
Esophagectomy for end-stage achalasia—is it too aggressive?
Introduction
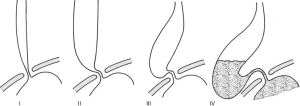
Achalasia is a rare primary motility disorder of the esophagus. Degeneration of the inhibitory neurons of the myenteric plexus within the esophagus leads to the characteristic loss of peristalsis and impaired relaxation of the lower esophageal sphincter. The subsequent abnormal emptying of esophageal contents into the stomach and stasis leads to a constellation of symptoms including dysphagia, regurgitation, aspiration, heart burn, and chest pain (1,2). Patients will often present with radiological features of esophageal dilatation on contrast studies which can be further categorized according to Rezende’s classification of Chagasic megaesophagus (Figure 1) (3).
The annual incidence of achalasia in South Australia has been estimated at 2.3 to 2.8 per 100,000 people (4). This is higher than the previously cited estimate of 0.5 to 1.6 per 100,000 that has been historically noted in studies throughout Europe, Asia, Canada and America. The management of this illness involves a multidisciplinary approach with endoscopic and surgical therapies which aim to palliate symptoms by improving gastrointestinal emptying and lowering the pressure gradient across the lower esophageal sphincter (5). Despite these treatments, 5% of patients with achalasia will progress to end-stage disease for which the management remains controversial (6).
The latest International Society for Diseases of the Esophagus (ISDE) guidelines released in 2018 state that management of recurrent symptoms should initially focus on less invasive treatments. In the instance that these methods fail, there should be progression to esophagectomy for management of end stage achalasia (7). In this report, we aim to review our own experience of end-stage achalasia by discussion of a representative case, review of the current literature, and discussion of our local results.
Case report
A 40-year-old male with type I achalasia was referred to our unit with progressively worsening dysphagia and regurgitation. He had undergone a laparoscopic cardiomyotomy and Dor fundoplication 4 years prior with symptom recurrence after approximately 12 months. He was initially treated by the original surgical team with four pneumatic dilatations with each providing only temporary control. At the time of referral, he was tolerating soft foods, with a stable BMI of 25. Other relevant co-morbidities included Type 1 diabetes mellitus which was increasingly difficult to control, and gastroparesis.
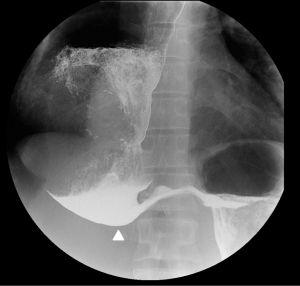
Initial investigations included esophageal manometry, barium swallow showing sump formation at the lower end and a dilated esophagus, and endoscopy. The decision was made to perform a diagnostic laparoscopy, with take-down of the original Dor fundoplication and possible redo cardiomyotomy. At surgery, a mal-positioned anterior fundoplication was found where the body of the stomach (rather than the fundus) had been inadvertently used to perform the wrap. Subsequently, there had been a band across the cardiomyotomy, causing gastro-esophageal obstruction. The patient initially reported improvement of his symptoms, but at follow-up 6 months later, reported worsening symptoms of dysphagia and regurgitation. A repeat endoscopy was performed showing copious amounts of fluid and food in the dilated esophagus despite a 48-hour fast. Fortuitously, the patient was intubated for the endoscopy to prevent aspiration. A barium swallow was also repeated showing end-stage achalasia (Figure 2). The patient agreed to proceed to an esophagectomy following discussion with a prior patient of the authors’ who had undergone esophagectomy for end-stage achalasia in 2016 with an excellent result.
An Ivor-Lewis esophagectomy and feeding jejunostomy was performed through an anterolateral thoracotomy and upper midline abdominal incision. The esophagus was mobilised and divided with a gastric pull-up and hand-sewn anastomosis performed. A pyloroplasty was performed and a feeding jejunostomy was inserted along with a routine chest drain. The intra-operative blood loss was approximately 500 mL. The post-operative period was complicated by lung atelectasis, elevated blood glucose levels, and a transient pyloric obstruction managed conservatively. The total length of stay was 14 days; 6 of which were in a high dependency unit.
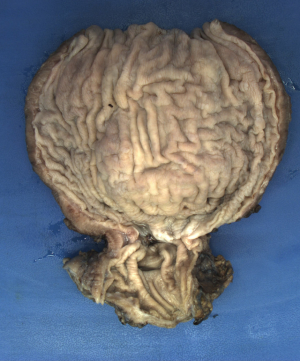
Histopathology of the resected specimen showed patchy lymphoplasmacytic inflammatory injury in the myenteric plexus with no definite ganglion cells detected (Figure 3). At 3 months, the patient reported no dysphagia, no regurgitation, and a normal diet. His weight had increased, and he reported being satisfied with the outcome of surgery.
Literature review
An extensive literature search was conducted of MEDLINE, Embase, Cochrane and ClinicalKey databases using the search terms “achalasia”, “end-stage achalasia”, “esophagectomy” and “esophageal resection” with “AND” and “OR”. English-written papers published between 1970 to 2019 were included. Abstracts and case reports of less than 5 patients were excluded.
Discussion
Achalasia is the consequence of T-cell mediated destruction of the myenteric plexus within the wall of the esophagus, with associated fibrous replacement (8). The precise etiology remains unclear. However, it is hypothesised to be multifactorial involving immunological and genetic factors (9). If left untreated, achalasia can lead to the progressive and irreversible elongation, dilatation, and loss of functionality of the esophagus (10). As a result, the diagnostic radiological feature of end-stage disease is a massively dilated and tortuous esophagus, usually greater than 6cm, known metaphorically as ‘sigmoid esophagus’ (11). The key finding on a contrast swallow, performed by an experienced radiologist, is the formation of a ‘sump’ in the lower esophagus, leading to pooling of fluid and food (Figure 2).
Further deterioration of function can then lead to significant morbidity including malnutrition, pulmonary complications from repeated aspiration, and chronic severe oesophagitis (12). Moreover, some authors have noted a 3 to 10% risk of developing esophageal squamous cell cancer due to the stasis of gastric contents inducing squamous hyperplasia with papillomatosis and basal cell hyperplasia (13,14). A recent meta-analysis in 2017 by Tustumi et al. also calculated an absolute risk increase of 18 cases per 100,000 per year for developing esophageal adenocarcinoma in patients with achalasia (15).
Management of end stage achalasia is challenging with many patients having undergone multiple failed therapeutic procedures. There is significant debate about the approach to patients with end-stage achalasia, but there is no doubt that each individual case requires thorough evaluation with repeat endoscopy, manometry, and contrast swallow to ascertain the potential cause of failure, and the appearance of the esophagus. Some of the various etiologies include inadequate myotomy (in particular, a myotomy which does not extend 2 to 3 cm onto the stomach), a tight or mal-positioned partial fundoplication, inappropriate hiatal repair, reflux esophagitis resulting in a peptic stricture, or development of a lower esophageal or junctional cancer (16).
The ISDE guidelines provide some advice on this matter. First, they recommended that failed pneumatic dilatations should undergo a cardiomyotomy, either laparoscopic or endoscopic (i.e., per oral endoscopic myotomy). Second, if there is recurrent achalasia despite a laparoscopic Heller’s myotomy, pneumatic dilatation is advised. Third, if a peroral endoscopic myotomy fails to alleviate symptoms, the patient should undergo either a laparoscopic myotomy or pneumatic dilation. In the event that each of these strategies fail, or there is radiological progression to end-stage achalasia (Figure 1), the guidelines indicate that esophagectomy should be performed. Unfortunately, these recommendations are of low-grade evidence which reflects the absence of high-quality research and lack of consensus amongst authors (7).
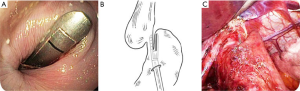
Our local institution has also looked at another option for end-stage achalasia: a laparoscopic cardioplasty. This operation was based on the treatment for a Zenker’s diverticulum, where a stapler is used to divide the common wall between the dilated esophagus and stomach, thereby divided the lower esophageal sphincter and optimizing drainage into the stomach (Figure 4). Unfortunately, our later results published in 2016 showed that most patients failed this approach due to ongoing dysphagia or unremitting reflux necessitating esophagectomy (17,18).
We believe that esophagectomy is a reasonable and appropriate option for patients with end-stage achalasia. This view is supported by many others, particularly in the setting of significant dilatation of the esophagus, and formation of a sump at the lower end (19,20). There are those who disagree, and still advocate less aggressive measures if possible (21,22). Our local experience includes 12 documented cases of patients with end-stage achalasia who have progressed to esophagectomy following either laparoscopic cardiomyotomy, or pneumatic balloon dilatation, or a combination of both. The patient in our case report had previously undergone a laparoscopic cardiomyotomy with anterior fundoplication and multiple subsequent pneumatic dilatations for management of recurrent dysphagia and regurgitation.
Esophagectomy for management of end-stage achalasia can be technically challenging with several pathological changes distorting the anatomy within the pleural and abdominal cavities. Deviation of the megaesophagus into the right chest is common, increasing the risk of pleural and tracheal injury (23). Second, the increased vascularity of hypertrophied esophageal muscle in achalasia necessitates meticulous care to ensure haemostasis of the mediastinal vessels (16). Several studies have documented cases of slow mediastinal bleeding requiring reoperation within 24 hours (13,16,23,24). Third, prior surgery at the hiatus can cause adhesions and scarring of the lower esophagus and proximal stomach. As a result, adhesions to the adjacent aorta and left lung can complicate a transhiatal mobilisation, and adhesions in the abdomen can shorten the gastric conduit, sometimes obviating an anastomosis in the neck (16).
Our preferred approach is an Ivor-Lewis esophagectomy with an open thoracotomy, and laparotomy, either synchronous or as a 2-stage procedure, for the reasons listed above. A 3-stage procedure, with a thoracoscopic approach in the chest, has also been performed in our institution. However, this approach is only possible if (I) the original myotomy was done via the abdomen, and not via the chest (to avoid adhesions between the myotomy and the lung), and (II) an anterior partial fundoplication was performed at the original procedure, not a posterior fundoplication (Toupet or Nissen) which can limit the length of the gastric conduit. We always perform a pyloromyotomy or pyloroplasty to help with gastric emptying, and we prefer a handsewn anastomosis due to the dilated esophageal lumen, which is too wide for a stapler anvil.
Open transthoracic (13,25-27), open transhiatal (11,16,20,23,24,28), and laparoscopic transhiatal (29,30) are three of the most common operations used for resection in this patient population, with much controversy regarding which is the superior approach. Miller et al. report that transhiatal esophageal resection was associated with increased morbidity and mortality and should only be reserved for patients who have had multiple prior operations due to the increased bleeding risk (20). In contrast, Orringer and co-workers state that a transhiatal approach was the most reliable technique and could be performed safely with a good level of morbidity (24). Furthermore, a left sided thoracoabdominal approach has been advocated by Hsu et al. as it provided an excellent operative field with less dissection needed of the intrathoracic esophagus and easier mobilisation of the wrapped esophagogastric junction (27).
We have had no immediate mortalities in our series, and our morbidity rate is 50%. Several studies have cited morbidity rates ranging from up to 50% (30) and mortality from 0% (11,24-29) to 9% (30). Post-operative complications including pneumonia, anastomotic leak, bleeding, chylothorax, and wound infection have all been reported (16). Anastomotic leaks were noted in the post-operative period in 4% (24) to 18% (30) of the population. These values have potentially been overstated given the lower incidence of this disease resulting in smaller sample sizes. Furthermore, the average length of stay for our patients was 20 days, which is comparable to other publications (11,13,16,20,23,24,26-30).
In summary, esophagectomy is a viable and safe option to manage end-stage achalasia. Our representative case study reported a considerable improvement in his symptoms and quality of life and wishes he had undergone esophagectomy much sooner. We are in the process of obtaining ethics to retrospectively contact all 12 patients to assess their current quality of life, and gastrointestinal function, and these results will be published in the coming year. Nevertheless, our anecdotal findings echo the outcomes observed in previous studies, which suggests that esophageal resection is not as aggressive a measure as previously believed!
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Esophagus for the series “Achalasia”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2020.03.06). The series “Achalasia” was commissioned by the editorial office without any funding or sponsorship. SKT served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Esophagus from Sep. 2019 to Aug. 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lo WK, Mashimo H. Further commentary: Pathophysioloy of Achalasia. In: Fisichella PM, Herbella FAM, Patti MG. Achalasia Diagnosis and treatment. Springer, Cham, 2016:9-14.
- Allaix M, Ramirez M, Patti MG. Further commentary: Clinical Presentation and Diagnostic Evaluation. In: Fisichella PM, Herbella FAM, Patti MG. Achalasia Diagnosis and treatment. Springer, Cham, 2016:15-21.
- Neto JG, de Cleva R, Zilberstein B, et al. Surgical risk for patients with Chagasic achalasia and its correlation with the degree of esophageal dilation. World J Gastroenterol 2005;11:5840-4. [Crossref] [PubMed]
- Duffield JA, Hamer PW, Heddle R, et al. Incidence of achalasia in South Australia based on esophageal manometry findings. Clin Gastroenterol Hepatol 2017;15:360-5. [Crossref] [PubMed]
- Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet 2014;383:83-93. [Crossref] [PubMed]
- Vela MF, Richter JE, Wachsberger D, et al. Complexities of managing achalasia at a tertiary referral center: use of pneumatic dilatation, Heller myotomy, and botulinum toxin injection. Am J Gastroenterol 2004;99:1029-36. [Crossref] [PubMed]
- Zaninotto G, Bennett C, Boeckxstaens G, et al. The 2018 ISDE achalasia guidelines. Dis Esophagus 2018;31: [Crossref] [PubMed]
- Clark SB, Rice TW, Tubbs RR, et al. Immunohistochemical analysis of the myenteric infiltrate in achalasia. Mod Pathol 1998;11:349A.
- Furuzawa-Carballeda J, Torres-Landa S, Valdovinos MÁ, et al. New insights into the pathophysiology of achalasia and implications for future treatment. World J Gastroenterol 2016;22:7892-907. [Crossref] [PubMed]
- Ellis FG. The natural history of achalasia of the cardia. Proc R Soc Med 1960;53:663-6. [Crossref] [PubMed]
- Banbury MK, Rice TW, Goldblum JR, et al. Esophagectomy with gastric reconstruction for achalasia. J Thorac Cardiovasc Surg 1999;117:1077-84. [Crossref] [PubMed]
- Eckardt VF, Hoischen T, Gudrun B. Life expectancy, complications, and causes of death in patients with achalasia: Results of a 33-year follow-up investigation. Eur J Gastroenterol Hepatol 2008;20:956-60. [Crossref] [PubMed]
- Molena D, Yang SC. Surgical management of end-stage achalasia. Semin Thorac Cardiovasc Surg 2012;24:19-26. [Crossref] [PubMed]
- Lehman MB, Clark SB, Ormsby AH, et al. Squamous mucosal alterations in esophagectomy specimens from patients with end-stage achalasia. Am J Surg Pathol 2001;25:1413-8. [Crossref] [PubMed]
- Tustumi F, Bernardo WM, da Rocha JRM, et al. Esophageal achalasia: a risk factor for carcinoma. A systematic review and meta-analysis Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Devaney EJ, Lannettoni MD, Orringer MB, et al. Esophagectomy for achalasia: patient selection and clinical experience. Ann Thorac Surg 2001;72:854-858. [Crossref] [PubMed]
- Griffiths EA, Devitt PG, Jamieson GG, et al. Laparoscopic stapled cardioplasty for end-stage achalasia. J Gastrointest Surg 2013;17:997-1001. [Crossref] [PubMed]
- Hamer PW, Griffiths EA, Devitt PG, et al. Laparoscopic Stapled Cardioplasty-Room for Improvement. J Gastrointest Surg 2016;20:1078-9. [Crossref] [PubMed]
- Ellis FH. Failure after esophagomyotomy for esophageal motor disorders: causes, prevention, and management. Chest Surg Clin N Am 1997;7:477-87. [PubMed]
- Miller DL, Allen MS, Trastek VF, et al. Esophageal resection for recurrent achalasia. Ann Thorac Surg 1995;60:922-5. [Crossref] [PubMed]
- Patti MG, Feo CV, Diener U, et al. Laparoscopic Heller myotomy relieves dysphagia when the esophagus is dilated. Surg Endosc 1999;13:843-7. [Crossref] [PubMed]
- Mineo TC, Pompeo E. Long-term outcome of Heller myotomy in achalasic sigmoid esophagus. J Thorac Cardiovasc Surg 2004;128:402-7. [Crossref] [PubMed]
- Tank AK, Kumar A, Babu TL, et al. Resectional surgery in achalasia cardia. Int J Surg 2009;7:155-8. [Crossref] [PubMed]
- Orringer MB, Stirling MC. Esophageal resection for achalasia: indications and results. Ann Thorac Surg 1989;47:340-5. [Crossref] [PubMed]
- Peters JH, Kauer WK, Crookes PF, et al. Esophageal resection with colon interposition for end-stage achalasia. Arch Surg 1995;130:632-6. [Crossref] [PubMed]
- Glatz SM, Richardson JD. Esophagectomy for End Stage Achalasia. J Gastrointest Surg 2007;11:1134-7. [Crossref] [PubMed]
- Hsu HS, Wang CY, Hsieh CC, et al. Short-segment colon interposition for end-stage achalasia. Ann Thorac Surg 2003;76:1706-10. [Crossref] [PubMed]
- Lewandowski A. Diagnostic criteria and surgical procedure for megaesophagus–a personal experience. Dis Esophagus 2009;22:305-9. [Crossref] [PubMed]
- Schuchert MJ, Luketich JD, Landreneau RJ, et al. Minimally invasive surgical treatment of sigmoidal esophagus in achalasia. J Gastrointest Surg 2009;13:1029-35. [Crossref] [PubMed]
- Palanivelu C, Rangarajan M, Jategaonkar PA, et al. Laparoscopic transhiatal esophagectomy for ‘sigmoid’ megaesophagus following failed cardiomyotomy: experience of 11 patients. Dig Dis Sci 2008;53:1513-8. [Crossref] [PubMed]
Cite this article as: Kundu NR, Thompson SK. Esophagectomy for end-stage achalasia—is it too aggressive? Ann Esophagus 2020;3:23.


