there are conditions which may mimic achalasia. The classic example of this is ‘pseudoachalasia’ due to a distal esophageal malignancy (136), but recently eosinophilic esophagitis has also emerged as a potential mimic of achalasia (137-139). The use of adjunct testing during manometry, additional investigations, and expert consensus can facilitate correct diagnosis of achalasia.
To clarify observations and to overcome the limitations of dysmotility not revealed by small bolus swallows (140), at least one form of adjunct testing is recommend by the British Society of Gastroenterology (53,58,141). A review of the literature to date supports a 200 mL RDC in upright posture (65,74,107). However, if high aspiration risk or elderly, consider three MRS or five upright swallows (73,142). ‘Which adjunct test is most appropriate to clarify achalasia?’ is an important research question for a future version of the Chicago classification!
Beyond HRM, additional investigations include use of a functional luminal imaging probe (FLIP) during endoscopy (143-145), dynamic video fluoroscopic swallowing studies (VFSS) (146,147), and a quantitative advancement, timed barium esophagram (TBE) (148) (Table 2). These technological advancements (discussed in other articles of this issue) provide the additional necessary evidence for definitive diagnosis of achalasia.
Several additional recent advancements in manometry may provide future direction relevant to patients with dysphagia and achalasia: (I) a new metric, EGJ contractile integral (EGJ-CI), which applies the concept of contractile integral (amplitude, axial length, duration) to the EGJ to quantify EGJ barrier pressure (unit: mmHg.cm), with potential to assess adequacy of achalasia treatments (149); (II) use of HRM with impedance, a logical extension of HRM, to enable detection of bolus movement e.g., a metric for bolus flow time across the EGJ, and also impedance bolus height, to assess bolus retention with promising utility for severity of impaired EGJ outflow and correlation with dysphagia in achalasia (150,151). Of note, impedance hardware is an additional expense and the inclusion of impedance rings makes for a stiffer catheter, which can cause difficulty traversing the EGJ; (III) automated impedance manometry (AIM) plot with pressure-flow analysis, which uniquely quantifies bolus movement relative to pressure events of the esophagus and EGJ (128). Recently applied to a paediatric achalasia cohort, the automated analysis generates HRIM metrics. When used in conjunction with software-driven diagnosis, supplemented by experienced observer interpretation, shows high intra-and inter- reliability for achalasia subtyping (kappa 0.98; 0.92 respectively) (152); (IV) Upright IRP, useful to unmask cases of subtle LES/EGJ obstruction (66,77). An IRP-4s above 12 mmHg identifies patients with radiographic EGJ outflow obstruction (98% sensitivity; 16% specificity) (ManoScan™ system).
Conclusions
High resolution manometry is recommended for achalasia diagnosis and specific spatio-temporal metrics informs the structured approach of the Chicago classification for diagnosis of primary achalasia, categorized into three subtypes I, II or III. Achalasia subtypes provide greater understanding of the severity of achalasia at presentation and guide treatment decisions. Adjunct swallow challenge tests overcome the limitations of dysmotility not revealed by small bolus swallows. More research is needed to advance our understanding of the underlying pathophysiology. Meanwhile, whenever HRM reveals a non-relaxing EGJ, achalasia subtypes are front and center of the hierarchical Chicago classification process, with improved consistency of diagnosis.
Acknowledgments
With gratitude to Rachel Davey, Certified Reference Librarian, SA Health, for support with implementing the literature search strategy.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sarah Thompson) for the series “Achalasia” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2019-ach-10). The series “Achalasia” was commissioned by the editorial office without any funding or sponsorship. JCM reports personal fees from Medtronic Australasia Pty Ltd., outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carlson DA, Gyawali CP. Is High-Resolution Manometry Always Needed for the Diagnosis of Achalasia? Clin Gastroenterol Hepatol 2018;16:480-2. [Crossref] [PubMed]
- Pandolfino JE, Gawron AJ. Achalasia: a systematic review. JAMA 2015;313:1841-52. [Crossref] [PubMed]
- Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005;128:209-24. [Crossref] [PubMed]
- Howard PJ, Maher L, Pryde A, et al. Five year prospective study of the incidence, clinical features, and diagnosis of achalasia in Edinburgh. Gut 1992;33:1011-5. [Crossref] [PubMed]
- Akaishi T, Nakano T, Machida T, et al. Clinical Usefulness of Endoscopy, Barium Fluoroscopy, and Chest Computed Tomography for the Correct Diagnosis of Achalasia. Intern Med 2020;59:323-8. [Crossref] [PubMed]
- Niebisch S, Hadzijusufovic E, Mehdorn M, et al. Achalasia-an unnecessary long way to diagnosis. Dis Esophagus 2017; [Crossref] [PubMed]
- Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology 2013;145:954-65. [Crossref] [PubMed]
- Pohl D, Tutuian R. Achalasia: an overview of diagnosis and treatment. J Gastrointestin Liver Dis 2007;16:297-303. [PubMed]
- Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut 2008;57:405-23. [Crossref] [PubMed]
- Soudagar AS, Sayuk GS, Gyawali CP. Learners favour high resolution oesophageal manometry with better diagnostic accuracy over conventional line tracings. Gut 2012;61:798-803. [Crossref] [PubMed]
- Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol 2008;103:27-37. [Crossref] [PubMed]
- Pandolfino JE, Fox MR, Bredenoord AJ, et al. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil 2009;21:796-806. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Yadlapati R, Keswani RN, Ciolino JD, et al. A System to Assess the Competency for Interpretation of Esophageal Manometry Identifies Variation in Learning Curves. Clin Gastroenterol Hepatol 2017;15:1708-14.e3. [Crossref] [PubMed]
- Kraft C, Kathpalia P, Baumgardner JM, et al. How to Incorporate Esophageal Manometry Teaching in Your Fellowship Program. Gastroenterology 2019;156:2120-3. [Crossref] [PubMed]
- Beck WC, Sharp KW. Achalasia. Surg Clin North Am 2011;91:1031-7. [Crossref] [PubMed]
- Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet 2014;383:83-93. [Crossref] [PubMed]
- Hertz AF. Achalasia of the Cardia (so-called Cardio-spasm). Proc R Soc Med 1915;8:22-5. [Crossref] [PubMed]
- D'Journo XB, Ferraro P, Martin J, et al. Lower oesophageal sphincter dysfunction is part of the functional abnormality in epiphrenic diverticulum. Br J Surg 2009;96:892-900. [Crossref] [PubMed]
- Dellon ES. Eosinophilic esophagitis. Gastroenterol Clin North Am 2013;42:133-53. [Crossref] [PubMed]
- Greig DM. OEsophageal Achalasia. Edinb Med J 1922;29:217-28. [PubMed]
- Code CF, Fyke FE Jr, Schlegel JF. The gastroesophageal sphincter in healthy human beings. Gastroenterologia 1956;86:135-50. [Crossref] [PubMed]
- Code CF, Creamer B, Schlegel JF, et al. An Atlas of Esophageal Motility in Health and Disease. Springfield, Illinois, USA: 1958.
- Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut 2001;49:145-51. [Crossref] [PubMed]
- Richter JE. Oesophageal motility disorders. Lancet 2001;358:823-8. [Crossref] [PubMed]
- Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol 1991;261:G677-84. [PubMed]
- Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil 2004;16:533-42. [Crossref] [PubMed]
- Gyawali CP. High resolution manometry: the Ray Clouse legacy. Neurogastroenterol Motil 2012;24:2-4. [Crossref] [PubMed]
- Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: the Chicago Classification. J Clin Gastroenterol 2008;42:627-35. [Crossref] [PubMed]
- Nicodème F, Pandolfino JE. Esophageal Disorders Not Yet Addressed by High-resolution Manometry. J Neurogastroenterol Motil 2013;19:114-5. [Crossref] [PubMed]
- Schizas D, Kapsampelis P, Tsilimigras DI, et al. The 100 most cited manuscripts in esophageal motility disorders: a bibliometric analysis. Ann Transl Med 2019; [Crossref] [PubMed]
- Basilisco G, Bharucha AE. High-resolution anorectal manometry: An expensive hobby or worth every penny? Neurogastroenterol Motil 2017; [Crossref] [PubMed]
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20; quiz 43.
- Reid BJ, Li X, Galipeau PC, et al. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer 2010;10:87-101. [Crossref] [PubMed]
- Navaneethan U, Eubanks S. Approach to Patients with Esophageal Dysphagia. Surg Clin North Am 2015;95:483-9. [Crossref] [PubMed]
- Katz PO, Dalton CB, Richter JE, et al. Esophageal testing of patients with noncardiac chest pain or dysphagia. Results of three years' experience with 1161 patients. Ann Intern Med 1987;106:593-7. [Crossref] [PubMed]
- Fisichella PM, Raz D, Palazzo F, et al. Clinical, radiological, and manometric profile in 145 patients with untreated achalasia. World J Surg 2008;32:1974-9. [Crossref] [PubMed]
- Rohof WOA, Bredenoord AJ. Chicago Classification of Esophageal Motility Disorders: Lessons Learned. Curr Gastroenterol Rep 2017; [Crossref] [PubMed]
- Jeon HH, Kim JH, Youn YH, et al. Clinical Characteristics of Patients with Untreated Achalasia. J Neurogastroenterol Motil 2017;23:378-84. [Crossref] [PubMed]
- Newberry C, Vajravelu RK, Pickett-Blakely O, et al. Achalasia Patients Are at Nutritional Risk Regardless of Presenting Weight Category. Dig Dis Sci 2018;63:1243-9. [Crossref] [PubMed]
- Patel DA, Naik R, Slaughter JC, et al. Weight loss in achalasia is determined by its phenotype. Dis Esophagus 2018; [Crossref] [PubMed]
- Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 1992;103:1732-8. [Crossref] [PubMed]
- Taft TH, Carlson DA, Triggs J, et al. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterol Motil 2018; [Crossref] [PubMed]
- Dakkak M, Bennett JR. A new dysphagia score with objective validation. J Clin Gastroenterol 1992;14:99-100. [Crossref] [PubMed]
- McElhiney J, Lohse MR, Arora AS, et al. The Mayo Dysphagia Questionnaire-30: documentation of reliability and validity of a tool for interventional trials in adults with esophageal disease. Dysphagia 2010;25:221-30. [Crossref] [PubMed]
- Urbach DR, Tomlinson GA, Harnish JL, et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol 2005;100:1668-76. [Crossref] [PubMed]
- Ponds FA, van Raath MI, Mohamed SMM, et al. Diagnostic features of malignancy-associated pseudoachalasia. Aliment Pharmacol Ther 2017;45:1449-58. [Crossref] [PubMed]
- Kaindlstorfer A, Pointner R. An appraisal of current dysphagia diagnosis and treatment strategies. Expert Rev Gastroenterol Hepatol 2016;10:929-42. [PubMed]
- Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol 2013;108:1238-49; quiz 50. [Crossref] [PubMed]
- Dempsey DT. Barium upper GI series in adults: a surgeon's perspective. Abdom Radiol (NY) 2018;43:1323-8. [Crossref] [PubMed]
- El-Takli I, O'Brien P, Paterson WG. Clinical diagnosis of achalasia: how reliable is the barium x-ray? Can J Gastroenterol 2006;20:335-7. [Crossref] [PubMed]
- Yamasaki T, Tomita T, Mori S, et al. Esophagography in Patients With Esophageal Achalasia Diagnosed With High-resolution Esophageal Manometry. J Neurogastroenterol Motil 2018;24:403-9. [Crossref] [PubMed]
- Trudgill NJ, Sifrim D, Sweis R, et al. British Society of Gastroenterology guidelines for oesophageal manometry and oesophageal reflux monitoring. Gut 2019;68:1731-50. [Crossref] [PubMed]
- Bredenoord AJ, Hebbard GS. Technical aspects of clinical high-resolution manometry studies. Neurogastroenterol Motil 2012;24:5-10. [Crossref] [PubMed]
- Gyawali CP, de Bortoli N, Clarke J, et al. Indications and interpretation of esophageal function testing. Ann N Y Acad Sci 2018;1434:239-53. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. Expert consensus document: Advances in the management of oesophageal motility disorders in the era of high-resolution manometry: a focus on achalasia syndromes. Nat Rev Gastroenterol Hepatol 2017;14:677-88. [Crossref] [PubMed]
- Tariq H, Makker J, Chime C, et al. Revisiting the Reliability of the Endoscopy and Sedation-Assisted High-Resolution Esophageal Motility Assessment. Gastroenterology Res 2019;12:157-65. [Crossref] [PubMed]
- Sanagapalli S, Sweis R. Achalasia: It Is Not All Black and White. Curr Gastroenterol Rep 2017; [Crossref] [PubMed]
- Roman S, Kahrilas PJ, Boris L, et al. High-resolution manometry studies are frequently imperfect but usually still interpretable. Clin Gastroenterol Hepatol 2011;9:1050-5. [Crossref] [PubMed]
- Gyawali CP. Making the most of imperfect high-resolution manometry studies. Clin Gastroenterol Hepatol 2011;9:1015-6. [Crossref] [PubMed]
- Carlson DA, Kahrilas PJ. How to Effectively Use High-Resolution Esophageal Manometry. Gastroenterology 2016;151:789-92. [Crossref] [PubMed]
- Ask P, Tibbling L. Effect of time interval between swallows on esophageal peristalsis. Am J Physiol 1980;238:G485-90. [PubMed]
- Dhawan I, O'Connell B, Patel A, et al. Utility of Esophageal High-Resolution Manometry in Clinical Practice: First, Do HRM. Dig Dis Sci 2018;63:3178-86. [Crossref] [PubMed]
- Sweis R, Heinrich H, Fox M, et al. Variation in esophageal physiology testing in clinical practice: Results from an international survey. Neurogastroenterol Motil 2018; [Crossref] [PubMed]
- Carlson DA, Roman S. Esophageal provocation tests: Are they useful to improve diagnostic yield of high resolution manometry? Neurogastroenterol Motil 2018; [Crossref] [PubMed]
- Misselwitz B, Hollenstein M, Butikofer S, et al. Prospective serial diagnostic study: the effects of position and provocative tests on the diagnosis of oesophageal motility disorders by high-resolution manometry. Aliment Pharmacol Ther 2020; [Crossref] [PubMed]
- Fornari F, Bravi I, Penagini R, et al. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil 2009;21:718-e41. [Crossref] [PubMed]
- Price LH, Li Y, Patel A, et al. Reproducibility patterns of multiple rapid swallows during high resolution esophageal manometry provide insights into esophageal pathophysiology. Neurogastroenterol Motil 2014;26:646-53. [Crossref] [PubMed]
- Mauro A, Savarino E, De Bortoli N, et al. Optimal number of multiple rapid swallows needed during high-resolution esophageal manometry for accurate prediction of contraction reserve. Neurogastroenterol Motil 2018; [Crossref] [PubMed]
- Shaker A, Stoikes N, Drapekin J, et al. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol 2013;108:1706-12. [Crossref] [PubMed]
- Savojardo D, Mangano M, Cantu P, et al. Multiple rapid swallowing in idiopathic achalasia: evidence for patients' heterogeneity. Neurogastroenterol Motil 2007;19:263-9. [Crossref] [PubMed]
- Leopold A, Yu D, Bhuta R, et al. Multiple Rapid Swallows (MRS) Complements Single-Swallow (SS) Analysis for High-Resolution Esophageal Manometry (HREM). Dig Dis Sci 2019;64:2206-13. [Crossref] [PubMed]
- Kushnir V, Sayuk GS, Gyawali CP. Multiple rapid swallow responses segregate achalasia subtypes on high-resolution manometry. Neurogastroenterol Motil 2012;24:1069-e561. [Crossref] [PubMed]
- Woodland P, Gabieta-Sonmez S, Arguero J, et al. 200 mL Rapid Drink Challenge During High-resolution Manometry Best Predicts Objective Esophagogastric Junction Obstruction and Correlates With Symptom Severity. J Neurogastroenterol Motil 2018;24:410-4. [Crossref] [PubMed]
- Marin I, Caballero N, Guarner-Argente C, et al. Rapid drink challenge test for the clinical evaluation of patients with Achalasia. Neurogastroenterol Motil 2018; [Crossref] [PubMed]
- Marin I, Serra J. Patterns of esophageal pressure responses to a rapid drink challenge test in patients with esophageal motility disorders. Neurogastroenterol Motil 2016;28:543-53. [Crossref] [PubMed]
- Triggs JR, Carlson DA, Beveridge C, et al. Upright Integrated Relaxation Pressure Facilitates Characterization of Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol 2019;17:2218-26.e2. [Crossref] [PubMed]
- Chuah SK, Lim CS, Liang CM, et al. Bridging the Gap between Advancements in the Evolution of Diagnosis and Treatment towards Better Outcomes in Achalasia. Biomed Res Int 2019; [Crossref] [PubMed]
- van Hoeij FB, Bredenoord AJ. Clinical Application of Esophageal High-resolution Manometry in the Diagnosis of Esophageal Motility Disorders. J Neurogastroenterol Motil 2016;22:6-13. [Crossref] [PubMed]
- Richter JE. High-resolution manometry in diagnosis and treatment of achalasia: help or hype. Curr Gastroenterol Rep 2014; [Crossref] [PubMed]
- Khan AA, Shah SW, Khan MA, et al. Hiatal hernia in achalasia. J Pak Med Assoc 1998;48:196-7. [PubMed]
- Ott DJ, Hodge RG, Chen MY, et al. Achalasia associated with hiatal hernia: prevalence and potential implications. Abdom Imaging 1993;18:7-9. [Crossref] [PubMed]
- Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol 2007;293:G878-85. [Crossref] [PubMed]
- Bredenoord AJ, Fox M, Kahrilas PJ, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil 2012;24:57-65. [Crossref] [PubMed]
- Herregods TV, Roman S, Kahrilas PJ, et al. Normative values in esophageal high-resolution manometry. Neurogastroenterol Motil 2015;27:175-87. [Crossref] [PubMed]
- Lin Z, Kahrilas PJ, Roman S, et al. Refining the criterion for an abnormal Integrated Relaxation Pressure in esophageal pressure topography based on the pattern of esophageal contractility using a classification and regression tree model. Neurogastroenterol Motil 2012;24:e356-63. [Crossref] [PubMed]
- Besanko LK, Burgstad CM, Cock C, et al. Changes in esophageal and lower esophageal sphincter motility with healthy aging. J Gastrointestin Liver Dis 2014;23:243-8. [Crossref] [PubMed]
- Cock C, Omari T. Systematic Review of Pharyngeal and Esophageal Manometry in Healthy or Dysphagic Older Persons (>60 years). Geriatrics (Basel) 2018. doi:
10.3390/geriatrics3040067 . - Jung KW, Jung HY, Myung SJ, et al. The effect of age on the key parameters in the Chicago classification: a study using high-resolution esophageal manometry in asymptomatic normal individuals. Neurogastroenterol Motil 2015;27:246-57. [Crossref] [PubMed]
- Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 2008;135:1526-33. [Crossref] [PubMed]
- Salvador R, Costantini M, Zaninotto G, et al. The preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. J Gastrointest Surg 2010;14:1635-45. [Crossref] [PubMed]
- Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 2013;144:718-25; quiz e13-4.
- Boeckxstaens G, Zaninotto G. Achalasia and esophago-gastric junction outflow obstruction: focus on the subtypes. Neurogastroenterol Motil 2012;24:27-31. [Crossref] [PubMed]
- Lee JY, Kim N, Kim SE, et al. Clinical characteristics and treatment outcomes of 3 subtypes of achalasia according to the chicago classification in a tertiary institute in Korea. J Neurogastroenterol Motil 2013;19:485-94. [Crossref] [PubMed]
- Pratap N, Kalapala R, Darisetty S, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil 2011;17:48-53. [Crossref] [PubMed]
- Hamer PW, Holloway RH, Heddle R, et al. Evaluation of outcome after cardiomyotomy for achalasia using the Chicago classification. Br J Surg 2016;103:1847-54. [Crossref] [PubMed]
- Crespin OM, Tatum RP, Xiao K, et al. The relationship between manometric subtype and outcomes of surgical treatment for patients with achalasia: Achalasia: manometric subtypes. Surg Endosc 2017;31:5066-75. [Crossref] [PubMed]
- Patel A, Patel A, Mirza FA, et al. Achalasia symptom response after Heller myotomy segregated by high-resolution manometry subtypes. J Gastroenterol 2016;51:112-8. [Crossref] [PubMed]
- Kim GH, Jung KW, Jung HY, et al. Superior clinical outcomes of peroral endoscopic myotomy compared with balloon dilation in all achalasia subtypes. J Gastroenterol Hepatol 2019;34:659-65. [Crossref] [PubMed]
- Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut 2016;65:732-9. [Crossref] [PubMed]
- Oude Nijhuis RAB, Prins LI, Mostafavi N, et al. Factors Associated With Achalasia Treatment Outcomes: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2020;18:1442-53. [PubMed]
- Andolfi C, Fisichella PM. Meta-analysis of clinical outcome after treatment for achalasia based on manometric subtypes. Br J Surg 2019;106:332-41. [Crossref] [PubMed]
- Hernandez JC, Ratuapli SK, Burdick GE, et al. Interrater and intrarater agreement of the chicago classification of achalasia subtypes using high-resolution esophageal manometry. Am J Gastroenterol 2012;107:207-14. [Crossref] [PubMed]
- Mittal RK, Hong SJ, Bhargava V. Longitudinal muscle dysfunction in achalasia esophagus and its relevance. J Neurogastroenterol Motil 2013;19:126-36. [Crossref] [PubMed]
- Zanoni A, Rice TW, Lopez R, et al. Timed barium esophagram in achalasia types. Dis Esophagus 2015;28:336-44. [Crossref] [PubMed]
- Nicodeme F, de Ruigh A, Xiao Y, et al. A comparison of symptom severity and bolus retention with Chicago classification esophageal pressure topography metrics in patients with achalasia. Clin Gastroenterol Hepatol 2013;11:131-7; quiz e15.
- Sanagapalli S, Roman S, Hastier A, et al. Achalasia diagnosed despite normal integrated relaxation pressure responds favorably to therapy. Neurogastroenterol Motil 2019; [Crossref] [PubMed]
- Fox MR, Pandolfino JE, Sweis R, et al. Inter-observer agreement for diagnostic classification of esophageal motility disorders defined in high-resolution manometry. Dis Esophagus 2015;28:711-9. [Crossref] [PubMed]
- Coss-Adame E, Vargas-Vorackova F, Valdovinos MA. Multiple Rapid Swallowing May Change Manometric Subtype Classification in Achalasia Patients. Gastrenterology 2011;140:S227-8. [Crossref]
- Hong SJ, Bhargava V, Jiang Y, et al. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology 2010;139:102-11. [Crossref] [PubMed]
- Park S, Zifan A, Kumar D, et al. Genesis of Esophageal Pressurization and Bolus Flow Patterns in Patients With Achalasia Esophagus. Gastroenterology 2018;155:327-36. [Crossref] [PubMed]
- Kim TH, Patel N, Ledgerwood-Lee M, et al. Esophageal contractions in type 3 achalasia esophagus: simultaneous or peristaltic? Am J Physiol Gastrointest Liver Physiol 2016;310:G689-95. [Crossref] [PubMed]
- Myers JC, Nguyen NQ, Jamieson GG, et al. Susceptibility to dysphagia after fundoplication revealed by novel automated impedance manometry analysis. Neurogastroenterol Motil 2012;24:812-20, e392-3.
- Biasutto D, Mion F, Garros A, et al. Rapid drink challenge test during esophageal high resolution manometry in patients with esophago-gastric junction outflow obstruction. Neurogastroenterol Motil 2018; [Crossref] [PubMed]
- Lynch KL, Yang YX, Metz DC, et al. Clinical presentation and disease course of patients with esophagogastric junction outflow obstruction. Dis Esophagus 2017; [Crossref] [PubMed]
- Ihara E, Muta K, Fukaura K, et al. Diagnosis and Treatment Strategy of Achalasia Subtypes and Esophagogastric Junction Outflow Obstruction Based on High-Resolution Manometry. Digestion 2017;95:29-35. [Crossref] [PubMed]
- Liu A, Woo M, Nasser Y, et al. Esophagogastric junction outflow obstruction on manometry: Outcomes and lack of benefit from CT and EUS. Neurogastroenterol Motil 2019; [Crossref] [PubMed]
- van Hoeij FB, Smout AJ, Bredenoord AJ. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil 2015;27:1310-6. [Crossref] [PubMed]
- Shin IS, Min YW, Rhee PL. Esophagogastric Junction Outflow Obstruction Transformed to Type II Achalasia. J Neurogastroenterol Motil 2016;22:344-5. [Crossref] [PubMed]
- Zikos TA, Triadafilopoulos G, Clarke JO. Esophagogastric Junction Outflow Obstruction: Current Approach to Diagnosis and Management. Curr Gastroenterol Rep 2020; [Crossref] [PubMed]
- Pérez-Fernández MT, Santander C, Marinero A, et al. Characterization and follow-up of esophagogastric junction outflow obstruction detected by high resolution manometry. Neurogastroenterol Motil 2016;28:116-26. [Crossref] [PubMed]
- Dimitriu A, Gheorghe C. High Resolution Manometry - A Mandatory Examination in the Pre and Postoperative Assessment of Patients with Achalasia. Chirurgia (Bucur) 2018;113:61-9. [Crossref] [PubMed]
- Gyawali CP, Bredenoord AJ, Conklin JL, et al. Evaluation of esophageal motor function in clinical practice. Neurogastroenterol Motil 2013;25:99-133. [Crossref] [PubMed]
- Babaei A, Szabo A, Yorio SD, et al. Pressure exposure and catheter impingement affect the recorded pressure in the Manoscan 360 system. Neurogastroenterol Motil 2018; [Crossref] [PubMed]
- Marsh JK, Hoffman SM, Dmuchowski CF. Effect of intravenous midazolam on esophageal motility testing in normal human volunteers. Am J Gastroenterol 1993;88:860-3. [PubMed]
- Brun R, Staller K, Viner S, et al. Endoscopically assisted water perfusion esophageal manometry with minimal sedation: technique, indications, and implication on the clinical management. J Clin Gastroenterol 2011;45:759-63. [Crossref] [PubMed]
- Christian KE, Morris JD, Xie G. Endoscopy- and Monitored Anesthesia Care-Assisted High-Resolution Impedance Manometry Improves Clinical Management. Case Rep Gastrointest Med 2018; [Crossref] [PubMed]
- Omari TI, Szczesniak MM, Maclean J, et al. Correlation of esophageal pressure-flow analysis findings with bolus transit patterns on videofluoroscopy. Dis Esophagus 2016;29:166-73. [Crossref] [PubMed]
- Huang L, Rezaie A. Progression of Jackhammer Esophagus to Achalasia. J Neurogastroenterol Motil 2016;22:348-9. [Crossref] [PubMed]
- Kwiatek MA, Post J, Pandolfino JE, et al. Transient lower oesophageal sphincter relaxation in achalasia: everything but LOS relaxation. Neurogastroenterol Motil 2009;21:1294-e123. [Crossref] [PubMed]
- Guillaumot MA, Leandri C, Leblanc S, et al. Three-Dimensional High-Resolution Esophageal Manometry Study of the Esophagogastric Junction in Patients with Achalasia. Dig Dis Sci 2020;65:1092-8. [PubMed]
- Galey KM, Wilshire CL, Niebisch S, et al. Atypical variants of classic achalasia are common and currently under-recognized: a study of prevalence and clinical features. J Am Coll Surg 2011;213:155-61; discussion 62-3. [Crossref] [PubMed]
- Ortiz V, Garcia-Campos M, Saez-Gonzalez E, et al. A concise review of opioid-induced esophageal dysfunction: is this a new clinical entity? Dis Esophagus 2018; [Crossref] [PubMed]
- Ratuapli SK, Crowell MD, DiBaise JK, et al. Opioid-Induced Esophageal Dysfunction (OIED) in Patients on Chronic Opioids. Am J Gastroenterol 2015;110:979-84. [Crossref] [PubMed]
- Babaei A, Shad S, Massey BT. Motility Patterns Following Esophageal Pharmacologic Provocation With Amyl Nitrite or Cholecystokinin During High-Resolution Manometry Distinguish Idiopathic vs Opioid-Induced Type 3 Achalasia. Clin Gastroenterol Hepatol 2020;18:813-821.e1. [PubMed]
- Sandler RS, Bozymski EM, Orlando RC. Failure of clinical criteria to distinguish between primary achalasia and achalasia secondary to tumor. Dig Dis Sci 1982;27:209-13. [Crossref] [PubMed]
- Savarino E, Gemignani L, Zentilin P, et al. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin Gastroenterol Hepatol 2011;9:1104-6. [Crossref] [PubMed]
- Spechler SJ, Konda V, Souza R. Can Eosinophilic Esophagitis Cause Achalasia and Other Esophageal Motility Disorders? Am J Gastroenterol 2018;113:1594-9. [Crossref] [PubMed]
- Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 1978;74:1298-301. [Crossref] [PubMed]
- Fox MR, Kahrilas PJ, Roman S, et al. Clinical measurement of gastrointestinal motility and function: who, when and which test? Nat Rev Gastroenterol Hepatol 2018;15:568-79. [Crossref] [PubMed]
- Nikaki K, Ooi JL, Sifrim D. Chicago Classification of Esophageal Motility Disorders: Applications and Limits in Adults and Pediatric Patients with Esophageal Symptoms. Curr Gastroenterol Rep 2016; [Crossref] [PubMed]
- Schnoll-Sussman F, Katz PO. Managing Esophageal Dysphagia in the Elderly. Curr Treat Options Gastroenterol 2016;14:315-26. [Crossref] [PubMed]
- Ponds FA, Bredenoord AJ, Kessing BF, et al. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil 2017; [Crossref] [PubMed]
- Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of Esophageal Motility Utilizing the Functional Lumen Imaging Probe. Am J Gastroenterol 2016;111:1726-35. [Crossref] [PubMed]
- Carlson DA, Gyawali CP, Kahrilas PJ, et al. Esophageal motility classification can be established at the time of endoscopy: a study evaluating real-time functional luminal imaging probe panometry. Gastrointest Endosc 2019;90:915-23.e1. [Crossref] [PubMed]
- Schima W, Stacher G, Pokieser P, et al. Esophageal motor disorders: videofluoroscopic and manometric evaluation - prospective study in 88 symptomatic patients. Radiology 1992;185:487-91. [Crossref] [PubMed]
- Amaravadi R, Levine MS, Rubesin SE, et al. Achalasia with complete relaxation of lower esophageal sphincter: radiographic-manometric correlation. Radiology 2005;235:886-91. [Crossref] [PubMed]
- de Oliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol 1997;169:473-9. [Crossref] [PubMed]
- Wang D, Patel A, Mello M, et al. Esophagogastric junction contractile integral (EGJ-CI) quantifies changes in EGJ barrier function with surgical intervention. Neurogastroenterol Motil 2016;28:639-46. [Crossref] [PubMed]
- Carlson DA, Lin Z, Kahrilas PJ, et al. High-Resolution Impedance Manometry Metrics of the Esophagogastric Junction for the Assessment of Treatment Response in Achalasia. Am J Gastroenterol 2016;111:1702-10. [Crossref] [PubMed]
- Cho YK, Lipowska AM, Nicodeme F, et al. Assessing bolus retention in achalasia using high-resolution manometry with impedance: a comparator study with timed barium esophagram. Am J Gastroenterol 2014;109:829-35. [Crossref] [PubMed]
- Singendonk MMJ, Rosen R, Oors J, et al. Intra- and interrater reliability of the Chicago Classification of achalasia subtypes in pediatric high-resolution esophageal manometry (HRM) recordings. Neurogastroenterol Motil 2017; [Crossref] [PubMed]
Cite this article as: Myers JC, Cock C. Achalasia subtypes are front and center of the Chicago classification—strategies to overcome limitations in clinical application. Ann Esophagus 2020;3:24.


